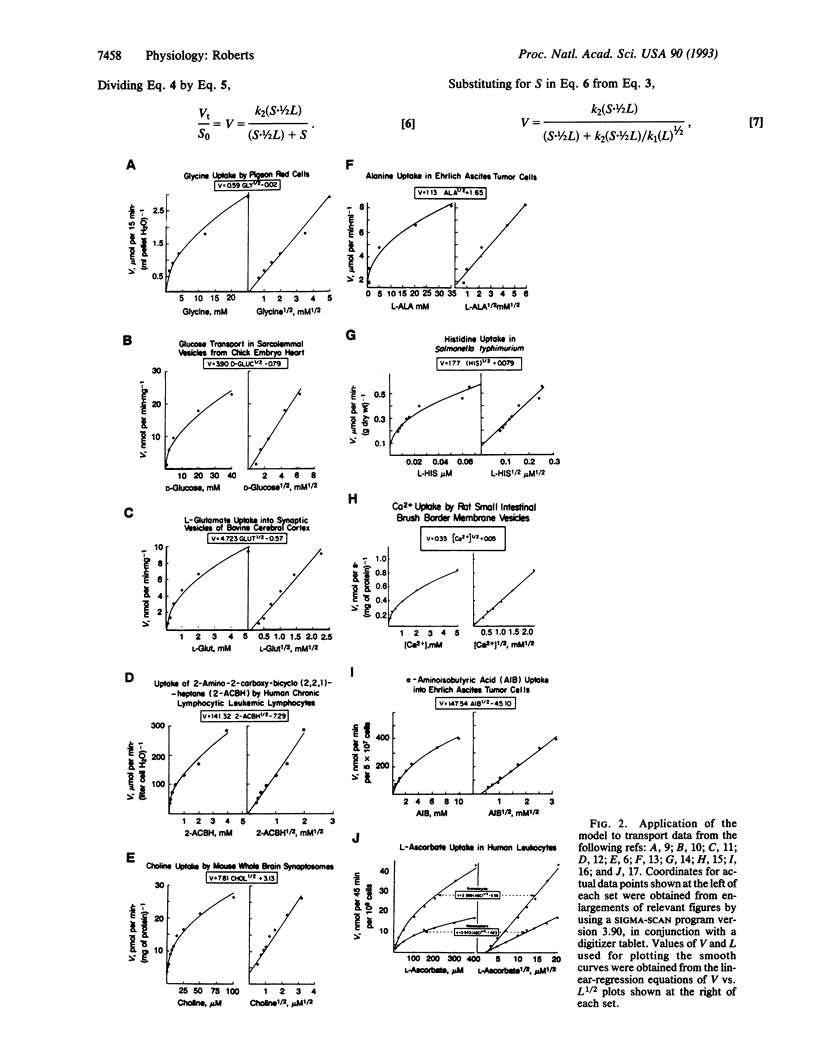
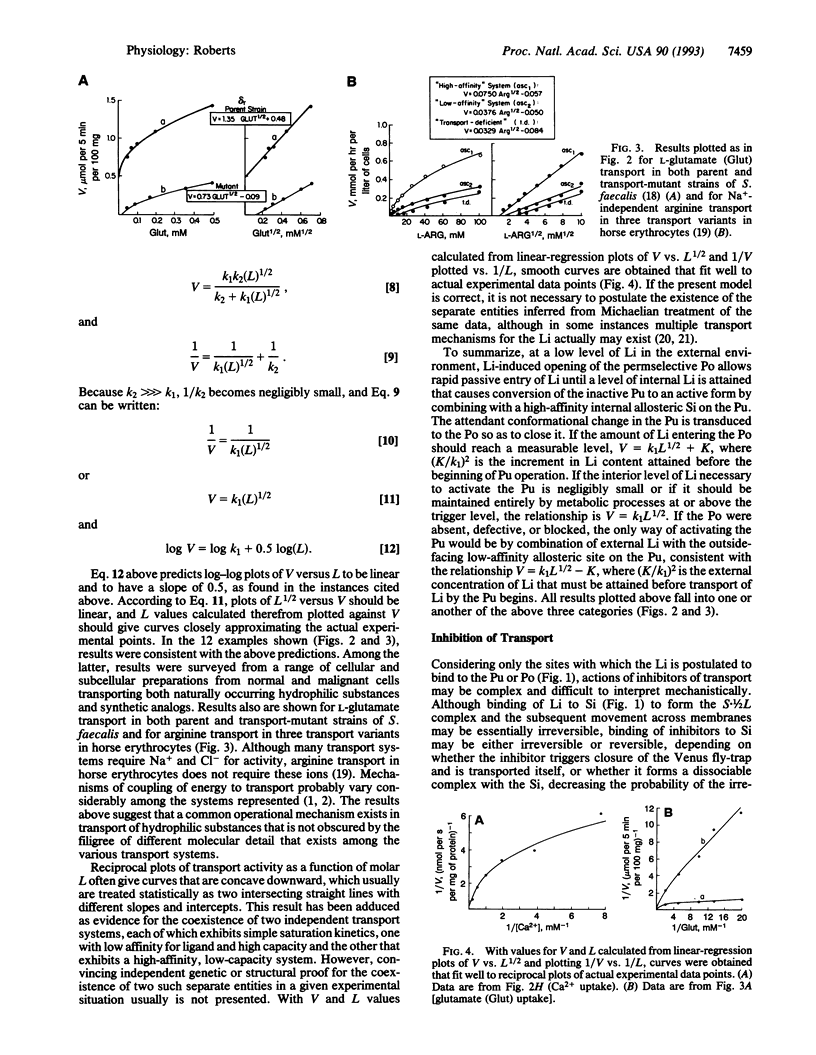
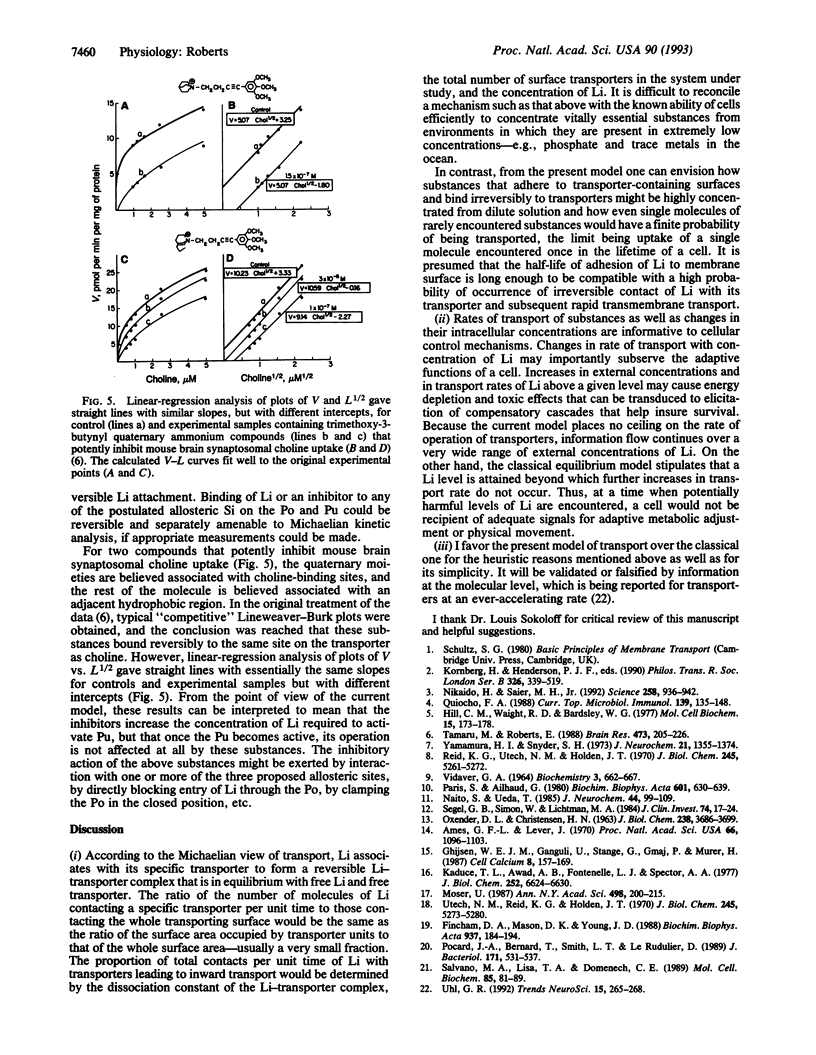
Abstract
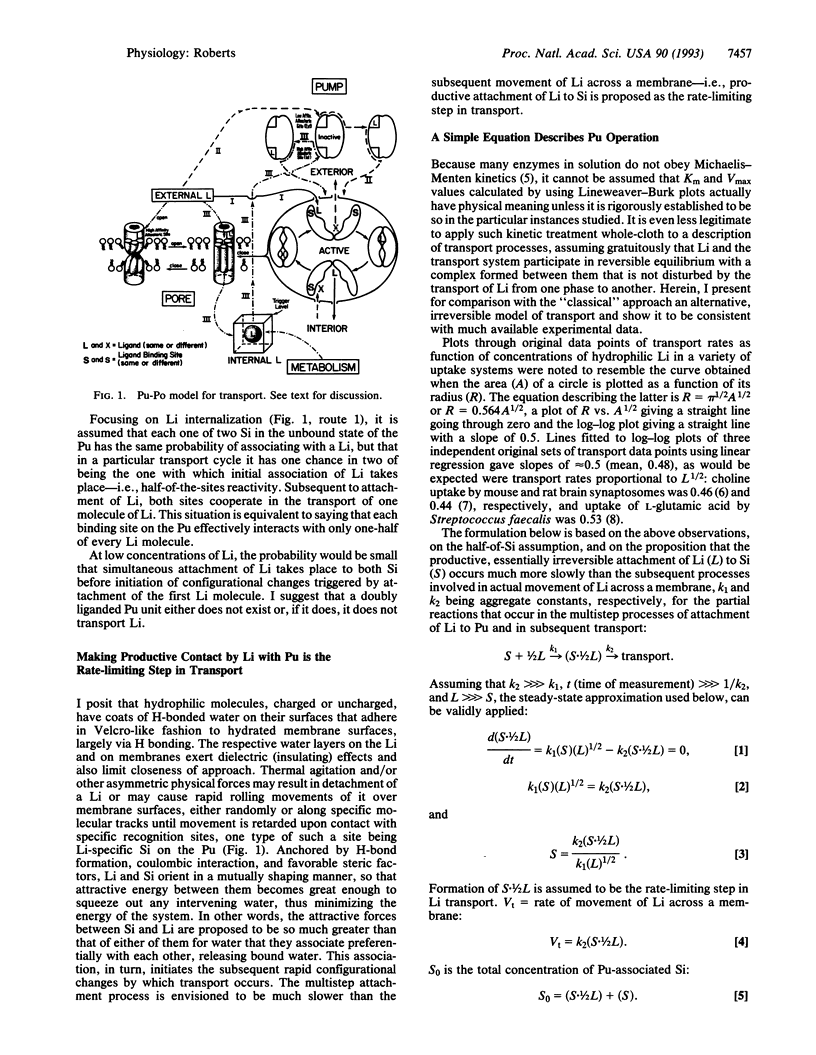
Transmembrane transport of a hydrophilic solute is presumed to begin when hydrated ligand adheres in Velcro-like fashion to hydrated membrane surface. Asymmetric physical forces cause rolling movements of ligand over membrane surface until contact occurs with appropriate transport machinery, consisting of a pump (Pu) to which is tethered a ligand (Li)-specific perm-selective pore (Po). The Po is in the open form when the Li is attached to an external high-affinity allosteric site on it. The active form of the Pu is stabilized by attachment of the Li to high-affinity internal or low-affinity external allosteric sites. The active form of the Pu induces closure of the Po, even when ligand is bound to it; the inactive conformation of the Pu permits Po opening. Attachment of Li to either one of two binding sites on the active Pu and irreversible envelopment by it in Venus fly-trap fashion trigger transmembrane transport of Li. Multistep attachment of Li is rate-limiting in the transport process. Application of a simple equation derived from relevant kinetic considerations relating velocity of transport (V) to concentration of Li (L), V = k1(L)1/2, gives V-L curves approximating transport data obtained in a variety of biological systems. This model is congruent with the ability of cells to concentrate substances from extremely dilute solutions and with the adaptive informational value to cells of rates of transport.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R. A., Hughey R. P. The identification of two subcellular sites for cleavage of gamma-glutamyltranspeptidase propeptide. Biochem Int. 1986 Dec;13(6):1009–1017. [PubMed] [Google Scholar]
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Bulle F., Mattei M. G., Siegrist S., Pawlak A., Passage E., Chobert M. N., Laperche Y., Guellaën G. Assignment of the human gamma-glutamyl transferase gene to the long arm of chromosome 22. Hum Genet. 1987 Jul;76(3):283–286. doi: 10.1007/BF00283624. [DOI] [PubMed] [Google Scholar]
- Cantin A. M., North S. L., Hubbard R. C., Crystal R. G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987 Jul;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Capraro M. A., Hughey R. P. Processing of the propeptide form of rat renal gamma-glutamyltranspeptidase. FEBS Lett. 1983 Jun 27;157(1):139–143. doi: 10.1016/0014-5793(83)81132-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P. Role of gamma-glutamyltranspeptidase in the renal metabolism of glutathione. Miner Electrolyte Metab. 1983;9(4-6):236–245. [PubMed] [Google Scholar]
- Dinsdale D., Green J. A., Manson M. M., Lee M. J. The ultrastructural immunolocalization of gamma-glutamyltranspeptidase in rat lung: correlation with the histochemical demonstration of enzyme activity. Histochem J. 1992 Mar;24(3):144–152. doi: 10.1007/BF01047464. [DOI] [PubMed] [Google Scholar]
- Fincham D. A., Mason D. K., Young J. D. Dibasic amino acid interactions with Na+-independent transport system asc in horse erythrocytes. Kinetic evidence of functional and structural homology with Na+-dependent system ASC. Biochim Biophys Acta. 1988 Jan 13;937(1):184–194. doi: 10.1016/0005-2736(88)90240-4. [DOI] [PubMed] [Google Scholar]
- Finidori J., Laperche Y., Haguenauer-Tsapis R., Barouki R., Guellaen G., Hanoune J. In vitro biosynthesis and membrane insertion of gamma-glutamyl transpeptidase. J Biol Chem. 1984 Apr 25;259(8):4687–4690. [PubMed] [Google Scholar]
- Gerard N. P., Eddy R. L., Jr, Shows T. B., Gerard C. The human neurokinin A (substance K) receptor. Molecular cloning of the gene, chromosome localization, and isolation of cDNA from tracheal and gastric tissues. J Biol Chem. 1990 Nov 25;265(33):20455–20462. [PubMed] [Google Scholar]
- Gerard N. P., Garraway L. A., Eddy R. L., Jr, Shows T. B., Iijima H., Paquet J. L., Gerard C. Human substance P receptor (NK-1): organization of the gene, chromosome localization, and functional expression of cDNA clones. Biochemistry. 1991 Nov 5;30(44):10640–10646. doi: 10.1021/bi00108a006. [DOI] [PubMed] [Google Scholar]
- Gerard N. P., Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991 Feb 14;349(6310):614–617. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- Ghijsen W. E., Ganguli U., Stange G., Gmaj P., Murer H. Calcium uptake into rat small intestinal brush border membrane vesicles: characterization of transmembrane calcium transport at short initial incubation times. Cell Calcium. 1987 Apr;8(2):157–169. doi: 10.1016/0143-4160(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Goodspeed D. C., Dunn T. J., Miller C. D., Pitot H. C. Human gamma-glutamyl transpeptidase cDNA: comparison of hepatoma and kidney mRNA in the human and rat. Gene. 1989 Mar 15;76(1):1–9. doi: 10.1016/0378-1119(89)90002-4. [DOI] [PubMed] [Google Scholar]
- Hill C. M., Waight R. D., Bardsley W. G. Dose any enzyme follow the Michaelis-Menten equation? Mol Cell Biochem. 1977 May 3;15(3):173–178. doi: 10.1007/BF01734107. [DOI] [PubMed] [Google Scholar]
- Kaduce T. L., Awad A. B., Fontenelle L. J., Spector A. A. Effect of fatty acid saturation on alpha-aminoisobutyric acid transport in Ehrlich ascites cells. J Biol Chem. 1977 Oct 10;252(19):6624–6630. [PubMed] [Google Scholar]
- Matsuda Y., Tsuji A., Katunuma N. Studies on the structure of gamma-glutamyltranspeptidase. III. Evidence that the amino terminus of the heavy subunit is the membrane binding segment. J Biochem. 1983 May;93(5):1427–1433. doi: 10.1093/oxfordjournals.jbchem.a134278. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Moser U. Uptake of ascorbic acid by leukocytes. Ann N Y Acad Sci. 1987;498:200–215. doi: 10.1111/j.1749-6632.1987.tb23762.x. [DOI] [PubMed] [Google Scholar]
- Nash B., Tate S. S. In vitro translation and processing of rat kidney gamma-glutamyl transpeptidase. J Biol Chem. 1984 Jan 10;259(1):678–685. [PubMed] [Google Scholar]
- Orning L., Hammarström S., Samuelsson B. Leukotriene D: a slow reacting substance from rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2014–2017. doi: 10.1073/pnas.77.4.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S., Ailhaud G. Amino acid and glucose transport in sarcolemmal vesicles from chick embryo heart. Biochim Biophys Acta. 1980 Oct 2;601(3):630–639. doi: 10.1016/0005-2736(80)90564-7. [DOI] [PubMed] [Google Scholar]
- Pawlak A., Cohen E. H., Octave J. N., Schweickhardt R., Wu S. J., Bulle F., Chikhi N., Baik J. H., Siegrist S., Guellaën G. An alternatively processed mRNA specific for gamma-glutamyl transpeptidase in human tissues. J Biol Chem. 1990 Feb 25;265(6):3256–3262. [PubMed] [Google Scholar]
- Pawlak A., Lahuna O., Bulle F., Suzuki A., Ferry N., Siegrist S., Chikhi N., Chobert M. N., Guellaen G., Laperche Y. gamma-Glutamyl transpeptidase: a single copy gene in the rat and a multigene family in the human genome. J Biol Chem. 1988 Jul 15;263(20):9913–9916. [PubMed] [Google Scholar]
- Pawlak A., Wu S. J., Bulle F., Suzuki A., Chikhi N., Ferry N., Baik J. H., Siegrist S., Guellaën G. Different gamma-glutamyl transpeptidase mRNAs are expressed in human liver and kidney. Biochem Biophys Res Commun. 1989 Oct 31;164(2):912–918. doi: 10.1016/0006-291x(89)91545-3. [DOI] [PubMed] [Google Scholar]
- Pocard J. A., Bernard T., Smith L. T., Le Rudulier D. Characterization of three choline transport activities in Rhizobium meliloti: modulation by choline and osmotic stress. J Bacteriol. 1989 Jan;171(1):531–537. doi: 10.1128/jb.171.1.531-537.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiocho F. A. Molecular features and basic understanding of protein-carbohydrate interactions: the arabinose-binding protein-sugar complex. Curr Top Microbiol Immunol. 1988;139:135–148. doi: 10.1007/978-3-642-46641-0_5. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E., Heisterkamp N., Groffen J. Cloning and nucleotide sequence of human gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8840–8844. doi: 10.1073/pnas.85.23.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. G., Utech N. M., Holden J. T. Multiple transport components for dicarboxylic amino acids in Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5261–5272. [PubMed] [Google Scholar]
- Sakamuro D., Yamazoe M., Matsuda Y., Kangawa K., Taniguchi N., Matsuo H., Yoshikawa H., Ogasawara N. The primary structure of human gamma-glutamyl transpeptidase. Gene. 1988 Dec 15;73(1):1–9. doi: 10.1016/0378-1119(88)90307-1. [DOI] [PubMed] [Google Scholar]
- Salvano M. A., Lisa T. A., Domenech C. E. Choline transport in Pseudomonas aeruginosa. Mol Cell Biochem. 1989 Jan 23;85(1):81–89. doi: 10.1007/BF00223517. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Simon W., Lichtman M. A. Multicomponent analysis of amino acid transport in human lymphocytes. Diminished L-system transport in chronic leukemic B lymphocytes. J Clin Invest. 1984 Jul;74(1):17–24. doi: 10.1172/JCI111398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stole E., Meister A. Interaction of gamma-glutamyl transpeptidase with glutathione involves specific arginine and lysine residues of the heavy subunit. J Biol Chem. 1991 Sep 25;266(27):17850–17857. [PubMed] [Google Scholar]
- Stole E., Seddon A. P., Wellner D., Meister A. Identification of a highly reactive threonine residue at the active site of gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1706–1709. doi: 10.1073/pnas.87.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaru M., Roberts E. Structure-activity studies on inhibition of choline uptake by a mouse brain synaptosomal preparation: basic data. Brain Res. 1988 Nov 15;473(2):205–226. doi: 10.1016/0006-8993(88)90850-5. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem. 1981 Sep 25;39:357–368. doi: 10.1007/BF00232585. [DOI] [PubMed] [Google Scholar]
- Uhl G. R. Neurotransmitter transporters (plus): a promising new gene family. Trends Neurosci. 1992 Jul;15(7):265–268. doi: 10.1016/0166-2236(92)90068-j. [DOI] [PubMed] [Google Scholar]
- Utech N. M., Reid K. G., Holden J. T. Properties of a dicarboxylic amino acid transport-deficient mutant of Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5273–5280. [PubMed] [Google Scholar]
- VIDAVER G. A. TRANSPORT OF GLYCINE BY PIGEON RED CELLS. Biochemistry. 1964 May;3:662–667. doi: 10.1021/bi00893a011. [DOI] [PubMed] [Google Scholar]
- Wetmore L. A., Gerard N. P., Herron D. K., Bollinger N. G., Baker S. R., Feldman H. A., Drazen J. M. Leukotriene receptor on U-937 cells: discriminatory responses to leukotrienes C4 and D4. Am J Physiol. 1991 Aug;261(2 Pt 1):L164–L171. doi: 10.1152/ajplung.1991.261.2.L164. [DOI] [PubMed] [Google Scholar]



