Abstract
Purpose
A minimally invasive approach for cochlear implantation involves drilling a narrow linear path through the temporal bone from the skull surface directly to the cochlea for insertion of the electrode array without the need for an invasive mastoidectomy. Potential drill positioning errors must be accounted for to predict the effectiveness and safety of the procedure. The drilling accuracy of a system used for this procedure was evaluated in bone surrogate material under a range of clinically relevant parameters. Additional experiments were performed to isolate the error at various points along the path to better understand why deflections occur.
Methods
An experimental setup to precisely position the drill press over a target was used. Custom bone surrogate test blocks were manufactured to resemble the mastoid region of the temporal bone. The drilling error was measured by creating divots in plastic sheets before and after drilling and using a microscope to localize the divots.
Results
The drilling error was within the tolerance needed to avoid vital structures and ensure accurate placement of the electrode; however, some parameter sets yielded errors that may impact the effectiveness of the procedure when combined with other error sources. The error increases when the lateral stage of the path terminates in an air cell and when the guide bushings are positioned further from the skull surface. At contact points due to air cells along the trajectory, higher errors were found for impact angles of 45° and higher as well as longer cantilevered drill lengths.
Conclusion
The results of these experiments can be used to define more accurate and safe drill trajectories for this minimally invasive surgical procedure.
Keywords: Drilling accuracy, Temporal bone, Cochlear implantation, Minimally invasive surgery
Introduction
Cochlear implantation (CI) is the current state-of-the-art for providing the ability to perceive sound for individuals with severe to profound sensorineural hearing loss. The CI system consists of several key components: an external microphone picks up sound from the environment, a processing unit filters the sound and sends the data to a subcutaneous receiver, and a transmitter then sends electrical impulses to an electrode array implanted within the cochlea, which ultimately stimulates the nerve. During CI surgery, the internal receiver is subcutaneously placed and the electrode array is implanted into the cochlea. The standard surgical approach for CI is invasive and begins with an incision behind the ear to expose the mastoid region of the temporal bone followed by a mastoidectomy, which involves removing a bulk portion of bone with a high-speed drill. Mastoidectomy allows identification of critical anatomical structures along the surgical path and access to the cochlea for insertion of the electrode array via a region known as the facial recess, which is bounded by the facial nerve and one of its branches, the chorda tympnai [1].
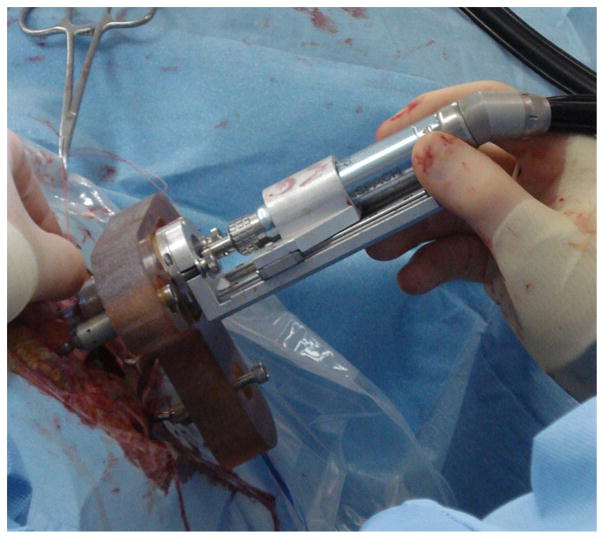
Several research groups have been exploring alternatives to the traditional CI surgical approach to reduce the invasiveness of the surgery [2–19]. One such minimally invasive approach for CI surgery is to drill a linear path from the exterior surface of the skull to the cochlea via the facial recess (Fig. 1) using custom microstereotactic frames [13,14,20–22]. A safe linear trajectory is chosen utilizing anatomical details in a computed tomography (CT) scan of the patient. The custom microstereotactic frame is used to constrain the drill along the desired trajectory. We utilize a microstereotactic frame system called the Microtable® that can be manufactured using a standard computer numeric control (CNC) milling machine within five minutes [20]. The Microtable attaches to the patient via bone-implanted markers and constrains a drill press, which is mounted on the Microtable, along the desired trajectory (Fig. 2). The drill press facilitates the linear slide of the surgical drill along the trajectory defined by the Microtable. A two-stage drilling approach is used for this technique [21,22]: a pilot hole through the lateral portion of the mastoid is made with a larger twist drill bit (~3.8 mm in diameter) and a narrower twist drill bit (~1.6 mm in diameter) is used medially to drill farther toward the cochlea through the facial recess. The narrow drill bit is required in the medial portion of the trajectory to avoid damage to critical anatomy; however, using this size, drill bit for the entire trajectory would lead to large deflections. Thus, a wider hole is drilled for the lateral stage. This wider hole also allows for a bushing to sit below the skull surface and guide the medial drill bit.
Fig. 1.
a Slice of CT scan showing amount of bone to be removed in traditional (dotted white outline) versus minimally invasive cochlear implantation surgery (shaded drill path). b Close-up of minimally invasive drill trajectory and surrounding anatomical structures
Fig. 2.
Microtable is anchored to skull using bone-implanted anchors, and the drill press mounts to the Microtable, allowing the surgeon to slide the drill into the skull along the desired trajectory
A challenge of this surgical approach is the close proximity of the facial nerve to the desired drill path, which necessitates submillimetric accuracy [23]. Damage to the facial nerve can cause weakness of the muscles in the face or facial paralysis and, therefore, must be avoided. Additionally, submillimetric accuracy is necessary to successfully gain access to the cochlea for insertion of the electrode. The drill bits are susceptible to deflections caused by transverse loadings at their tips. The medial bit, which must pass close to the facial nerve along its path, is particularly error prone due to its small diameter and the fact that it must extend deep into the skull. Thus, the drilling accuracy and potential errors must be understood to ensure patient safety and accurate placement of the implant using this surgical approach. Williamson et al. [24] used the correlation between force and bone density while drilling through the mastoid bone to estimate the location of the drill tip. Their algorithm also provided the surgeon with preoperative density information to assess the risk of the drill deviating from the planned path. Kobler et al. [25] presented a method for evaluating various types of drill bits for this procedure with respect to borehole accuracy. They measured the drilling error through bone surrogate materials for various bone surface angles and determined that a drilling strategy employing a gun barrel drill bit for the medial path resulted in better accuracy than twist drill bits and spherical surgical bits.
The work presented in this paper uses a similar approach to that used in [25] and expands upon this work to investigate the effect of several key factors on the drilling accuracy during minimally invasive CI, which can be used to assess the efficacy of the surgery and define safer drill paths. Specifically, the effects of the following parameters on drilling accuracy are assessed for the system that is currently used clinically: (1) skull surface angles, or impact angles, at the entry point; (2) the use of bushings to guide the drill and the bushing locations with respect to the trajectory; (3) the composition of bone at the transition between drilling stages (i.e., the point where the lateral hole ends and medial drilling begins); (4) cantilevered length of the medial drill bit when passing through air cells; and (5) angle of bone at the air/bone interface of an air cell along the trajectory. Some of these factors are discussed in a general sense in prior works; however, a thorough investigation has not yet been performed. Finally, the errors measured in this study are discussed in the context of the overall accuracy of the surgical procedure and used to identify anatomical conditions that may lead to unacceptably large error.
Experimental methods
Setup and procedure
The experimental setup was designed to mimic the minimally invasive CI approach using the Microtable while isolating the error caused by deviations in the drill path. In practice, there are multiple additional sources of error, including error in (1) identification of vital structures and target points, (2) registration, (3) manufacture and assembly of the Microtable, and (4) mounted position of the Microtable relative to the patient. In the current work, our intent was to analyze the error in targeting due to drilling through bone with air cells. The presence of mastoid air cells differs considerably among patients based on individual temporal bone anatomy. Thus, it is necessary to evaluate various anatomical effects on this particular component of the surgical error.
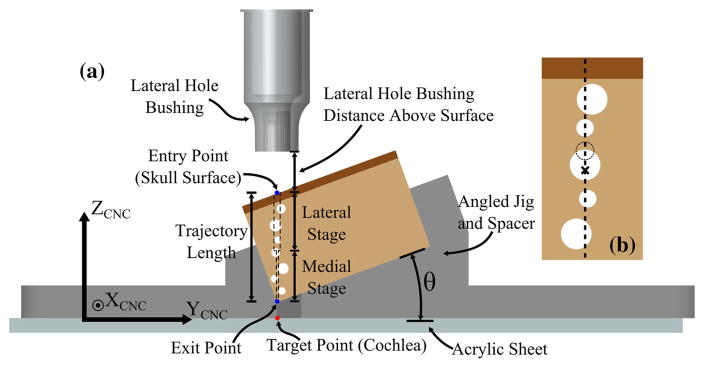
Drilling accuracy was measured by making divots in an acrylic sheet that is positioned under a test piece before and after drilling. For the study, the drill press was mounted to a CNC milling machine (Exact Jr. 3-axis CNC, Broussard Enterprises, Inc., Santa Fe Springs, California, USA, with a Fagor 8040 M CNC controller (Fagor automation, Elk Grove Village, IL) using a custom bracket (Fig. 3). This setup allowed precise positioning and movement of the drill press as the maximum CNC positioning error is 0.0005 in (0.0127 mm). The drill press was placed in the bracket mounted to the CNC machine such that the drill press was along the z-axis of the CNC machine. However, only the x- and y-axes of the CNC were used for positioning the drill press. The z-axis of the CNC was fixed throughout the experiments, and the surgical drill press used for moving the drill up and down. Test blocks made of a bone surrogate material were placed in a jig held by the milling machine vise. An acrylic sheet was placed under the test block and held in place by the vise. For each target point, two predrilling divots were made in the acrylic sheet using the drill bit tip with the drill powered off before inserting the test block and performing the drilling. These divots were located 1 mm in either direction of the target point along the y-axis of the CNC. Then, the test block was inserted, the drill press moved to the target point (the center of the line segment formed by the two predrilling divots), and the two-stage drilling was performed. After completion of the drilling through the test block, the drill was turned off and the tip of the drill bit pushed into the acrylic sheet to make another divot (Fig. 4). The jig held the test block slightly above the acrylic sheet because there is an air gap between the end of the drill path and the cochlea in a clinical case. This existence of space also allowed for the drilling to stop in this gap prior to making the postdrilling divot.
Fig. 3.
a Experimental setup showing drill press mounted to bracket on CNC milling machine. b Bone surrogate material made from short-fiber-filled epoxy (top layer representing cortical/surface bone) and solid rigid polyurethane foam (bottom layer representing mastoid bone)
Fig. 4.
Steps in the experimental procedure for a single targeting trial: a creation of predrilling divots. The drill press is moved along the negative y-axis of the CNC milling machine such that the y-coordinate of CNC, YCNC, is Yoffset. The drill is moved down along the z-axis such that the drill tip makes a divot in the acrylic sheet. This divot is called the predrilling divot 1. The same process is repeated by moving the drill press along the positive y-axis to YCNC = Yoffset to make the predrilling divot 2. b Creation of the postdrilling divot. The jig and test block are inserted, and the drill press is moved to YCNC = 0. Drilling is performed through the test block. With the drill turned off, the drill is moved down into acrylic to make postdrilling divot
Drill system
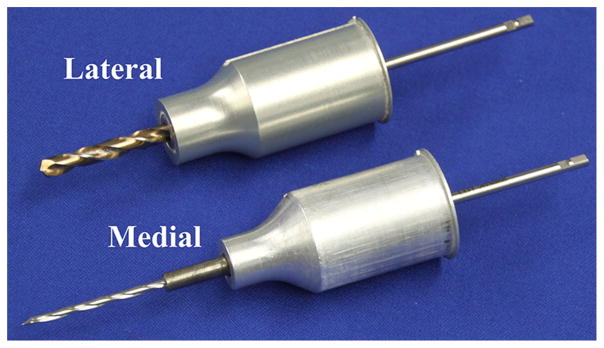
The drill press mounted to the CNC milling machine using the same coupling used to mount it to the Microtable. A surgical drill (Anspach eMax 2, Synthes, Inc., West Chester, PA, USA) was fixed in a clamp on the linear slide of the drill press with its length calibrated preoperatively so that it drills to the correct depth. Two drill bits were used: a 3.8-mm twist drill bit made from cobalt steel for the lateral stage and a 1.59-mm CingleBitTM drill bit made from hardened stainless steel for the medial hole (Orchid Orthopedic Solutions, Holt, MI, USA). For each drill bit, there was also a bushing assembly that mounted to the Microtable to control the movement of the drill bits. Both drill bits and bushings are shown in Fig. 5.
Fig. 5.
Lateral (top) and medial (bottom) drill bits and bushings used for minimally invasive cochlear implantation
Bone surrogate materials
The test blocks (Fig. 3b) were Sawbones biomechanical test materials (Pacific Research Laboratories, Inc., Vashon Island, Washington, USA). The temporal bone consists of a thin layer of dense cortical bone at the surface and a mastoid bone region with air cells deeper into the skull. Hence, a custom laminated block was manufactured consisting of a 3-mm top layer made from short-fiber- filled epoxy representing the cortical bone and a 12- to 25-mm bottom layer of solid rigid polyurethane foam (ρ = 50 lb/ft2) representing the mastoid bone. This material was previously used as a temporal bone surrogate in studies by Kobler et al. [25,26]. Holes (2–3.5 mm in diameter) were drilled in the bottom layer of the test blocks to represent the mastoid air cells present in the temporal bone. Two different patterns were chosen for the locations of the air cells with the difference between the two being the composition of bone at the location where the lateral drilling ends (air cell versus solid bone). The size of the air cells were chosen based on a review of clinical CT data. The air cell patterns are not intended to represent specific patient anatomy; however, it has been observed in clinical cases that the lateral hole ends in an air cell for some patients and solid bone for others. Thus, this characteristic is differentiated in the two patterns.
Divot localization and error calculation
The target point was defined as the midpoint of the line segment connecting the two predrilling divots. The error was therefore the vector displacement between this target and the divot made after drilling (Fig. 6). An image of the three divots was acquired using an optical microscope, and then, the divots were localized in the image using a custom, semiautomated program written in MATLAB (The MathWorks, Inc., Natick, MA, USA). A calibration grid slide (Thorlabs, Inc, Newton, NJ, USA, part #R1L3S3P) was placed on the acrylic sheet during the acquisition of the microscope image, and various points on the grid were selected in software to calculate the scale of the image. For each set of points, the distance between the predrilling divots was checked to make sure that it was 2 mm as prescribed (with a tolerance of ±0.01 mm).
Fig. 6.
a Photograph of predrilling and postdrilling divots in acrylic sheet under microscope. b Schematic of error calculation. The virtual target is defined as the midpoint of the two predrilling divots
Validation of method
A control study with 12 trials was performed to validate the measurement method. The acrylic sheet was held in place with the vise, and no test block was used in this control study. For each trial in this control study, the following four steps were performed: (1) The CNC machine was moved to desired position along the x- and y-axes for the trajectory, and x and y values were zeroed. (2) With x-axis of the CNC machine held constant at zero, two predrilling divots were made as described earlier by moving the CNC machine along the y-axis to −Yoffset and +Yoffset positions and moving the drill in the drill press down to make the divots. (3) A target divot was made by moving the CNC machine to y = 0 (x still at zero). (4) The error in the target divot location was calculated as described above. All the divots were made with the medial drill bit. The calculated error for these control trials represents a combination of the CNC positioning error, error in localizing the predrilling divots in the image and calculation of the target point based on these locations, and error localizing the postdrilling divot. If the localization of the divots was perfect, the errors measured in these control trials would fall within the tolerance of the CNC. The control study trials (n = 12) had a mean error of 0.012 ± 0.008 mm, which is slightly outside the positioning tolerance of the CNC, indicating that there is a small amount of error between the various error sources described above. However, the drill tip errors measured in these trials are considerably lower than the errors in the drilling experiments and the required accuracy of the surgery, confirming that this is a suitable method of measuring drill deviation for this study. Additional trials were performed to check for errors that may result from repeated mounting and removal of the drill press from the Microtable mount (e.g., between trials and when changing drill bits). The errors observed in these additional trials were also inconsequential.
Drilling accuracy experiments
Two groups of drilling experiments were performed as part of this study. First, the full, two-stage drilling was completed on the laminated test blocks to assess the drilling accuracy and examine the effects of various clinical parameters on the accuracy. Next, experiments were performed to further isolate the drilling error at individual bone contact points along the medial drill path (air/bone interfaces at air cells) to identify specific anatomical features within the mastoid that may influence drilling accuracy.
Two-stage drilling experiments
Data from prior clinical studies of the minimally invasive image-guided CI system [22] were used to select drilling parameters used in these experiments, including the depth of drilling for the lateral and medial drilling, positioning of drill guide bushings, angle of drill relative to skull surface, and location of the cochlea relative to the end of the drilling path (Table 1). In the experiments, three variables were adjusted: angle of drill relative to the skull surface, location of bushings relative to skull surface, and composition of bone at the end of the lateral hole (solid bone vs. air cell). Distance between target point (cochlea), where we wanted to measure the error, and the end of the medial drilling was fairly consistent among patients and therefore kept constant in these experiments. Figure 7 provides a schematic of the test setup with the various parameters displayed. A total of ten combinations of parameters were tested with twelve trials per set. Statistical analysis was performed on the error data to determine which parameters significantly affected the drilling accuracy. The data for each set of parameters was first checked for normality, and then, comparative tests were used to determine whether the means were significantly different. Additionally, the results from these experiments can be used, along with an estimate of other sources of error in the system, to estimate the overall error of the procedure, etotal (RMS). In a prior study [20], the free-space positioning error, epos (RMS), resulting from image processing, Microtable manufacture, and Microtable assembly errors, was measured. Assuming both error sources are normally distributed, the root-mean-square (RMS) errors for free-space positioning and drilling can be added in quadrature to calculate total RMS error.
Table 1.
Parameters for accuracy evaluation (two-stage drilling) experiments
| Parameter | Average from clinical data | Value(s) used in experiments |
|---|---|---|
| Drill depth for lateral drilling | 13.0 mm | 13.0 mm |
| Drill depth for medial drilling | 9.9 mm | 10.0 mm |
| Angle of drill relative to skull surface at point of entry | 40° | 0°, 20°, 40°, 60° |
| Distance of the base of the bushing above the skull surface along the trajectorya | Lateral: +9.3 mm | Lateral: +6, +9, +14 mm |
| Medial: −2.7 mm | Medial: −6, −3, +2 mm | |
| Composition of bone when the lateral drilling ends | Solid bone or air cell | Solid bone or air cell |
| Drill speed | 20,000–80,000 rpm | 30,000 rpm |
A positive value indicates that the bushing ends above the skull surface. A negative value indicates that the bushing is within the temporal bone
Fig. 7.
a Schematic of test setup indicating the various parameters used in the experiments. The length of drill path and location of target point relative to the skull surface were held constant. The angle of the surface, height of bushings, and composition of bone at the end of the lateral hole were varied. Note the lateral bushing is shown in this figure. During the medial drilling stage, a different guide bushing is used, which extends into the hole created by the lateral drilling. b Close-up of the mastoid air cell pattern for the case of the lateral stage ending in an air cell. For the case of the lateral stage ending in solid bone, the large middle air cell is replaced with a smaller air cell located at a shallower depth (dashed line)
The edrill (RMS) value was measured by the two-stage drilling experiments. The RMS errors of the free-space positioning and drilling errors are given by and , respectively.
Medial drilling experiments
Major contribution to the drill deviation error was assumed to be due to the deflection of the medial drill bit since it is narrower and is used to drill deeper than the lateral drill bit. This effect has been observed in some of the cadaveric testing where the lateral hole was aligned well to the desired trajectory, but the end of the medial hole missed the target. Therefore, experiments to isolate the error during the medial drilling were performed. Most of the deflection of the drill tip, which leads to inaccurate hole placement, occurs at the initial penetration of the material [27]. For the case of solid, homogeneous material, this penetration is at the surface of the material only. In the mastoid, the presence of air cells results in many air/bone interfaces along the path and thus numerous penetration (deflection) points. The overall drilling error is a result of the combination of these numerous sources of error. This overall error is captured in the first group of experiments discussed above. The purpose of this second group of experiments is to identify the parameters that lead to large errors during the medial stage of drilling. Again, various angles were tested (0°–75°) as well as different cantilevered lengths of the drill bit (i.e., length of drill bit extended out from bushing when contacting bone). The same protocol described above in the Experimental Methods section was used for these trials with the exceptions that drilling was performed only using the medial drill bit and thinner blocks made of only solid rigid polyurethane foam (representing mastoid region only) were used for this study.
Experimental results and discussion
Two-stage drilling experiments
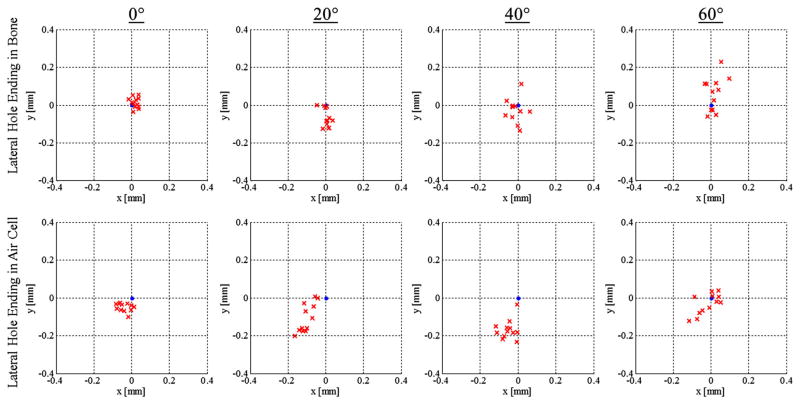
The experimental results for the ten cases tested in the first group of experiments are provided in Table 2, and scatter plots for this data are given in Fig. 8. The range of errors meets the accuracy requirements of the surgery; however, there are some parameter sets that lead to larger errors that, when combined with the other sources of error in the surgery, could lead to insufficient targeting accuracy. Specifically, combining the RMS errors observed in these experiments (0.039–0.247mm) with the free- space positioning data (epos(RMS) = 0.40mm) [20], yields an overall RMS error of 0.41–0.48 mm. The results for the different parameters are discussed in the sections below. Note that in all trials, the block was angled such that the positive y-axis of the CNC machine pointed up the slope.
Table 2.
Drilling accuracy data for two-stage drilling experiments
| Casea | Parametersb | Targeting error magnitude (mm)
|
X–Y position of error (mm)
|
|||
|---|---|---|---|---|---|---|
| μ ± σ | RMS | Max. | X, μ ± σ | Y, μ ± σ | ||
| 1 | 0°/Bone/9 mm | 0.036 ± 0.015 | 0.039 | 0.064 | 0.019 ± 0.018 | 0.012 ± 0.028 |
| 2 | 20°/Bone/9 mm | 0.074 ± 0.044 | 0.086 | 0.126 | 0.003 ± 0.021 | −0.068 ± 0.048 |
| 3 | 40°/Bone/9 mm | 0.066 ± 0.039 | 0.077 | 0.136 | −0.012 ± 0.036 | −0.027 ± 0.063 |
| 4 | 60°/Bone/9 mm | 0.093 ± 0.063 | 0.112 | 0.235 | 0.017 ± 0.036 | 0.060 ± 0.089 |
| 5 | 0°/Air/9 mm | 0.070 ± 0.021 | 0.073 | 0.100 | −0.039 ± 0.032 | −0.049 ± 0.022 |
| 6 | 20°/Air/9 mm | 0.153 ± 0.071 | 0.169 | 0.258 | −0.101 ± 0.036 | −0.106 ± 0.076 |
| 7 | 40°/Air/9 mm | 0.178 ± 0.054 | 0.186 | 0.231 | −0.052 ± 0.039 | −0.166 ± 0.051 |
| 8 | 60°/Air/9 mm | 0.072 ± 0.045 | 0.085 | 0.167 | −0.018 ± 0.057 | −0.031 ± 0.055 |
| 9 | 40°/Air/14 mm | 0.241 ± 0.055 | 0.247 | 0.320 | −0.058 ± 0.017 | −0.234 ± 0.055 |
| 10 | 40°/Air/6 mm | 0.170 ± 0.060 | 0.180 | 0.259 | −0.020 ± 0.024 | −0.168 ± 0.058 |
12 trials were performed for each case
Parameters are angle of drill relative to skull surface at entry point/composition of bone at end of lateral drilling/height of lateral bushing from skull surface. The height of the medial bushing is 12 mm deeper than the lateral bushing (e.g., 3 mm inside skull when lateral bushing is 9 mm from skull surface)
Fig. 8.
Scatter plots for the eight experimental cases comparing skull surface angle as well as lateral stage stopping location. The target point for each plot is at the origin
Angle of skull surface
The effect of the angle of the drill shaft relative to the skull surface was examined for both lateral drilling ending locations with a constant height for the bushings (solid bone: case 1 vs. 2 vs. 3 vs. 4, air cell: case 5 vs. 6 vs. 7 vs. 8). For both sequences, an ANOVA test indicated a significant difference in the means between the groups. Subsequent comparisons were performed between the individual groups using the Bonferroni correction factor, indicated that only some parameter sets were significantly different. For the cases in which the lateral drilling ends in solid bone, the difference between the 0° and 60° cases was statistically significant at the 5 For the cases in which the lateral hole stops in air, the results of the 20° and 40° cases were statistically different from the 0° ( p < 0.05).
These data indicate that there is some effect of drill/bone angle on the drilling accuracy; however, it is difficult to determine the extent of the effect since the results are not consistent. This is likely due to the fact that there are many other factors influencing the drilling accuracy, most notably the location and size of the air cells in the test blocks. Different deflections at the surface lead to the medial trajectory passing through air cells at different points and thus leading to a range of deflections deeper along the trajectory. In particular, the 60° case for the lateral hole stopping in air seems to not follow the general trend of the rest of the data. Looking at the test blocks after drilling indicates that the location of the drill path with respect to the final air cell in the test block was slightly offset compared to the other blocks, which may have caused a different deflection at this point in the trajectory. It is clear that steeper angles at the skull surface negatively affect the drilling accuracy but this is only one component of the drilling error and must be considered with the parameters along the medial stage as discussed in more detail in the Medial Drilling Experiments section below.
Composition of bone at end of lateral hole
For each drill angle, a comparison was made between the cases in which the lateral stage ends in solid bone and the lateral hole ends in an air cell (cases 1 vs. 5, 2 vs. 6, 3 vs. 7, 4 vs. 8) using a two-sample t test. The difference between results (bone vs. air cell stopping point) for the 0°, 20°, and 40° cases are statistically significant ( p < 0.001, p = 0.003 and p < 0.001, for angles of 0°, 20°, and 40°, respectively) and all indicating that drill deviation error is greater when the lateral stage ends in an air cell. The difference in results for the 60° case is not statistically significant at the 5 percent level. This may be caused by the medial bit passing through a different part of a deeper air cell as discussed above.
These results show that higher accuracy can be achieved by planning the drill trajectory such that the lateral drilling ends in solid bone. This finding is likely due to the fact that when the lateral stage stops in an air cell, the medial drill bit is cantilevered out from the bushing a longer distance before initially contacting bone. Therefore, any transverse force on the tip of the drill results in a larger bending moment on the drill bit shaft. Additionally, when the lateral hole ends in solid bone, the shape of the drill bit provides a conical shape at the end of the hole, which helps to center the medial drill bit.
Bushing location relative to skull surface
Three different bushing locations were tested (note: The medial bushing extends 12 mm beyond the lateral bushing, so raising/lowering one affects the other the same amount). In each case, the bone was fixed at an angle of 40° relative to the drill and the lateral hole was planned to end in an air cell (cases 7, 9, and 10). An ANOVA test indicated a difference between the group means and subsequent individual comparisons with Bonferroni correction performed. The error difference between the two cases with the medial bushing located inside the skull (cases 7 and 10) is not statistically significant. However, there is a statistical significance between these two cases and the case for which the medial bushing is located outside of the skull ( p = 0.006 for case 10 vs. case 9 and p = 0.009 for case 7 vs. case 9). This implies that there will be a decrease in accuracy when the Microtable is placed higher above the skull surface, particularly when the medial bushing does not reach the skull surface. This agrees with the finding that accuracy decreases when the lateral drilling ends in an air cell. In both situations, the longer cantilevered length of the narrow medial drill bit leads to more deflection.
Medial drilling experiments
The results for the medial drilling experiments are provided in Table 3. In these experiments, the errors increased along the direction of the angle of the block and with increase in cantilevered length of the medial drill bit as expected. For most of the parameter sets, the errors were very low relative to the positional accuracy required for successful surgery; however, it is important to note that this error represents the deflection at one point along the medial trajectory. In a typical trajectory, there are several deflection points so it is possible for the cumulative deflection to result in more significant drilling error. These results are discussed in more detail in the following paragraphs.
Table 3.
Drilling accuracy data for medial drilling experiments
| Parametersa,b | Targeting error magnitude (mm)
|
X-Y position of error (mm)
|
|||
|---|---|---|---|---|---|
| μ ± σ | RMS | Max. | X, μ ± σ | Y, μ ± σ | |
| 0°/6 mm | 0.035 ± 0.011 | 0.037 | 0.060 | −0.031 ± 0.014 | 0.012 ± 0.007 |
| 15°/6 mm | 0.027 ± 0.009 | 0.029 | 0.042 | −0.019 ± 0.011 | 0.094 ± 0.017 |
| 30°/6 mm | 0.028 ± 0.021 | 0.035 | 0.071 | −0.023 ± 0.024 | 0.003 ± 0.012 |
| 45°/6 mm | 0.053 ± 0.023 | 0.058 | 0.090 | −0.028 ± 0.021 | −0.041 ± 0.020 |
| 60°/6 mm | 0.098 ± 0.036 | 0.105 | 0.166 | −0.056 ± 0.029 | −0.079 ± 0.028 |
| 75°/6 mm | 0.116 ± 0.011 | 0.117 | 0.131 | −0.037 ± 0.021 | −0.109 ± 0.012 |
| 45°/3 mm | 0.072 ± 0.017 | 0.074 | 0.104 | −0.052 ± 0.022 | −0.046 ± 0.011 |
| 45°/9 mm | 0.117 ± 0.043 | 0.125 | 0.185 | −0.054 ± 0.022 | −0.103 ± 0.041 |
12 trials were performed for each case
Parameters are angle of bone at contact point/cantilevered length of drill bit from end of bushing to contact point
The medial drill deflection increased significantly for angles of 45° and greater compared to the 0°–30° cases. The data for the three cases with lower angles are very similar and, accounting for the experimental error and number of trials, can be considered approximately equal for the purpose of predicting deflections in surgery. The magnitude of these deflections is considerably low compared to the required positional accuracy and would only need to be considered if the addition of other factors was likely to result in higher error (e.g., the presence of large air cell or bushing placement that leads to long cantilevered length of drill bit). Higher angles, on the other hand, (45° and above) may result in much higher errors and affect the success of the surgery, especially when coupled with other parameters that lead to higher errors. The errors in these trials were primarily directed along the negative y-axis in scatter plots, which is the downward direction of the angled blocks, and the negative x-axis (Fig. 9). The angle of the block causes the bit to first contact only part of the bone, which results in an unbalance of the forces between the drill bit flutes and the material. The direction of the cumulative force is dependent on the spindle direction of the drill. The drill bit spins clockwise (as viewed looking down the drill shaft toward the tip) in this system so the error is directed in the negative x-direction as well as down the angled face.
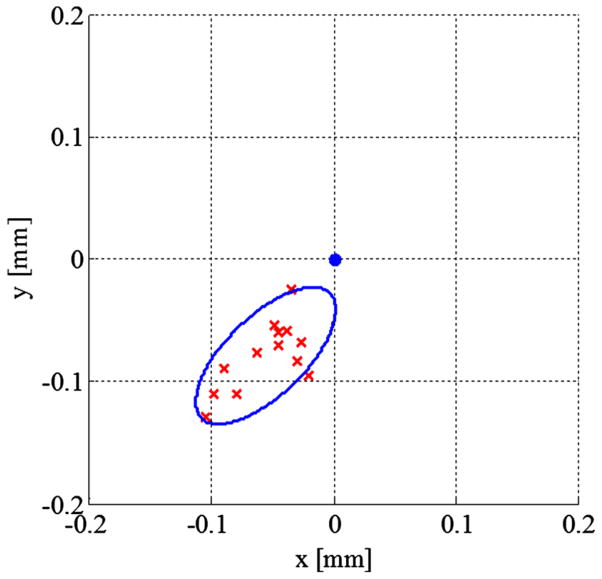
Fig. 9.
Scatter plot for medial drilling error at a single bone contact point with the bone at an angle of 60°. The ellipse around the data represents and encloses two standard deviations along the principal axes
Additional experiments comparing the cantilevered length of the drill bit at the bone contact point indicated that the accuracy decreases with longer extension of the medial drill bit. These experiments were performed with a bone angle of 45°. The error increased significantly for a cantilevered length of 9 mm as compared to 3 and 6 mm lengths. The error was primarily in the same direction as the higher-angle trials discussed above. For most points along the medial trajectory, the cantilevered length will be low since the bone serves as a constraint similar to the bushings; however, for large air cells and the initial contact point, the drill bit may be extended up to 10 mm. These longer extensions must be accounted for when predicting medial drill deflection.
Conclusion and future work
In this paper, a method for evaluating the drilling accuracy of a minimally invasive CI surgical technique was presented. The drilling accuracy of this approach was evaluated in bone surrogate material and found to be within the tolerance of the procedure and within the range seen in cadaveric and clinical trials for most parameter sets; however, it is clear that specific anatomical features can lead to inaccurate drilling and must be accounted for when planning the surgery. Furthermore, these errors must be considered in the context of other error sources. Given the overall RMS error range of 0.41–0.48 mm predicted by combining the data from the prior free-space accuracy study [20] and this work, it is especially important to avoid certain trajectories and keep the expected error within that range.
The material used in this study is similar to bone; however, it is obviously not a perfect substitute, and there can be large variations in the properties of bong among patients. Additionally, the specific drill bits used in this system affect the drilling accuracy. Thus, these results are best used as a guide to determining a range of possible errors and identifying which factors affect the drilling accuracy during surgery. Specifically, it is clear that the lateral drilling stage should end in solid bone. If this is not possible given the patient’s anatomy, particular attention should be paid to minimize the cantilevered length of the medial bit when it first contacts bone and avoid drilling trajectories that lead to steep air/bone contact angles along the medial stage. The use of and position of the guide bushings also play a significant role in drilling accuracy and is likely the primary reason for the increased accuracy observed in this study compared to the work of Kobler at al., as the authors of that paper also hypothesized. Based on the results of this study, the most accurate percutaneous drilling approach is to employ guide bushings and design the apparatus such that they are placed as deep as possible in the bone.
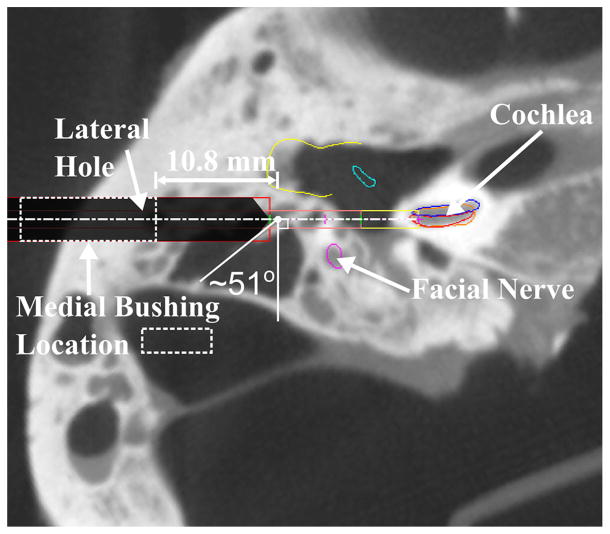
The results of this study help explain the reason for the inaccurate placement of an electrode array in a previous cadaveric trial performed with this system [28]. The electrode was inserted into the scala vestibuli subcomponent of the cochlea instead of the scala tympani as had been planned. The preoperative CT scan of this cadaveric temporal bone specimen along with the planned drilling trajectory is shown in Fig. 10. The lateral stage of the trajectory ended in a very large air cell causing the medial drill bit to be extended quite far from the bushing at the initial contact point. The angle at this initial contact point was also very steep. This trial would be best modeled by parameter set 10 from the two-stage drilling experiments. Additionally, the results from the medial drilling experiments with higher angles at the air/bone contact point and long cantilevered length of the medial drill bit help to explain the large error. These factors likely contributed to a deflection from the planned path that resulted in the deviation of the drill bit into the scala vestibuli. Using the information learned from this study, an alternative trajectory could be planned for future cases in which the patient anatomy indicates a possible large deflection. In this particular case, a shallower lateral stage would allow the lateral hole to center the medial drill bit and limit the cantilevered length of this bit along the deeper portion of the trajectory. A deeper lateral stage would also help center the medial bit and would remove the bone in the area of the steep angle.
Fig. 10.
Preoperative scan from a prior cadaver trial in which the drill path deviated from the target path and the electrode was inserted into the scala vestibuli instead of the scala tympani. The data presented in this study explain the reasoning for the large drilling error for this case. The medial drill extended 10.8 mm from the bushing before first contacting bone and the large air cell at the end of the lateral stage resulted in a steep initial contact angle of 51°. The dashed lines in this figure represent the location of the medial bushing
Future work on this project will include translating the results from this study to improvements in the trajectory planning of clinical cases. As a first step, checks to the current drill plans should be implemented to identify any possible larger deflections based on the patient’s anatomy. This can be extended to estimate drill deviations from the plan near the facial nerve which would result from drill deflections lateral to this point in the trajectory. The likelihood of safety near the facial nerve and accuracy of the drill tip at the cochlea will provide the surgeon guidance as to choosing between this method of minimally invasive CI surgery and the traditional approach. Furthermore, creating and verifying a model for the drill deflection based on the patient’s preoperative CT scan would allow for choosing a drill path that combines the current trajectory planning method [29] with probable drilling errors to identify a safer and more effective path. Finally, the results from this study can also be applied to other image-guided surgical procedures requiring accurate drilling through bone, both within the field of otolaryngology (e.g., percutaneous access to the petrous apex) and in other areas of medicine (e.g., pedicle screw placement in spinal surgery).
Acknowledgments
The project described was supported by Award Number R01DC008408 and R01DC012593 from the National Institute on Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health. The authors would like to thank Jan-Philip Kobler and Leuder Kahrs (Institute of Mechatronic Systems, Leibniz Universität Hannover, 30167 Hannover, Germany) for supplying information on the test blocks and sharing insight from their experiments.
Footnotes
Conflict of interest: Neal P. Dillon and Ramya Balachandran declare that they do not have any conflicts of interest pertaining to this work. Robert F. Labadie has served as a consultant for Medtronic Inc. and Advanced Bionics within the past 12 months.
References
- 1.House WF. Surgical considerations in cochlear implantation. Ann Otol Rhinol Laryngol. 1981;91(2 Pt 3):15–20. [PubMed] [Google Scholar]
- 2.Roberson JB, Stidham KR, Scott KM, Tonokawa L. Cochlear implantation: minimal hair removal technique. Otolaryngol-Head Neck Surg. 2000;122(5):625–629. doi: 10.1016/S0194-5998(00)70186-0. [DOI] [PubMed] [Google Scholar]
- 3.O’Donoghue GM, Nikolopoupos TP. Minimal access surgery for pediatric cochlear implantation. Otol Neurotol. 2002;23(6):891–894. doi: 10.1097/00129492-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Dalchow CV, Werner JA. A new instrument for minimal access surgery in cochlear implantation. Otol Neurotol. 2005;26(4):678–679. doi: 10.1097/01.mao.0000178135.74106.39. [DOI] [PubMed] [Google Scholar]
- 5.Djalilian HR, Roy S, Benson AG, Regala C, McDonald TB, Leman T. Transcanal cochlear implantation under monitored anesthesia care. Otol Neurotol. 2005;26(4):674–677. doi: 10.1097/01.mao.0000178127.58859.e0. [DOI] [PubMed] [Google Scholar]
- 6.Caner G, Olgun L, Gultekin G, Aydar L. Local anesthesia for middle ear surgery. Otolaryngol-Head Neck Surg. 2005;133(2):295–297. doi: 10.1016/j.otohns.2004.09.112. [DOI] [PubMed] [Google Scholar]
- 7.Kroenenberg J, Migriov L, Dagan T. Suprameatal approach: new surgical approach for cochlear implantation. J Laryngol Otol. 2001;115(04):283–285. doi: 10.1258/0022215011907451. [DOI] [PubMed] [Google Scholar]
- 8.Kronenberg J, Baumgartner W, Migriov L, Dagan T, Hildesheimer M. The suprameatal approach: an alternative surgical approach to cochlear implantation. Otol Neurotol. 2004;25(1):41–45. doi: 10.1097/00129492-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hausler R. Cochlear implantation without mastoidectomy: the pericanal electrode insertion technique. Acta Otolaryngologica. 2002;122(7):715–719. doi: 10.1080/00016480260349773. [DOI] [PubMed] [Google Scholar]
- 10.Sargent EW, Bucholz RD. Middle cranial fossa surgery with image-guided instrumentation. Otolaryngol-Head Neck Surg. 1997;117(1):131–134. doi: 10.1016/S0194-59989770222-5. [DOI] [PubMed] [Google Scholar]
- 11.Raine CH, Strachan DR, Gopichandran T. How we do it: using a surgical navigation system in the management of the ossified cochlea. Cochlear Implants Int. 2003;4(2):96–101. doi: 10.1179/cim.2003.4.2.96. [DOI] [PubMed] [Google Scholar]
- 12.Caversaccio M, Romualdez J, Baechler R, Nolte LP, Kompis M, Hausler R. Valuable use of computer-aided surgery in congenital bony aural atresia. J Laryngol Otol. 2003;117(04):241–248. doi: 10.1258/00222150360600814. [DOI] [PubMed] [Google Scholar]
- 13.Labadie RF, Choudhury P, Cetinkaya E, Balachandran R, Haynes DS, Fenlon MR, Jusczyzck AS, Fitzpatrick JM. Minimally invasive, image-guided, facial recess approach to the middle ear: demonstration of the concept of percutaneous cochlear access in vitro. Otol Neurotol. 2005;26(4):557–562. doi: 10.1097/01.mao.0000178117.61537.5b. [DOI] [PubMed] [Google Scholar]
- 14.Labadie RF, Balachandran R, Noble JH, Blachon GS, Mitchell JE, Reda FA, Dawant BM, Fitzpatrick JM. Minimally invasive image-guided cochlear implantation surgery: First report of clinical implementation. Laryngoscope. 2014;124(8):1915–1922. doi: 10.1002/lary.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kratchman LB, Blachon GS, Withrow TJ, Balachandran R, Labadie RF, Webster RJ. Design of a bone-attached parallel robot for percutaneous cochlear implantation. Biomed Eng IEEE Trans. 2011;58(10):2904–2910. doi: 10.1109/TBME.2011.2162512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobler JP, Kotlarski J, Oltjen J, Baron S, Ortmaier T. Design and analysis of a head-mounted parallel kinematic device for skull surgery. Int J Comput Assist Radiol Surg. 2012;7(1):137–149. doi: 10.1007/s11548-011-0619-8. [DOI] [PubMed] [Google Scholar]
- 17.Baron S, Eilers H, Munske B, Toennies JL, Balachandran R, Labadie RF, Ortmaier T, Webster RJ. Percutaneous inner-ear access via an image-guided industrial robot system. Proc Inst Mech Eng H: J Eng Med. 2010;224(5):633–649. doi: 10.1243/09544119JEIM781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell B, Stieger C, Gerber N, Arnold A, Nauer C, Hamacher V, Kompis M, Nolte L, Caversaccio M, Weber S. A self-developed and constructed robot for minimally invasive cochlear implantation. Acta Otolaryngologica. 2012;132(4):355–360. doi: 10.3109/00016489.2011.642813. [DOI] [PubMed] [Google Scholar]
- 19.Bell B, Gerber N, Williamson T, Gavaghan K, Wimmer W, Caversaccio M, Weber S. In vitro accuracy evaluation of image-guided robot system for direct cochlear access. Otol Neurotol. 2013;34(7):1284–1290. doi: 10.1097/MAO.0b013e31829561b6. [DOI] [PubMed] [Google Scholar]
- 20.Labadie RF, Mitchel JE, Balachandran R, Fitzpatrick JM. Customized, rapid-production microstereotactic table for surgical targeting: description of concept and in vitro validation. Int J Comput Assist Radiol Surg. 2009;4(3):273–280. doi: 10.1007/s11548-009-0292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balachandran R, Mitchell JE, Blachon GS, Noble JH, Dawant BM, Fitzpatrick JM, Labadie RF. Percutaneous cochlear implant drilling via customized frames: an in vitro study. Otolaryngol-Head Neck Surg. 2010;142(3):421–426. doi: 10.1016/j.otohns.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labadie RF, Balachandran R, Mitchell JE, Noble JH, Majdani O, Haynes DS, Bennett M, Dawant BM, Fitzpatrick JM. Clinical validation study of percutaneous cochlear access using patient customized microstereotactic frames. Otol Neurotol. 2010;31(1):94. doi: 10.1097/MAO.0b013e3181c2f81a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labadie RF, Majdani O, Fitzpatrick JM. Image-guided technique in neurotology. Otolaryngol Clin North Am. 2007;40(3):611–624. doi: 10.1016/j.otc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Williamson TM, Bell BJ, Gerber N, Salas L, Zysset P, Caversaccio M, Weber S. Estimation of tool pose based on force-density correlation during robotic drilling. Biomed Eng IEEE Trans. 2013;60(4):969–976. doi: 10.1109/TBME.2012.2235439. [DOI] [PubMed] [Google Scholar]
- 25.Kobler JP, Schoppe MG, Lexow J, Rau TS, Majdani O, Kahrs LA, Ortmaier T. Temporal bone borehole accuracy for cochlear implantation influenced by drilling strategy: an in vitro study. Int J Comput Assist Radiol Surg. 2014;9(6):1033–1043. doi: 10.1007/s11548-014-0997-9. [DOI] [PubMed] [Google Scholar]
- 26.Kobler JP, Prielozny L, Lexow GJ, Rau TS, Majdani O, Ortmaier T. Mechanical characterization of bone anchors used with a bone-attached, parallel robot for skull surgery. Med Eng Phys. 2015;37(5):460–468. doi: 10.1016/j.medengphy.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Eman KF, Wu SM. An analysis of the drill wandering motion. J Manuf Sci Eng. 1987;109(4):297–305. [Google Scholar]
- 28.Rohani P, Pile J, Kahrs LA, Balachandran R, Blachon GS, Simaan N, Labadie RF. Forces and trauma associated with minimally invasive image-guided cochlear implantation. Otolaryngol-Head Neck Surg. 2014;150(4):638–645. doi: 10.1177/0194599813519747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noble JH, Majdani O, Labadie RF, Dawant BM, Fitzpatrick JM. Automatic determination of optimal linear drilling trajectories for cochlear access accounting for drill-positioning error. Int J Med Robot Comput Assist Surg. 2010;6(3):281–290. doi: 10.1002/rcs.330. [DOI] [PMC free article] [PubMed] [Google Scholar]