Abstract
Purpose
Pancreatic cancer stromal microenvironment is considered to be the major reason for failure of conventional and targeted therapy for this disease. The desmoplastic stroma, comprising mainly of collagen and glycosaminoglycans like hyaluronan (HA), is responsible for compression of vasculature in the tumor resulting in impaired drug delivery and poor prognosis. Minnelide, a water-soluble pro-drug of triptolide currently in Phase I clinical trial, has been very effective in multiple animal models of pancreatic cancer. However, whether Minnelide will have efficacious delivery into the tumor in spite of the desmoplastic stroma, has not been evaluated before.
Experiment design
Patient tumor derived xenografts (PDX) and spontaneous pancreatic cancer mice were treated with 0.42 mg/kg and 0.21 mg/kg body weight for 30 days. Stromal components were determined by IHC and ELISA based assays. Vascular functionality and drug delivery to the tumor were assessed following treatment with Minnelide.
Result
Our current study shows that treatment with Minnelide resulted in reduction of ECM components like hyaluronan (HA) and collagen in the pancreatic cancer stroma of both the spontaneous KPC mice as well as in patient tumor xenografts. Further, treatment with Minnelide improved functional vasculature in the tumors resulting in 4- times more functional vessels in the treated animals compared to untreated animals. Consistent with this observation, Minnelide also resulted in increased drug delivery into the tumor compared to untreated animals. Along with this, Minnelide also decreased viability of the stromal cells along with the tumor cells in pancreatic adenocarcinoma.
Conclusion
In conclusion, these results are extremely promising as they indicate that Minnelide, along with having anti-cancer effects is also able to deplete stroma in pancreatic tumors, which makes it an effective therapy for pancreatic cancer.
Keywords: Minnelide, stroma, pancreatic cancer, ECM, collagen, hyaluronan
Introduction
Pancreatic cancer is among the most devastating of all cancers with a dismal survival rate. In United States alone, the estimated cases for pancreatic cancer is almost 48000. (www.cancer.gov). 5-year survival in patients with pancreatic cancer is about 5% and this figure has remained relatively unchanged over the past 25 years (1, 2). Pancreatic cancer stromal microenvironment is considered to be the major reason behind the failure of conventional and targeted therapy for this disease (3). It has been observed that the dense stroma comprising mostly of proteoglycans like hyaluronan and collagen forms a physical barrier for drugs targeting the tumor cells (4, 5). Recent efforts directed towards development of new therapies have thus been targeted towards depletion of stroma in order to improve drug delivery (6, 7).
A molecular analysis of the pancreatic ductal adenocarcinoma stroma revealed that it was extremely heterogeneous, comprising of cellular and acellular components, like fibroblasts, myofibroblasts, pancreatic stellate cells, immune cells, blood vessels, extracellular matrix (ECM) made of collagen and hyaluronan, and soluble proteins such as cytokines and growth factors. As a result of accumulation of ECM components, the normal architecture of the pancreatic tissue is distorted resulting in an abnormal configuration of blood and lymphatic vessels in the tumor (4, 5, 8-10). Based on this observation it was hypothesized that the desmoplastic stroma acted as a barrier to drug delivery in the tumor (5, 10). Hyaluronan (HA) and collagen are the two major components of the pancreatic tumor stroma. HA is a high molecular weight glycosaminoglycan comprising of a polymer of N-Acetyl Glucosamine and glucuronic acid, that retains water due to its high colloid osmotic pressure (11). Excessive HA accumulation in solid tumors has been reported to raise interstitial fluid pressure and compress blood vessels (3, 6, 7). Collagen, particularly type IV collagen, is the main component of the basement membrane of the ECM that provides a scaffold for its assembly and mechanical stability. Along with providing an extensive support, the collagen network in the stroma also plays a significant role in cell adhesion, migration, survival, proliferation, and differentiation (12, 13). Sonic hedgehog (SHH) signaling has been shown to be restricted to the stromal compartment (10, 14). Thus, pharmacologic inhibition of the Shh pathway was thought to have a positive impact on gemcitabine delivery, by reducing the desmoplastic stroma. Though a study involving combination of the Smoothened inhibitor (IPI-926) and gemcitabine caused depletion of tumor stroma and resulted in increased microvessel density (5), the compound failed to offer significant survival benefit in a clinical trial.
In another study, hyaluronan degradation by hyaluronidase PEGPH20 decreased interstitial fluid pressure in murine pancreatic ductal adenocarcinoma (PDAC). Consequently, increased vessel patency, drug delivery, and survival were also observed (3, 7). Even though “anti-stromal” therapy has been emerging as a therapeutic option, the role of stroma in pancreatic cancer has remained controversial. It is also proposed that the stroma is more “re-straining” in nature for the tumor, thereby acting to prevent metastasis by constricting blood vessels (15). Thus, an ideal therapy for pancreatic cancer would be one that on one hand will have a potent anti-cancer effect and at the same time will ensure its efficacious delivery into the tumor.
Triptolide, an active compound isolated from a chinese herb has been used as an anticancer compound since the 1990's. Its efficacy in reducing a number of tumors including pancreatic tumors has been reported over last few years (16-21). Owing to its limited solubility in aqueous medium, triptolide had restricted use in clinical and preclinical studies. Recently, a water-soluble analog of triptolide, Minnelide has been effectively used as an anti-cancer therapy (22-27). Minnelide is currently being evaluated in a Phase1 clinical trial against gastrointestinal cancers. Our pre-clinical studies with Minnelide has shown that this compound is able to increase survival in a number of mouse models both orthotopic as well as patient-tumor derived xenografts (PDX). Minnelide, apart from reducing the primary tumor burden also decreases metastasis in pancreatic as well as other cancers (24). Our previous studies have shown that Minnelide acts by inhibiting the transcriptional activity of Sp1, by altering its glycosylation status (22). Though there have been a number of studies on triptolide/Minnelide, its effect on the stromal architecture has not been studied till date.
In the current study we show that Minnelide, apart from having a profound anti-cancer effect as seen in our earlier studies, also effectively depletes the stromal architecture in pancreatic cancer by inhibiting collagen stabilization and hyaluronan synthesis. This eventually results in improved vascular function and better drug delivery, leading to an increased survival of treated KRasG12D; Trp53R172H; Pdx-1Cre (KPC) mice.
Methods
Quantitative Real Time PCR
Quantitative RTPCR for indicated primers was carried out using primers procured from Qiagen (Quantitect Primer Assay, Qiagen). PCR array for ECM and Adhesion pathway (Qiagen, SA Biosciences) was used to analyse transcripts of genes involved in the ECM. RNA was isolated from the tumor samples according to manufacturer's instructions using Trizol (Invitrogen). Total RNA (1ug) was used to perform real-time PCR using the Quantitect Sybr green PCR kit (Qiagen) according to the manufacturer's instructions using an Applied Biosystems 7300 real-time PCR system. All data were normalized to the housekeeping gene 18S (18S Quantitect Primer Assay; Qiagen).
Estimation of HA in tumors, HA synthase and Hyalouronidase assay
For estimating HA, Hyaluronidase and HA synthase activity, tumor tissue (from control animals and animals treated with Minnelide) was washed several times in ice cold PBS to remove traces of blood. The tissue was then homogenized in the assay buffer (20mM sodium phosphate buffer, 77mM sodium chloride) in the presence of protease inhibitors and used for assay. HA was estimated in the tumor samples using the ELISA based Hyaluronan Duo Set (R&D system) according to manufacturer's instruction. For Hyaluronidase assay, ELISA based hyaluronidase kit (AMS Biotech) was used according to manufacturers instruction. HA synthase assay was done by slightly modifying the protocol established by Itano (28). Briefly, the membrane fraction of the tumor tissue was incubated at 37 °C for 1 h in 0.2 ml of 25 mM Hepes-NaOH, pH 7.1, 5 mM dithiothreitol, 15 mM MgCl2, 0.1 mM UDP-GlcNAc (Sigma), 2 μM UDP-GlcA (Sigma), Reactions were terminated by adding SDS at 2% (w/v). For negative control, the total reaction mix was heat inactivated prior to incubation. HA formed was estimated by the ELISA based Hyalurona Duo set as described before. Results were expressed as the amount of HA formed per ug protein per min.
Collagen isolation and Hydroxyproline and hydroxylysine estimation
Tumor tissue from Minnelide treated and untreated mice were minced into 1-2mm pieces and extracted in 0.5M Acetic Acid for 72h at 4°C. The supernatant containing acid soluble collagen was dialyzed against 0.05M Na2HPO4. Hydroxyproline assay was performed on this extracted collagen using a Hydroxyproline assay kit (Sigma) and results were expressed as Hydroxyproline formed per ng collagen. Hydroxylysine in the collagen was estimated using hydroxylysine assay kit (Biosource) according to manufacturer's instruction. Results were expressed at hydroxylysine produced per ng of collagen.
Immunohistochemistry and immunofluorescence
For immunohistochemistry, paraffin tissue sections were deparaffinized in xylenes and hydrated through graded ethanol. Hematoxylin and Eosin (H&E) staining were used for evaluation of histological features. Sirius Red staining was used to visualize the collagen in the tumor stroma, Slides were steamed with Reveal Decloaker (Biocare Medical) to minimize background staining. Sniper Universal Blocking Sera (Biocare Medical) were used throughout the protocol. Primary antibodies for alpha SMA, CD31 (Abcam), HABP-Biotin (Sigma) were diluted according to vendor's instruction and incubated overnight at 4°C. The primary antibody was omitted for the negative controls. For immunofluorescence, fluorescent antibody conjugates were used after primary antibody staining. Slides were counterstained with DAPI and visualized in a Nikon fluorescent microscope. Tissue samples were incubated with mouse IgG1 isotype controls (BD Biosciences) and did not demonstrate any specific staining.
Human xenograft pancreatic cancer tumor model
Patient tumor derived xenografts were established by implanting de-identified human pancreatic tumors subcutaneously into NOD-SCID mice (Jackson Laboratories). Upon reaching a volume of 500 mm3, tumors were dissected and cut into 10 mm3 pieces, which were then subcutaneously implanted into both flanks of additional SCID mice (n=20 animals). Animals were randomized and tagged prior to treatment. Treatment with Minnelide (0.42 mg/kg body weight) was started when the tumors were ∼500mm3 in size. Animals were treated for 7 days prior to collecting tissues for histology, vascular function assay or drug delivery assay.
Transgenic mouse model of spontaneous pancreatic cancer
KRasG12D; Trp53R172H; Pdx-1Cre animals were generated by crossing Lox Stop Lox (LSL) KRasG12D; LSL Trp53R172H animals with Pdx-1 Cre animals. Minnelide treatment with 0.42 mg/kg body weight/day was started when animals were 4-6 weeks of age. Animals in saline and treatment groups were age-matched. Animals were weighed every week to monitor for weight loss during experiment and treatment doses were adjusted accordingly. Animals were treated for 30 days prior to collecting tissues for histology, vascular function assay or drug delivery assay.
Experiments were performed and animals sacrificed in accordance with animal care committee regulations at the University of Minnesota. For survival study, the survival data was represented as a Kaplan Meier plot using GraphPad Prism 6.
Vascular function assay
4-5 mice were evaluated for each experimental arm. Twenty-four hours after the final dose of Minnelide, mice received an intravenous infusion of fluorophore-labelled tomato lectin. Thirty minutes later, mice were terminally perfused sequentially with 50 ml saline followed by 30 ml of 4% paraformaldehyde/phosphate buffered saline (pH 7.4). Perfused tissues were harvested, fixed for 16-24 h in paraformaldehyde and transferred to 70% ethanol before paraffin embedding. Sections were deparaffinised, rehydrated and, for vascular patency assays, immunostained for CD31. All sections were counterstained with DAPI. Visualisation was performed on a Nikon Ti confocal microscope using standardized settings, and background signal intensities were established against unlabelled terminally perfused samples. The open/compressed vessels were counted for 10 non-adjacent 20× fields for each slide.
Drug Delivery assay
For the doxorubicin delivery assay, a minimum of five mice from saline and Minnelide were included for analysis. Mice received an intravenous infusion of doxorubicin 24 h after the final dose of Minnelide. Mice were euthanized and tissues processed as above. Sections were deparaffinised, rehydrated and counterstained with DAPI. Doxorubicin fluorescence was visualized under a Nikon Ti confocal microscope using standardized settings, and background signal intensities were established against samples without doxorubicin stain.
Viability of CAF and KPC tumor cells
Cancer associated fibroblasts were isolated from 5-7 tumors from KPC mice as described in Sharon et al (29). The purity of the fibroblasts were checked by flow-cytometry after staining isolated fibroblasts with FSP antibody and CK19 antibody. Population with FSP+CK19- staining was used for viability experiments.
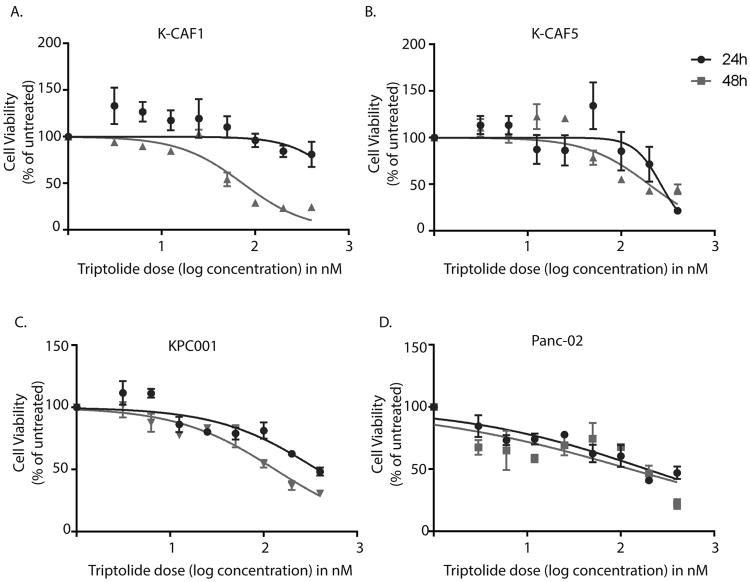
Both isolated CAFs from KPC tumors and mouse epithelial pancreatic ductal adenocarcinoma (PDAC) cell line KPC001 and Panc-02 were seeded on 96 well plates and allowed to grow for 48h prior to treating them with indicated doses of triptolide for 24h and 48h. Cell viability was measured using an MTT based assay.
Statistical Analysis
Values are expressed as mean ± SEM. All in vitro experiments were performed at least three times. The significance of the difference between any two samples was analysed by Student's t-test using GraphPad Prism 6; values of p<0.05 were considered statistically significant.
Results
Molecular components of desmoplastic stroma are upregulated in human and murine PDAC compared to normal pancreas
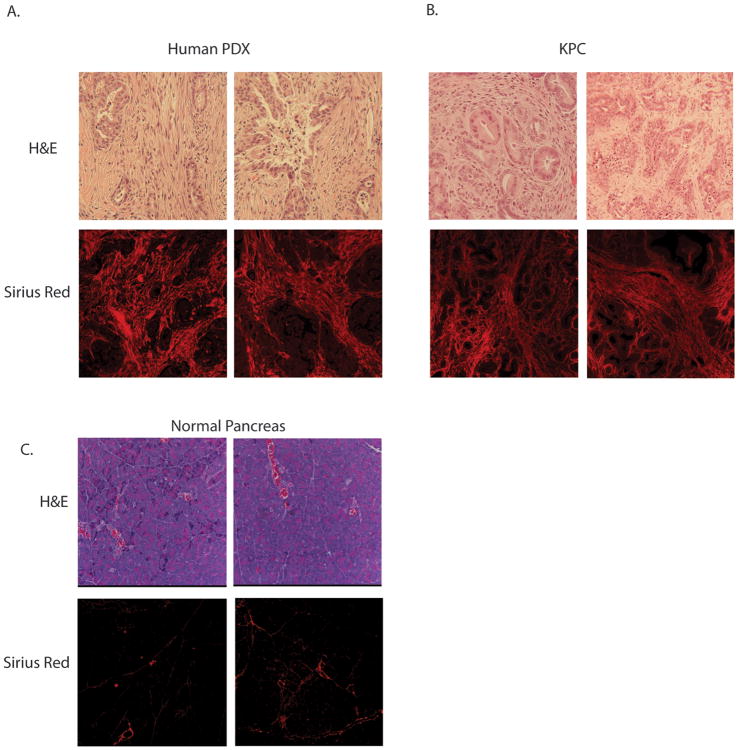
Pancreatic cancer is characterized by an intense desmoplastic stroma. The molecular components of the stroma include the activated stellate cells in the pancreas, the myofibroblasts, proteoglycans and glycosaminoglycans. Synthesis of this complex ECM involves a number of very tightly regulated pathways (30-32). PCR array of genes responsible for the extracellular matrix or ECM showed several fold increased expression in pancreatic ductal adenocarcinoma tissues (from KPC tumors) compared to normal ductal epithelial cells (Supplementary Fig 1). The genes that were significantly upregulated or downregulated are tabulated in Supplementary Table 1. Consistent with this, the histological sections of pancreatic tumors resected from patients or from the KPC mice showed presence of an extensive stroma as seen by H&E staining (Fig 1A) when compared to normal pancreas tissue (Fig 1C). Both patient tumor-derived xenografts (PDX) as well as tumors from KPC mice also stained heavily with collagen as visualized by Sirius Red staining (Fig 1B).
Figure 1.
Figure showing presence of extensive stroma in pancreatic ductal adenocarcinoma in (A) patient tumor derived xenografts (PDX) and (B) spontaneous KRasG12D,TP53,Pdx-Cre (KPC) mice in H&E stain, picrosirius red stain. Normal pancreas tissue showed very little staining with picrosirius red (C).
Patient tumor xenografts and KPC spontaneous tumors show reduced stroma in response to Minnelide treatment
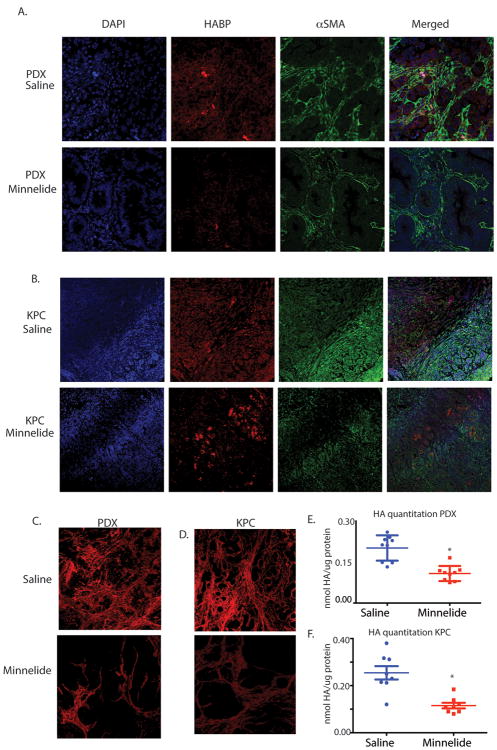
Minnelide has been extremely efficient in regressing tumor both in human tumor derived xenografts as well as in spontaneous KPC mice (KRASG12D,TP53-PdxCre mice) for pancreatic cancer (24). To see if Minnelide was also effective in reducing the stromal components, the animals were treated with Minnelide and the histological sections were stained with stromal markers and quantitated. Hyaluronan is one of the major constituents on the pancreatic tumor stroma, which is typically visualized in tissue sections by staining with HABP (hyaluronan binding protein) staining. HABP staining was significantly less in the Minnelide treated animals in both PDX (Fig. 2A) and KPC models (Fig. 2B). Minnelide also decreased alpha SMA staining in tissues indicating that activated stellate cells were also reduced (Fig. 2A,B). Quantitation of the alpha SMA staining indicated that this was significantly less than untreated tumors (Supplementary figure 2). Minnelide treatment reduced fibrosis in the stroma (as visualized by Sirius red staining) in both PDX and spontaneous KPC model as well (Fig 2C,D). Quantitation of staining showed that the fibrosis (collagen) was significantly decreased in the Minnelide treated tissues (Supplementary figure 2).
Figure 2.
Minnelide reduces stromal component alpha SMA and HA in (A) patient tumor derived xenograft as well as (B) in KPC tumors. (C) Picosirius red staining is also decreased in both models. (D) Quantitation of HA from the PDX tumors (E) and KPC tumor (F) also shows decreased HA in the tumors. The * represents p<0.05.
To further confirm if the HA in the treated tumor is indeed decreased by Minnelide, we quantitated the total HA in the tumors treated with Minnelide. Consistent with the histological observation, HA was decreased in the Minnelide treated tumors derived from both KPC (Fig. 2E) and PDX models (Fig. 2F).
Reduction in fibrosis is caused by impaired collagen synthesis and modification
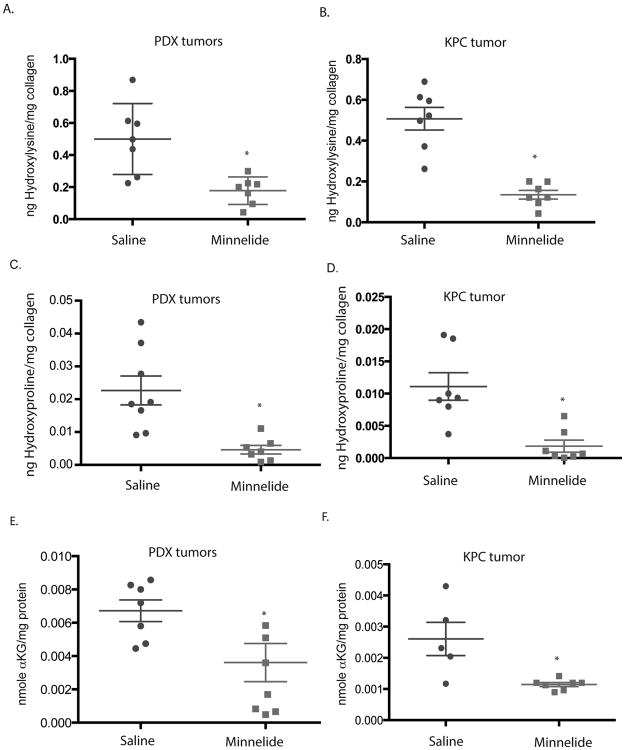
Since our histological sections revealed that Minnelide treatment significantly reduced fibrosis in the two models (as seen decreased Sirius Red staining), we studied the pathways involved in synthesis of collagen. RNA analysis revealed that the transcription of genes involved in the synthesis of collagen (ColE1-5), or stabilization of collagen (PLOD1-4, P4H), were not affected by Minnelide (Supplementary figure 3). Our results show that PLOD1-4 (procollagen lysine 5 dioxygenase) enzymes are very highly expressed in pancreatic tumors (Supplementary figure 3). These genes catalyze the hydroxylation of lysine to hydroxylysine, a key step in stabilization and formation of collagen. Since Minnelide did not alter the expression of PLOD genes, we measured the total hydroxylysine in the total collagen from treated tissues. Hydroxylysine is a rare amino acid only present in collagen, thus a measure of total hydroxylysine in tumors is a measure of the enzyme activity of the PLOD genes. Both KPC and PDX tumors treated with Minnelide showed a decrease in the total hydroxylysine content indicating that Minnelide prevented stabilization of collagen in the tumor thereby leading to depletion of stromal architecture (Fig. 3A,B).
Figure 3.
Minnelide decreased hydroxylysine in (A) patient tumor derived xenografts and (B) KPC tumors; hydroxyproline in patient tumor derived xenografts (C) and (D) KPC tumors; and alpha Ketoglutarate in (E) patient tumor derived xenografts and (F) KPC tumors. The * represents p<0.05.
The other class of enzymes responsible for the stabilization of collagen is procollagen hydroxylases that are overexpressed in pancreatic tumors. Similar to the procollagen lysine dioxygenase, prolyl hydroxylase predominantly catalyze hydroxylation of proline residues in collagen. Minnelide did not reduce the expression of P4H enzymes in KPC or PDAC tumors (Supplementary Figure 3). However, treatment with Minnelide did decrease the total hydroxyproline in the collagen of tumor tissues treated with Minnelide (Figure 3C,D).
Since both PLOD enzymes as well as P4H enzymes are oxygen dependent and require alpha ketoglutarate as the primary co-factor in their reaction, we assayed for the total alpha KG produced in response to Minnelide treatment. Our results indicated decreased alpha KG in response to Minnelide treatment in both KPC and PDX models (Fig 3E,F).
Decreased hyaluronan in stroma results from reduced HAS activity
Since Minnelide decreased hyaluronan in the tumors, we next evaluated if this was due to decreased HA synthesis or enhanced HA degradation in the tumors. HA is synthesized by the tumor cells by HA Synthase enzymes (HAS1-3). Analysis of mRNA showed that HAS1, HAS2 and HAS3 genes were decreased in response to Minnelide treatment in both models (Fig. 4A). Consistent with this observation, the HAS enzyme activity was significantly decreased in the tumors treated with Minnelide (Fig 4B) in both KPC mice and PDX tumor models (Supplementary Fig 4). HA degradationis orchestrated by the hyaluronidase enzymes (HYAL1-5, PH20). Of these, HYAL1, HYAL2 and PH20 were present in KPC and PDX tumors. Minnelide did not decrease the mRNA of these genes (Fig 4C). As expected, hyaluronidase activity also remained unaltered in the tumor tissues after treatment with Minnelide (Fig 4D) in both KPC mice and PDX tumor models (Supplementary Fig 4).
Figure 4.
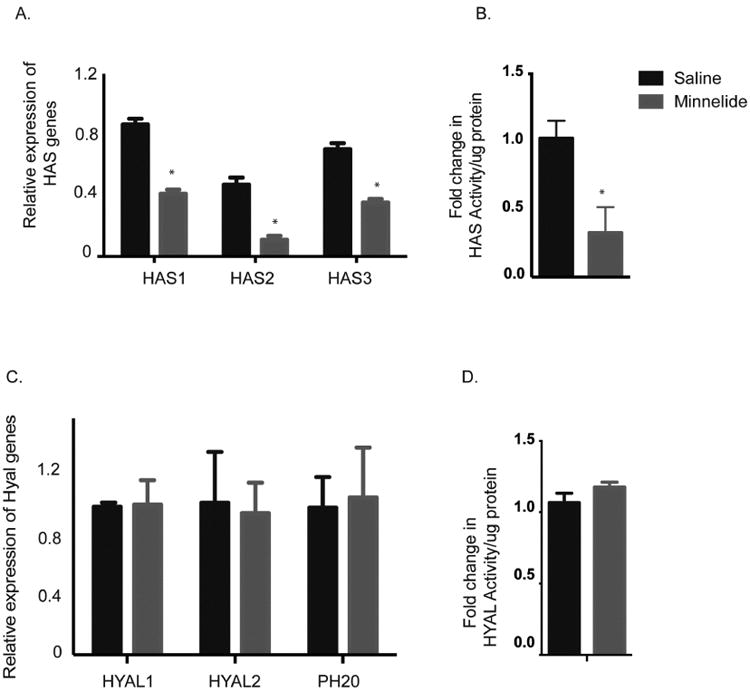
Minnelide decreased (A) HA synthase expression along with (B) the activity in KPC tumors. (C) Hyaluronidase expression as well as (D) activity was found to be unaltered in these tumors following treatment. The * represents p<0.05.
This indicated that decrease in HA in response to Minnelide was in response to decreased synthesis and not increased degradation.
Decreased stromal component result in improved drug delivery and survival
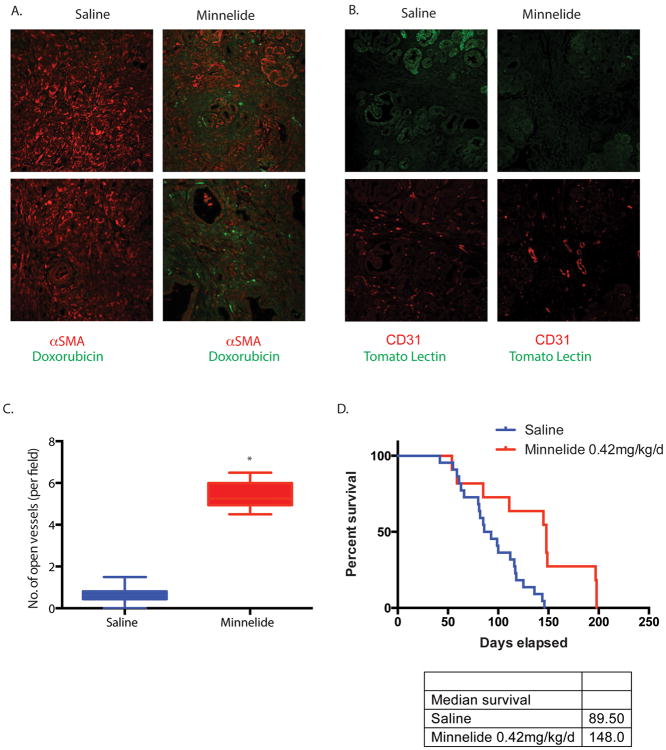
Inefficient drug delivery is a hallmark of pancreatic tumors. This phenomenon is often attributed to compression of tumor vasculature by dense stroma. To see if the physical depletion of stroma translated to an improved drug delivery by improving vascular function, we injected doxorubicin to study drug delivery in these animals. Doxorubicin fluorescence was observed inside the tumors of KPC animals treated with Minnelide (0.42 mg/kg/d), where as negligible doxorubicin fluorescence was present in the saline animals (Fig 5A). Compressed vasculature in the pancreatic tumors prevents drug delivery to the tumor cells in pancreatic cancer. To see if Minnelide induced stromal depletion relieved this vascular compression, we tested for vascular functionality in the tumors. Treatment with Minnelide resulted in greater number of open blood vessels in the tumor compared to the untreated tumors (Fig 5B,C).
Figure 5.
Minnelide improved (A) drug delivery as seen by doxorubicin fluorescence in both KPC and PDX tumors. Minnelide also reduced vascular compression (B) and resulted in (C) more “open” blood vessels compared to untreated tumors. This resulted in a (D) greater survival of the KPC mice receiving Minnelide compared to the untreated mice. The * represents p<0.05.
Our previous studies have shown that Minnelide improved survival and caused tumor regresssion of PDX model. To see if Minnelide indeed improved survival of the KPC model, we treated age matched KPC animals with 0.42 mg/kg Minnelide and plotted their survival. Minnelide indeed increased the median survival of these animals by 58 days following treatment (Fig. 5D).
Minnelide decreased viability of both tumor cells and stromal cells
Our previous studies have shown that Minnelide decreases viability of tumor cells in a number of animal models and pancreatic cancer cell lines, while having no effect on the normal pancreatic ductal cells. To study if it also reduced the viability of cancer associated fibroblasts (CAF) as efficiently as the cancer epithelial cells; we compared the cell viability of CAFs and cancer epithelial cells isolated from KPC animals. Our results showed that Minnelide indeed decreased the viability of CAFs (Fig. 6A, B) as efficiently as it decreased viability of mouse epithelial PDAC cells (Figure 6C,D), indicating that it not only depleted the stromal ECM but also induced stromal cell death. The IC50 values for tumor and stromal cells are indicated in Supplementary Table 2.
Figure 6.
Triptolide decreased viability of cancer-associated fibroblasts as well as epithelial tumor cells. Representative CAFs from KPC tumors, K-CAF1 (A), K-CAF5 (B) and epithelial mouse PDAC cell lines KPC001 (C) and Panc02 (D) were treated with indicated doses of triptolide for 24 and 48h. Viability was plotted as percent of untreated control. The IC50 values are represented in Supplementary table 2.
Discussion
Pancreatic cancer stromal microenvironment is considered to play a major role in chemo-resistance of the tumor by preventing efficient drug delivery into the tumor. The stroma has received a lot of focus in pancreatic cancer research over the last 6-8 years. There are conflicting evidences as to whether targeting stroma and its components are beneficial for regression of the tumor. On one hand, relieving the vascular compression in the tumor results in better drug delivery to the tumor (3, 33, 34), while on the other hand it is also argued that stroma is actually restraining the tumor by preventing metastasis (15, 35, 36). Thus, an ideal anti-pancreatic tumor drug would thus be the one that will cause stromal depletion to ensure efficacious drug delivery into the tumor and at the same time be able to inhibit metastasis by being tumoricidal.
The KrasG12D; TP53 mutated mice (KPC) has been extensively used to understand this phenomenon pancreatic tumor microenvironment (33, 37). A molecular analysis of the pancreatic ductal adenocarcinoma stroma in these mice revealed that it was extremely heterogeneous, comprising of cellular and acellular components, like fibroblasts, myofibroblasts, pancreatic stellate cells, immune cells, blood vessels, extracellular matrix (ECM), and soluble proteins such as cytokines and growth factors. Similar stromal architecture is also found in the resected pancreatic tumors from patients. Our studies showed that patient tumor derived xenografts in severely immune compromised (SCID) mice also show similar elaborate stromal architecture in the first 2-3 passages (Fig 1). Similar to the KPC mice, these tumors had extensive fibrosis (as visualized by Sirius red staining), cancer associated fibroblasts and activated stellate cells and an extensive extracellular matrix comprising of hyaluronan (Fig. 1). Hyaluronan is a glycosaminoglycan made of N-acetyl glucosamine and glucuronic acid (38). Apart from being instrumental in fluid retention and wound healing in normal tissues, hyaluronan is also involved in mediating a number of signaling in human body, one of which is the sperm-ova recognition (39). Interestingly, triptolide, the active drug of Minnelide, derived from a chinese herb has been used as a male contraceptive in China and has been shown to influence hyaluronan synthesis (40).
Previous studies from our group have shown that Minnelide, the water soluble pro-drug of triptolide is extremely effective as an anti-cancer compound in a number of animal models for pancreatic cancer, including the KPC model (24). Minnelide is currently under Phase I clinical trial at the University of Minnesota. In that context, it was imperative to test if treatment with Minnelide was able to breach the stromal barrier. Our histological studies showed that Minnelide significantly reduced both total collagen and hyaluronan in both the PDX and the KPC tumor models (Fig. 2).
The extracellular matrix in pancreatic tumor is predominantly made of collagen. Synthesized as a pro-collagen molecule, collagen assembly and cross-linking into tertiary structure provides the structural strength to the matrix. Interestingly, our studies revealed that the expression of the genes involved in synthesis or stability of collagen (for eg ColE genes, PLOD genes and P4H genes remained unchanged in response to Minnelide (Supplementary fig 3). The stability of the procollagen into cross-linked collagen fibrils is mediated by a group of two enzymes: Pro-collagen lysine 2-oxoglutarate dioxygenase (PLOD1-4) and Prolyl 4 hydroxylase (P4H). PLOD1-4 are a group of enzymes that catalyze the transfer of a hydroxyl group to the lysine residues in the collagen. The addition of hydroxyl groups is essential for collagen molecules to form stable interactions, called cross-links, with one another. Cross-links between these molecules allow collagen to form networks of strong, slender fibrils that eventually constitute the stroma. Since hydroxylysine is a rare amino acid only present in collagen, a measure of the total hydroxylysine is a measure of the total PLOD1-4 activity. P4H, on the other hand catalyze the formation of 4-hydroxyproline that is essential to the proper three-dimensional folding of newly synthesized procollagen chains. Once again, since the major source hydroxyproline is collagen, estimation of total hydroxyproline is a measure of the activity of P4H in the tumors. Minnelide decreased total hydroxylysine and hydroxyproline in the tumor tissues (Fig 4), indicating that the activity of these enzymes was inhibited by this compound.
Our results showed that Minnelide decreased total hyaluronan in the tumor tissues. We hypothesized this could either be due to increased degradation of hyaluronan or decreased synthesis of hyaluronan. To address this, we perform activity assays for both HA synthase (HAS) enzymes as well as hyaluronidase (HYAL1-4, PH20) enzymes. Our results showed that Minnelide decreased activity as well as expression of HAS genes, were as HYAL genes remained unaffected by Minnelide treatment (Fig. 4). Review of literature showed that activity of HA synthase was dependent on the availability of UDP-GlcNAc in the cell (41). Previous results from our group showed that triptolide/Minnelide downregulates the hexosamine biosynthesis pathway that results in production of UDP-GlcNAc (22). Further, this downregulation of hexosamine pathway by Minnelide also leads to inhibition of O-GlcNAc transferase that glycosylates Sp1, a transcription factor that is responsible for transcription of HAS genes (22, 41, 42). This suggested that Minnelide resulted in decreased synthesis of hyaluronan by (a) depleting the substrate pool of UDP-GlcNAc in the cells resulting in decreased activity of the HA synthase, as well as (b) downregulating activity of Sp1, which resulted in decreased transcription of HAS genes.
Initial studies on Minnelide indicated that this compound downregulated HSP70 in pancreatic cancer cells and as a result induced cell death by triggering multiple cell death pathways (21, 43). Follow up studies in this line have indicated that the downregulation of HSP70 is mediated by inhibition of transcriptional activity of Sp1 (22). Interestingly, Sp1 has been reported to be instrumental in regulating HA synthase genes as well (42, 44). Thus, downregulation of the HA in the stroma of the pancreatic tumor by HA may be a result of inhibition of Sp1 transcriptional activity.
Since the stromal “compression” was relieved following treatment with Minnelide, the next question was to determine if it translated to better intra-tumoral drug delivery. Our results showed that treatment with Minnelide resulted in more number of “open” blood vessels in the tumor (Fig 5A). This further facilitated a better delivery of doxorubicin (a fluorescent compound that is studied routinely to determine efficacy of drug delivery) in the tumors (Fig. 5B). The current statistics show that the presence of the abundant stroma in pancreatic tumor models like KPC, results in hypo-vascularized tumors with very poor drug perfusion. Our study showed that Minnelide not only decreased the stromal components, it also improved the functional vasculature in the tumor and increased drug delivery into the tumor that resulted in a better median survival of the KPC mice (Fig 5C).
The understanding of the role of stroma in the pancreatic carcinogenesis, progression and treatment is under evolution. Studies have shown that stromal elements can not only enhance cancer cell proliferation and invasion but can also help cancer evade immune system (45). Furthermore, studies suggest that desmoplastic stroma of pancreatic cancer may also impede deliver of chemotherapeutics. In a study by Olive et al, treatment of mice with pancreatic cancer in KPC genetically engineered mouse model of pancreatic cancer with gemcitabine in combination with a Shh inhibitor led to decrease stromal content and better delivery of gemcitabine into the tumor, as compared to gemcitabine treatment alone. This was ascribed to presence of increased functional vessel on depletion of stroma. However, recent studies from Rhim et al (15) have shown that stroma may have a restraining effect on the tumor In this study chronic depletion of stroma by embryonic deletion of Shh in pancreatic epithelial cells or by chronic administration of smoothened inhibitor to KPC mice led to aggressive tumors with increased vascularity, heightened proliferation and increased metastases. Furthermore this effect was reversed, at least partially by VEGFR blocking antibodies, suggesting that the stroma may also restraint tumor by suppressing tumor angiogenesis.
In our study we have shown that Minnelide is very effective in reducing stromal content of tumors as well as in improving survival of mice in KPC model of pancreatic cancer. Though this seems contrary to previous study by Rhim et al where stromal depletion led to worse survival of KPC mice, we believe that this effect is due to a combination of anti-tumor and anti-stromal effect of Minnelide. We have shown in multiple previous studies that Minnelide is very effective in killing tumor cells. Thus Minnelide's stromal depletion properties ensure the intra-tumoral delivery of Minnelide, and of another anti-tumor agent when used in combination with Minnelide, for enhanced killing of tumor cells and possibly increased survival. This data supports use of stroma depletion strategy only in combination with a strong anti-cancer agent.
Conclusion
Minnelide has shown tremendous promise in the pre-clinical studies. Since August 2013, it is being evaluated in a Phase1 clinical trial against GI cancers. In this respect, evaluating its effect on stroma in pancreatic cancer is extremely relevant and timely. This study shows that Minnelide is not only tumoricidal at the given doses as we have seen before, but it also depletes the stromal architecture and induces stromal cell death, thus increasing the intra-tumoral concentration of the drug and finally eradicating them. Pancreatic cancer has extremely very poor prognosis owing to very poor drug delivery into the tumor. In this context, our study is a step forward in addressing the current challenges that exist in conquering this devastating disease.
Supplementary Material
Statement of Translational Relevance.
The prevalence of stroma in pancreatic tumors is the primary reason behind impaired drug delivery in pancreatic cancer. The desmoplastic stroma constricts the blood vessels in the tumor, leading to inefficient vasculature and poor drug delivery. Thus there is an urgent need to develop and evaluate therapy that along with having anti-cancer effect will also ensure better drug delivery into the tumors. Minnelide, a potent anti-cancer compound is currently in Phase I clinical trial. In this study, we have evaluated the effect of Minnelide on stromal lysis in pancreatic cancer. Our studies show that Minnelide depletes the extracellular matrix components by depleting hyaluronan and collagen in the stroma, leading to better drug delivery and improved survival in a spontaneous animal model of pancreatic cancer. This is translationally relevant as it suggests that Minnelide can be developed as an effective therapy against pancreatic cancer.
Acknowledgments
Grant Support: This study was funded by NIH grants R01-CA170946 and CA124723 (to AKS); NIH grant R01-CA184274 (to SB); Katherine and Robert Goodale foundation support (to AKS).
Abbreviation
- PDAC
Pancreatic Adenocarcinoma
- HA
Hyaluronan
Footnotes
Conflict of Interest: University of Minnesota has filed a patent for Minnelide, which has been licensed to Minneamrita Therapeutics, LLC. AKS is the co-founder and the Chief Scientific Officer of this company; SB is a consultant with this company. This relationship has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–20. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 5.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY) 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan VP, Boucher Y, Ferrone CR, Roberge S, Martin JD, Stylianopoulos T, et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell. 2014;26:14–5. doi: 10.1016/j.ccr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer cell. 2012;21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Molecular cancer therapeutics. 2007;6:1186–97. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 9.Wehr AY, Furth EE, Sangar V, Blair IA, Yu KH. Analysis of the human pancreatic stellate cell secreted proteome. Pancreas. 2011;40:557–66. doi: 10.1097/MPA.0b013e318214efaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4254–9. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI. Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Seminars in cancer biology. 2008;18:288–95. doi: 10.1016/j.semcancer.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Ohlund D, Lundin C, Ardnor B, Oman M, Naredi P, Sund M. Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. Br J Cancer. 2009;101:91–7. doi: 10.1038/sj.bjc.6605107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohlund D, Franklin O, Lundberg E, Lundin C, Sund M. Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer. 2013;13:154. doi: 10.1186/1471-2407-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–47. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter BZ, Mak DH, Schober WD, Dietrich MF, Pinilla C, Vassilev LT, et al. Triptolide sensitizes AML cells to TRAIL-induced apoptosis via decrease of XIAP and p53-mediated increase of DR5. Blood. 2008;111:3742–50. doi: 10.1182/blood-2007-05-091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan EW, Cheng SC, Sin FW, Xie Y. Triptolide induced cytotoxic effects on human promyelocytic leukemia, T cell lymphoma and human hepatocellular carcinoma cell lines. Toxicology letters. 2001;122:81–7. doi: 10.1016/s0378-4274(01)00353-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen YW, Lin GJ, Chia WT, Lin CK, Chuang YP, Sytwu HK. Triptolide exerts anti-tumor effect on oral cancer and KB cells in vitro and in vivo. Oral oncology. 2009;45:562–8. doi: 10.1016/j.oraloncology.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, et al. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology. 2009;136:1772–82. doi: 10.1053/j.gastro.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krosch TC, Sangwan V, Banerjee S, Mujumdar N, Dudeja V, Saluja AK, et al. Triptolide-mediated cell death in neuroblastoma occurs by both apoptosis and autophagy pathways and results in inhibition of nuclear factor-kappa B activity. Am J Surg. 2013;205:387–96. doi: 10.1016/j.amjsurg.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer research. 2007;67:9407–16. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee S, Sangwan V, McGinn O, Chugh R, Dudeja V, Vickers SM, et al. Triptolide-induced Cell Death in Pancreatic Cancer Is Mediated by O-GlcNAc Modification of Transcription Factor Sp1. J Biol Chem. 2013;288:33927–38. doi: 10.1074/jbc.M113.500983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee S, Thayanithy V, Sangwan V, Mackenzie TN, Saluja AK, Subramanian S. Minnelide reduces tumor burden in preclinical models of osteosarcoma. Cancer letters. 2013 doi: 10.1016/j.canlet.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, et al. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Science translational medicine. 2012;4:156ra39. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsaied OA, Sangwan V, Banerjee S, Krosch TC, Chugh R, Saluja A, et al. Sorafenib and triptolide as combination therapy for hepatocellular carcinoma. Surgery. 2014;156:270–9. doi: 10.1016/j.surg.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee S, Nomura A, Sangwan V, Chugh R, Dudeja V, Vickers SM, et al. CD133+ tumor initiating cells in a syngenic murine model of pancreatic cancer respond to Minnelide. Clin Cancer Res. 2014;20:2388–99. doi: 10.1158/1078-0432.CCR-13-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rousalova I, Banerjee S, Sangwan V, Evenson K, McCauley JA, Kratzke R, et al. Minnelide: a novel therapeutic that promotes apoptosis in non-small cell lung carcinoma in vivo. PLoS One. 2013;8:e77411. doi: 10.1371/journal.pone.0077411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itano N, Sawai T, Atsumi F, Miyaishi O, Taniguchi S, Kannagi R, et al. Selective expression and functional characteristics of three mammalian hyaluronan synthases in oncogenic malignant transformation. J Biol Chem. 2004;279:18679–87. doi: 10.1074/jbc.M313178200. [DOI] [PubMed] [Google Scholar]
- 29.Sharon Y, Alon L, Glanz S, Servais C, Erez N. Isolation of normal and cancer-associated fibroblasts from fresh tissues by Fluorescence Activated Cell Sorting (FACS) J Vis Exp. 2013:e4425. doi: 10.3791/4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waghray M, Yalamanchili M, di Magliano MP, Simeone DM. Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol. 2013;29:537–43. doi: 10.1097/MOG.0b013e328363affe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–9. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 33.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer. 2013;108:1–8. doi: 10.1038/bjc.2012.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bijlsma MF, van Laarhoven HW. The conflicting roles of tumor stroma in pancreatic cancer and their contribution to the failure of clinical trials: a systematic review and critical appraisal. Cancer metastasis reviews. 2015;34:97–114. doi: 10.1007/s10555-014-9541-1. [DOI] [PubMed] [Google Scholar]
- 36.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, Passi A. Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta. 2014;1840:2452–9. doi: 10.1016/j.bbagen.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Meizel S. Molecules that initiate or help stimulate the acrosome reaction by their interaction with the mammalian sperm surface. The American journal of anatomy. 1985;174:285–302. doi: 10.1002/aja.1001740309. [DOI] [PubMed] [Google Scholar]
- 40.Yan SX, Wang Y. Inhibitory effects of Triptolide on interferon-gamma-induced human leucocyte antigen-DR, intercellular adhesion molecule-1, CD40 expression on retro-ocular fibroblasts derived from patients with Graves' ophthalmopathy. Clin Experiment Ophthalmol. 2006;34:265–71. doi: 10.1111/j.1442-9071.2006.01190.x. [DOI] [PubMed] [Google Scholar]
- 41.Jokela TA, Makkonen KM, Oikari S, Karna R, Koli E, Hart GW, et al. Cellular content of UDP-N-acetylhexosamines controls hyaluronan synthase 2 expression and correlates with O-linked N-acetylglucosamine modification of transcription factors YY1 and SP1. J Biol Chem. 2011;286:33632–40. doi: 10.1074/jbc.M111.265637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monslow J, Williams JD, Fraser DJ, Michael DR, Foka P, Kift-Morgan AP, et al. Sp1 and Sp3 mediate constitutive transcription of the human hyaluronan synthase 2 gene. J Biol Chem. 2006;281:18043–50. doi: 10.1074/jbc.M510467200. [DOI] [PubMed] [Google Scholar]
- 43.Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, et al. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology. 2009;136:1772–82. doi: 10.1053/j.gastro.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michael DR, Phillips AO, Krupa A, Martin J, Redman JE, Altaher A, et al. The human hyaluronan synthase 2 (HAS2) gene and its natural antisense RNA exhibit coordinated expression in the renal proximal tubular epithelial cell. J Biol Chem. 2011;286:19523–32. doi: 10.1074/jbc.M111.233916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z, Pothula SP, Wilson JS, Apte MV. Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol. 2014;20:11216–29. doi: 10.3748/wjg.v20.i32.11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.