Abstract
Surgery represents the main curative therapeutic modality for gastric cancer, and it is occasionally considered for palliation as well as prophylaxis. Most frequently, surgical outcomes are conveyed in terms of oncological outcomes such as recurrence and survival. However, quality of life (QoL) is also important and should be considered when making treatment decisions - including the extent of and approach to surgery. Measurement of QoL usually involves the application of questionnaires. While there are multiple QoL questionnaires validated for use in oncology patients, there are very few that have been validated for use in those with gastric cancer. In this review, we discuss and compare the current status of QoL questionnaires in gastric cancer. More importantly, the impact of surgery for treatment, palliation and prophylaxis of gastric cancer on QoL will be described. These data should inform the surgeon on the optimal approach to treating gastric cancer, taking into account oncological outcomes. Knowledge gaps are also identified, providing a roadmap for future studies.
Keywords: Quality of life, Gastric cancer, Palliation, Surgery, Oncology
Core tip: Quality of life is an important determinant in the optimal management of patients with malignancy. This is no different for gastric cancer where surgery is considered in cases of resection-for cure, palliation and prophylaxis. This review summarizes the available evidence surrounding the impact of surgery on quality of life in gastric cancer. In general, there has been an improved appreciation of the importance of quality of life as an outcome that must be considered in the context of survival and performance status.
INTRODUCTION
Gastric cancer is the fifth most common cancer worldwide and is the third leading cause of cancer-related mortality according to the World Health Organization (WHO). There are over 22000 new gastric cancer cases diagnosed yearly in the United States[1,2]. Current estimates from Western countries show a 5-year survival ranging from 30%-40% after R0 resection[3]. In order to improve this, multimodality therapy has been employed. Curative measures may therefore be comprised of subtotal or total gastrectomy (TG), lymphadenectomy and perioperative chemotherapy or postoperative chemoradiation[4,5].
In addition to survival, other outcomes must be considered when measuring the effectiveness of therapies for gastric cancer. Surgery is associated with short-term morbidity and mortality. In long-term survivors, gastric surgery may have functional outcomes[6,7]. Chemotherapy and radiation therapy are also associated with a number of toxicities that, at times, limit their use and effectiveness[4,5]. Performance status, an objective measure of overall function, can be impacted by treatments. Finally, quality of life (QoL) can be impacted by any treatments for gastric cancer.
QoL is a construct that describes the subjective well-being of an individual. It has physical and psychological dimensions and therefore is generally poorly understood by physicians. As a result, QoL is generally underreported and poorly documented, even though, to the patient, this outcome may be of paramount importance, and despite the recognition that almost any cancer treatment can adversely affect QoL. In fact, while most oncologists believe QoL is an important clinical endpoint, only 50% measure it in clinical practice[8]. This may be because the QoL construct has frequently been confused with functional outcomes in the past. The instruments used to measure the multiple dimensions of QoL have only recently been validated.
For a disease with a limited survival such as gastric cancer, consideration of QoL is paramount when considering any treatment strategy. Surgery plays a dominant role in the treatment of gastric cancer. Its effects on QoL are obvious and intuitive. What is less obvious is the magnitude of any adverse effects on QoL, the duration of this impaired QoL, and the comparative effects of various surgical options on QoL.
In thinking about the role of surgery for any individual, one must consider the present QoL and the likelihood that that QoL can be restored or improved in a reasonably short period of time, taking into account the expected length of survival (Figure 1). Surgery will undoubtedly have a QoL “cost”, a temporary deterioration of QoL that would be exacerbated by complications or by disease progression; this cost must be evaluated in the specific clinical context. For example, in an individual undergoing a prophylactic gastrectomy [for hereditary diffuse gastric cancer (HDGC), for example], preoperative QoL will likely be excellent and it will be imperative to restore QoL to normal levels (Figure 1, line A). In a patient undergoing curative resection for gastric cancer, baseline QoL may be lower (Figure 1, line B). Given the more limited survival of such a patient, strategies that reduce the recovery time should be explored. Finally, in a patient with incurable disease, baseline QoL may be particularly impaired; expected survival is short (Figure 1, line C). Any surgical procedure under consideration should have a reasonable likelihood of improving QoL within a short period of time after the procedure, with little QoL “cost”. Using recently validated QoL instruments to serially evaluate the QoL throughout a patient’s clinical course will provide the surgeon with an objective measure of whether a QoL benefit has been realized.
Figure 1.
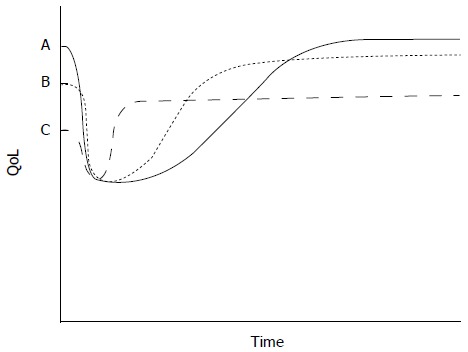
A model to conceptualize the effects of gastric procedures on quality of life. For any given situation, surgery will have a quality of life (QoL) “cost” which is proportional to the magnitude of the reduction in QoL and the duration of this impaired QoL. Ideally, QoL should be restored to preoperative levels in individuals undergoing prophylactic gastrectomy (line A). In patients undergoing curative procedures, QoL should be return to baseline within a short period (line B). In patients undergoing a palliative procedure, QoL should be improved soon after surgery, with little QoL “cost” (line C).
QOL IN GASTRIC CANCER
Methods to measure QoL
According to Schipper et al[9], QoL is “the functional effect of a disease and its consequent therapy upon a patient, as perceived by the patient”. In gastric cancer, it has been defined as the subjective evaluation of a patient’s physical, emotional, social and functional well-being and perceived symptom burden[10]. Both definitions account for the fact that QoL is a patient-centered variable that must take into account the patient’s perception of their current situation. Clinicians likely have their own opinions regarding the effects of various treatments on patients’ QoL, but these are highly influenced by “hard signs” such as recurrence rate and overall survival. In fact the “best” treatment for gastric cancer would be the one that provides the longest overall survival with the least toxicity and the best QoL[11]. Assessing QoL is thus a vital statistic in the determination of optimal therapy and outcomes, in curative and palliative settings.
Measurement and reporting of prognostic factors (e.g., tumor stage) and survival outcomes are commonplace in most gastric cancer clinical trials. However, QoL is less well understood, infrequently documented, and at times improperly measured. In a review by Kaptein et al[12], twenty-six studies addressing QoL in gastric cancer were identified. Twenty (77%) of these studies examined the impact of surgical procedures on QoL. Nearly a quarter of these 20 studies utilized novel and unvalidated QoL instruments while others used multiple instruments or ones not specifically validated for surgical oncology patients.
The European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 is a generic oncology QoL questionnaire focused mainly on physical symptoms[13]. This is a robust questionnaire, validated for multiple cancer sites and in numerous languages. The Functional Assessment of Cancer Therapy-General (FACT-G) is a similar questionnaire that focuses on a broader range of areas important to QoL including social and emotional factors[14]. It is comprised of four general well being subtypes: physical, social, emotional and functional. In comparing these two instruments, Kemmler et al[15] found that despite considerable overlap in the questionnaires, they measured markedly different aspects of QoL.
In recent years, it has also become apparent that some dimensions of QoL are disease-specific. A lack of disease-specific QoL elements may limit the sensitivity of detecting changes or differences in QoL. One example of how this may have been important was in a cohort treated with systemic therapy. Webb et al[16] utilized the QLQ-C30 to measure QoL differences in patients with advanced gastroesophageal cancer undergoing two different chemotherapeutic protocols. Despite differences in survival and chemotherapeutic effect, there were no measurable differences in QoL except for the global scores at 24 wk. It is possible that this reflected a need for newer and more focused QoL instruments - ones specific for the needs of those patients with gastric cancer.
Measuring QoL in gastric cancer
There are many factors that determine the QoL in patients with gastric cancer. Symptoms (including pain and dysphagia), emotional well-being, social and financial status, and body image may all play a role. In this regard, creating a valid and robust questionnaire has presented some challenges. Early attempts failed because they were not applicable to multiple treatment modalities and did not undergo formal validation[17-19].
The first validated gastric cancer-specific QoL instrument developed was the EORTC QLQ-STO22[20] (Table 1). This is a 22 item questionnaire that is administered in conjunction with the generic QLQ-C30. As in the parent questionnaire, the STO-22 focuses mainly on patient symptoms including pain, dysphagia, reflux and early satiety. However, it also touches on emotional issues including body image, weight loss and a patient’s reflection on their illness. It has been validated across multiple countries and languages, over numerous treatment modalities, and in both the curative setting as well as patients receiving palliative or best supportive care[21].
Table 1.
Comparison of gastric cancer-specific quality of life questionnaires
| EORTC QLQ-STO22 | FACT-Ga | DAUGS32 | |
| Year introduced | 2001 | 2004 | 2005 |
| Validated? | Yes | Yes | Yes |
| Parent questionnaire | EORTC QLQ-C30 | FACT-G | None |
| Number of items/questions | 22 | 19 | 32 |
| Focus | Patient symptoms | Emotional and physical symptoms | Gastrointestinal dysfunction |
| Applicable to all treatment modalities? | Yes | Yes | No (surgery only) |
A second gastric cancer-specific instrument is the FACT-Gastric instrument (FACT-Ga)[22]. This 19-item tool was developed to pair with the FACT-G generic cancer questionnaire. The FACT-Ga includes questions regarding physical symptoms (pain, energy etc.) but focuses more on patients’ emotional and physical well-being as well as their reaction to illness. Just like the STO-22, the FACT-Ga has been validated for use in patients with gastric adenocarcinoma, in Western countries[23] and abroad[24,25].
The STO-22 and the FACT-Ga are relatively broad questionnaires that cover a range of QoL issues affecting gastric cancer patients. Additionally, they have the advantage of being applicable to nearly all treatment modalities. There are other scoring systems that are narrower in scope. The Dysfunction after Upper Gastrointestinal Surgery for Cancer (DAUGS32) questionnaire is a 32-item study designed to elicit post-operative gastrointestinal dysfunction issues[26]. The DAUGS32 focuses strictly on gastrointestinal symptoms including reflux, gastric dumping, digestive difficulties, nausea and vomiting and lower gastrointestinal symptoms. Unlike the STO-22 and the FACT-Ga, the DAUGS32 is a stand-alone test, not partnered with a more general cancer QoL questionnaire. It has been validated for use in postoperative patients[26-28]. However, there are two major limitations to this questionnaire. Firstly, it is not designed to study patients receiving non-surgical treatment or those being considered for palliative measures. Secondly, it has not been utilized outside of a Japanese patient population. Therefore, its utility is limited, as it is unclear how the DAUGS32 applies to a more general gastric cancer population. A more recently developed instrument is the Postgastrectomy Syndrome Assessment Scale (PGSAS-45)[29-31], a 45 item survey that borrows questions from the Gastrointestinal Symptom Rating Scale - a system designed for benign gastric conditions[32]. In contrast to the DAUGS32, the PGSAS-45 touches on general QoL factors (general health, mental health, social functioning etc.) in addition to pointed questions aimed at determining post-operative gastric function. This questionnaire is ideally suited to study the effects of varied surgical techniques on post-operative QoL and may gain a wider usage in the near future. It has not been validated in non-Asian patients.
In summary, there are many means to measure QoL in gastric cancer patients (Table 2). These range from questionnaires specific to a certain patient population to the more broadly applied STO-22 and FACT-Ga surveys. This plethora of options could make comparison of QoL across studies difficult. On the other hand, gastric cancer is a complex disease and QoL is a multifaceted outcome that may not be adequately measured with a single device. In the end, most researchers will likely choose the questionnaire they have the most experience with, provided it has been validated for their patient population.
Table 2.
Quality of life tools utilized in the included references
| Author | Year | Main QoL outcome | QoL tool |
| Korenaga et al[37] | 1992 | QoL - single time point | Interview, non-validated |
| Davies et al[40] | 1998 | Comparison of extent of surgery (DG vs TG) | RSCL, Troidl, HAD, ADLs |
| Spector et al[63] | 2002 | Comparison of approaches to GEJ tumors | GIQLI, LAGS |
| Díaz De Liaño et al[68] | 2003 | Comparison of extent of resection and nodal dissection | QLQ-C30 |
| Barbour et al[62] | 2008 | Comparison of approaches to GEJ tumors | QLQ-C30 |
| Kim et al[71] | 2008 | Comparison of lap vs open for DG | QLQ-C30, QLQ-STO22 |
| Tyrväinen et al[38] | 2008 | Long-term QoL | SF-36, 15D |
| Avery et al[34] | 2010 | Longitudinal follow-up after TG and DG | QLQ-C30, QLQ-STO22 |
| Lee et al[36] | 2010 | Long-term QoL | QLQ-C30, QLQ-STO22 |
| Jeurnink et al[90] | 2010 | Surgical GJ vs stent for GOO | QLQ-C30, EuroQoL-5D, EuroQoL-VAS, QLQ-PAN26 |
| Kobayashi et al[42] | 2011 | Comparison of extent (DG vs TG)and method (lap vs open) of surgery | QLQ-C30, QLQ-STO22 |
| Kim et al[35] | 2012 | Longitudinal follow-up after TG and DG | QLQ-C30, QLQ-STO22 |
| Kulig et al[85] | 2012 | QoL in non-curative resection | QLQ-C30 |
| Lee et al[48] | 2012 | Comparison of reconstruction after DG (BI vs BII vs R-Y) | GIQLI |
| Munene et al[11] | 2012 | Longitudinal follow-up, comparison of extent of surgery (DG vs TG) | FACT-Ga |
| Takiguchi et al[49] | 2012 | Comparison of reconstruction after DG (BI vs R-Y) | QLQ-C30, DAUGS 20 |
| Karanicolas et al[33] | 2013 | Comparison of extent of surgery (DG vs TG vs PG) | QLQ-C30, QLQ-STO22 |
| Rausei et al[43] | 2013 | Comparison of extent of surgery (DG vs TG) | QLQ-C30, QLQ-STO22 |
| Park et al[44] | 2014 | Comparison of extent of surgery (DG vs TG) | QLQ-C30, QLQ-STO22 |
| Takiguchi et al[29] | 2014 | TG vs PG for proximal gastric tumors | PGSAS-45 |
| Worster et al[99] | 2014 | Longitudinal study, prophylactic gastrectomy patients | QLQ-C30, QLQ-STO22 |
| Ronellenfitsch et al[57] | 2015 | Longitudinal follow-up after PG | FACT-E |
ADL: Activities of daily living; TG: Total gastrectomy; DG: Distal gastrectomy; FACT: Functional assessment of cancer therapy (-G: Gastric, -E: Esophageal); GEJ: Gastroesophageal junction; GJ: Gastrojejunostomy; GIQLI: Gastrointestinal Quality of Life Index; GOO: Gastric outlet obstruction; HAD: Hospital anxiety and depression scale; LAGS: Life after gastric surgery; PGSAS: Postgastrectomy syndrome assessment scale; RSCL: Rotterdam symptom checklist; QoL: Quality of life.
EFFECT OF SURGERY FOR CURE ON QOL
Surgery forms the mainstay of current therapy for gastric cancer and, as such, also forms one of the main determinants of patient QoL. In addition, given the high likelihood of disease recurrence, the benefit of any surgical procedure must exceed any detriment to the patient’s QoL. Therefore, while surgical resection for gastric cancer should abide by sound oncologic principles, it must also take into account the effect on the patient’s QoL.
In general, surgery itself causes a decrease in QoL. Munene et al[11] showed that QoL scores decreased after surgery and normalized by 6 mo. This decrease was not affected by the extent of surgical resection. The group from Memorial Sloan Kettering, followed 134 patients prospectively and showed similar results[33]. Over 50% of patients experienced post-operative impairment in their global QoL. Of note, nearly one-third of patients continued to have worse QoL than before surgery, even after the 6 mo point. Other studies have shown similar results, underlining the importance of addressing the QoL of patients in the postoperative period[34,35].
Some studies suggest that gastric resection has even more sustained detrimental effects. Lee et al[36] studied a group of long-term survivors after distal gastrectomy (DG). They compared 126 patients 5 years after surgery to a group of healthy controls. Using the QLQ-C30 and STO-22 they found that the surgical patients scored higher for emotional functioning and fatigue but scored worse for nausea and vomiting, financial difficulties, eating restrictions and body image[36]. While there may be issues with the methodology of comparing gastric cancer survivors to healthy individuals awaiting a routine screening exam, the study does underline the persistence of impaired QoL after resection. Earlier studies had found similar results in long-term survivors, although the specific elements of QoL affected by surgery varied across the studies[37,38].
DG vs TG for distal gastric cancers
For distal gastric lesions, the decision to perform a DG vs a TG is typically made based on oncologic principles, but the effects of the procedure on QoL should also be considered. In his review published in 1992, Bozzetti et al[39] surmised that “when two surgical procedures are compared, if the oncological results are the same, the operation which is associated with the least discomfort and impairment of the QoL should be chosen”. Early advocates for the routine use of TG for gastric cancer quoted the reduced risk of recurrence and elimination of the risk of a second cancer[40]. While there is a statistically significant yet small risk of recurrence in the remaining distal stomach, there is high quality evidence showing equivalence in 5-year survival between the two procedures[7,41]. Moreover, there is no increased risk of mortality with either procedure[7]. Therefore, as long as oncologic margins are negative, the “best” procedure would be that which affords the best QoL.
Using a number of indices, Davies and coworkers studied QoL over the first year after surgery in 47 patients undergoing either DG or TG[40]. At one-year, those who had undergone DG had a significantly better QoL than those in the TG group. In fact, the QoL in the DG group was better after surgery than it was preoperatively. The inferior QoL associated with TG has been confirmed over multiple studies in the years since[35,42-44]. Kim et al[35] followed a cohort of over 450 Korean patients through the first post-operative year. Those patients that underwent TG developed alterations in social functioning, pain, insomnia, reflux and dry mouth that did not return to baseline, although those same symptoms normalized in those undergoing DG. Overall QoL was also affected, being more likely to return to baseline in the DG group as compared to the TG group. Similarly, Rausei et al[43] showed that TG is associated with a number of upper-gastrointestinal tract symptoms, including reflux, leading to a negative impact on overall QoL.
Not every study has shown a difference in QoL between the two procedures. Munene et al[11] followed 43 patients after distal or TG in a Canadian centre. Using the FACT-Ga instrument, there was no difference in QoL between DG and TG over the study period. On the other hand, in patients undergoing TG, there was a measurable decline in function as evaluated by the Karnofsky performance status. A more recent study from the United Kingdom found similar values in post-operative global QoL although those undergoing TG had worse dysphagia and eating restrictions. While these latter two studies are likely hampered by low sample sizes and survey response rates, they do add to the portrait of overall impairment in those undergoing TG as compared to DG. In all, if it is possible to perform an adequate resection with negative margins by DG, then this will result (on average) in an improved QoL at no cost to overall survival.
Influence of method of reconstruction after DG
There are multiple methods for re-establishing gastrointestinal continuity after DG. The Billroth I (BI), Billroth II (BII) and Roux-en-Y (R-Y) reconstructions are most frequently employed, although there are many variations of each technique and the “best” reconstruction option is controversial (Figure 2A). The BII anastomosis (gastrojejunostomy) is associated with increased bile reflux, biliary gastritis, dumping symptoms and the possibility of remnant stomach cancer[43,45]. Bile reflux and biliary gastritis are substantially less common following a R-Y reconstruction[33,43,46,47]. The advantage of a BI anastomosis (gastroduodenostomy) is that it is more “physiologic”, maintaining the normal stream of gastric contents into the duodenum. However, this is not technically feasible in the majority of instances of resections for gastric cancer, where a small gastric remnant is remaining. That is, following a radical DG, the distance between proximal gastric remnant and duodenal stump is too great to enable a tension-free anastomosis. In addition to these technical considerations, there are regional preferences (as well as preferences by surgeons). For example, a BII anastomosis is most frequently performed in North America and Europe; in the East, BI and R-Y reconstructions are more common[33,45].
Figure 2.
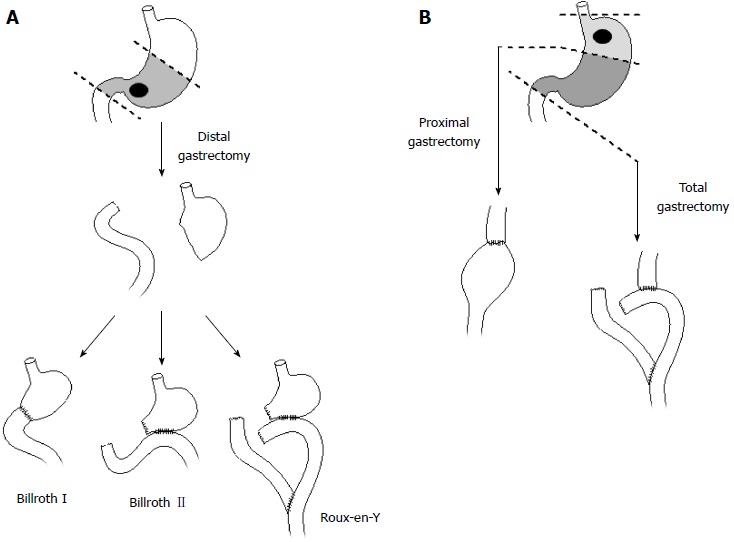
Resection and reconstruction options for distal (A) and proximal gastric cancers (B).
There is no evidence that the method of reconstruction has any influence on oncological outcomes. Therefore, the choice of anastomosis should be heavily influenced by functional considerations and QoL. Non-randomized studies have not shown convincing differences in QoL, although the incidence of bile reflux symptoms and endoscopic evidence of reflux are least common following R-Y[33,43,46,47]. There are also at least two randomized controlled trials evaluating the influence of anastomotic technique on functional and QoL outcomes. Lee et al[48] reported on 159 DG patients randomized to BI, BII or R-Y reconstruction. There was no overall difference in QoL between the three techniques, although those with a R-Y developed less frequent biliary reflux as determined by endoscopy. Similarly, Takiguchi et al[49] randomized patients to BI and R-Y and studied postoperative QoL. Despite worse reflux symptoms in the BI group, there was no difference in QoL in the two groups. It is interesting that reflux and its symptomatic manifestations have little impact on QoL. This may be because, in the majority of individuals, these symptoms can be effectively managed by pharmacological therapy.
Role of proximal gastrectomy
For tumors of the cardia and proximal stomach, the surgeon is similarly faced with the decision on the best approach to extirpating the tumor. It is unclear whether patients derive any benefit from the remaining distal stomach with a proximal gastrectomy (PG) as opposed to performing a TG (Figure 2B). Oncologically, PG and TG should be equivalent procedures, provided clear resection margins are achieved, as the chance of metastasis to distal gastric nodes is uncommon[50]. Indeed, multiple studies have demonstrated equivalence in survival[51-55]. The operative decision may then revolve around QoL and functional differences.
While multiple studies have been conducted to attempt to answer this question, many of them are hampered by a lack of validated QoL data[52-54,56]. Takiguchi et al[29] reviewed nearly 400 patients of which 193 underwent proximal gastrectomy. Overall, QoL after PG was similar to QoL after TG, although PG patients benefited from reduced dumping and less need for additional meals. Some would argue that the removal of the lower esophageal sphincter in the setting of an intact distal stomach would predispose to reflux. While this could lead to a reduced QoL, a recent study showed that, even though one third of patients with PG had endoscopic signs of esophagitis, only two patients reported symptoms[57]. Moreover, QoL measured with the FACT-E questionnaire was not reduced in the early post-operative period and increased steadily over time. Reflux was only reported as “mild” by most patients, possibly because all were prescribed a proton-pump inhibitor. Conversely, Karanicolas and coworkers found that patients undergoing PG developed significantly more clinical reflux and nausea, as well as a diminished global QoL compared to those undergoing TG or DG[33]. A large meta-analysis incorporating nearly 1100 patients reported that PG was associated with higher morbidity, including increased reflux esophagitis and anastomotic stenosis[55]. Still another study found increased rates of severe esophagitis in the TG group[58].
In summary, the role of proximal gastrectomy is still uncertain. While PG is an equivalent oncologic procedure to TG, it may predispose to worsened clinical reflux and QoL. There do not seem to be any obvious QoL benefits to PG, and patients seem to manage reasonably well without a remnant distal stomach.
Surgery for gastroesophageal junction tumors
The surgical approach to gastroesophageal junction (GEJ) tumors can depend on pre-operative workup, the exact location of the tumor, and surgeon preference. Siewert et al[59] divided these tumors into three subtypes based on their anatomic location, a classification that has been updated in the most recent TNM staging system[60]. Surgical options include extended TG or esophagectomy with both transabdominal and transthoracic approaches.
From an oncologic standpoint, for GEJ tumors there is no apparent difference in survival between gastrectomy and esophagectomy. While there are no randomized controlled trials, a recent systematic review supports this claim[61]. Likewise, morbidity and mortality rates are comparable for the two techniques[61].However, there may be differences in their effects on QoL. Barbour et al[62] compared pre-operative QoL to post-operative values in those undergoing either esophagectomy or gastrectomy for Siewert I-III tumors. Using the QLQ-C30 questionnaire, they found that those undergoing gastrectomy had better overall QoL and social function. Fatigue was also less common following gastrectomy than following esophagectomy. However, all those selected for gastrectomy in this study had Siewert III tumors, and this inherent difference in the groups may interfere with any conclusions that can be drawn. Spector and colleagues also reported that patients undergoing esophagogastrectomy had gastrointestinal symptoms more frequently than those who received a gastrectomy with R-Y reconstruction[63]. While it is difficult to make concrete recommendations based on these data, the preponderance of information suggests that QoL is inferior after esophagectomy compared to after gastrectomy. The reasons for this are likely multifactorial including type of incision, difficulties in post-operative food intake, patient selection and tumor location.
Influence of the extent of lymphadenectomy
It is known that metastasis to lymph nodes is an early event in gastric cancer and as such, lymph node dissection is recommended as part of a curative resection. The optimal extent of lymph node dissection is less clear. The minimal procedure would include removal of the perigastric lymph nodes, a D1 lymph node dissection. In comparison, a D2 nodal dissection would include those in a D1 dissection in addition to nodes surrounding the celiac axis, left gastric artery, common hepatic artery, splenic artery and splenic hilum. It is still unclear whether a D2 lymphadenectomy is beneficial. In non-Asian countries, the available randomized series have not demonstrated a survival benefit associated with extended lymphadenectomy[64,65]. However, these studies have been criticized for high mortality rates and study protocol violations[66]. In Asian centers, D2 lymphadenectomy is routinely performed and the procedure is associated with a survival benefit when performed by experienced surgeons[67].
The effects of extended lymphadenectomy on QoL are poorly understood. In the Dutch trial, there was a higher mortality rate, complication rate and reoperation rate in the group that underwent a D2 lymphadenectomy, but QoL was not directly measured[65]. In a retrospective study from Spain in which QoL was determined using the QLQ-C30 questionnaire, there was no difference in QoL between patients who had had a D1 and D2 lymphadenectomy[68]. Rausei et al[43] found a significant increase in patient-reported nausea and belching with D2 lymphadenectomy. More studies are warranted in Asia and the West on the effects of extended lymphadenectomy on operative outcomes, survival and QoL, particularly with more contemporary adjunctive therapies such as postoperative radiotherapy or perioperative chemotherapy.
Laparoscopic vs open gastrectomy
Laparoscopic procedures for gastric cancer are now becoming more widely utilized. In Eastern centers, the laparoscopic approach is commonly applied. However, in the West the adoption has been much slower. This is likely due to the steep learning curve, the complexity of the procedure, as well as patient characteristics[69].
There have now been a number of randomized controlled trials[70-72] and at least one meta-analysis[73] showing equivalent oncologic outcomes between laparoscopic and open approaches. Additional reported benefits of the procedure include decreased length of hospital stay, reduced blood loss, and earlier resumption of oral intake. An additional important reason to perform minimally invasive techniques is to improve patient QoL. In fact there is good evidence supporting this. Kim et al[71] performed a randomized controlled trial comparing laparoscopic DG to open DG. Using validated QoL questionnaires, they found that a laparoscopic DG was associated with a significantly better global QoL in the first 3 mo after surgery, as well as higher scores in the physical, emotional and social subscales during this immediate postoperative period. Kobayashi et al[42] found that the laparoscopic approach led to superior QoL in the early postoperative period but that these differences disappeared by one year after surgery.
PALLIATIVE PROCEDURES AND QOL
For those with locally unresectable or metastatic disease, treatment is directed towards palliation. Palliation has been defined by the WHO as an approach that improves QoL, provides relief from pain and intends neither to hasten nor postpone death[74]. Bleeding, obstruction, malnutrition and pain must be identified and treated if possible[75]. It is in these patients that it is especially imperative that any treatments have a minimal impact on QoL, as lifespan may be measured in weeks to months. Unfortunately, there is a paucity of data to inform the clinician on the best approach to palliation, including the QoL cost-to-benefit ratio for any treatment. Indeed, palliative treatments are presently largely decided by a physician based on prior experiences in conjunction with the patients’ wishes[75]. In these circumstances, great clinical judgment is an asset to the surgeon considering a palliative procedure.
Palliative resection
There is evidence that gastric resection in the palliative setting can lead to a survival benefit, but only in a highly selected group of patients[76-79]. A meta-analysis of 10 studies showed that those undergoing resection had a 2.5-fold higher overall survival rate than those treated conservatively[80]. The subset that derived a survival benefit consisted of younger patients with a higher performance status, lower tumor burden and a favorable histology. In addition, the best overall survival was observed in those who were able to receive postoperative chemotherapy[80,81]. Individuals with disseminated peritoneal disease, bilobar liver metastases, and metastases in more than one organ were least likely to derive a survival benefit. Emergency surgery for bleeding, obstruction or perforation is associated with a shorter survival than operations done electively[82].
Unfortunately, there are few studies employing validated and reliable QoL questionnaires that definitively direct clinical decisions on when palliative gastric surgery is effective. Mahar and coworkers summarized the available studies to date in a recent systematic review[83]. Nine studies were included, with over 75% of patients with stage IV disease. However, results of this review were limited as none of the included studies employed validated QoL measurement tools. Instead they used surrogate markers of QoL such as time to oral intake, length of postoperative hospital stay, hospital re-admission and analgesic use[79,84]. Since that review, Kulig et al[85] published a study comparing QoL in those undergoing curative resection for gastric cancer to those with metastatic disease undergoing either non-resectional surgery or gastric resection. Those undergoing a resection in the face of metastatic disease survived 6 mo longer than patients undergoing non-resectional surgery, although the role of selection on this observation could not be measured. On the other hand, the post-operative QoL in those with metastatic disease declined progressively following surgery, even if a non-curative resection was performed. This study would have perhaps benefited from inclusion of a group of non-surgical patients (receiving best supportive care) for comparison, although the role of selection bias remains significant in any such retrospective studies.
Palliative surgical bypass vs stenting
Gastric outlet obstruction (GOO) is a troublesome complication of advanced pancreatic and distal gastric cancer. Traditionally, this has been treated with gastrojejunostomy, but endoscopic stenting is another alternative that should be considered. Gastrojejunostomy can be associated with good functional outcome but is also associated with significant morbidity[86,87]. The laparoscopic approach is an option but is not necessarily associated with a significant benefit[88]. More recently, endoscopic stenting has become an attractive alternative that forgoes the need for an abdominal procedure. A meta-analysis that included multiple cancer types, showed resumption of oral intake in 89% with minimal morbidity[89]. Late stent failure, mainly due to tumor infiltration, was seen in 18%. In 2010, a randomized trial was conducted comparing gastrojejunostomy to endoscopic stenting in those with GOO[90]. Gastrojejunostomy was superior in terms of longer-term patency and a less common need for reintervention. Of note, < 10% of these patients had gastric cancer. QoL was measured using the QLQ-C30 and did not differ between the two methods.
It is very difficult, at this point, to make sweeping recommendations on the best method of surgical palliation in terms of improving and/or preserving QoL. Highly selected patients will derive a survival benefit from resection, usually months at best; the QoL benefits over best supportive care are poorly documented. As illustrated in Figure 1, the goal of any palliative procedure is to provide an improvement of QoL while minimizing complications. The time course for improvements of QoL should also be quicker than for a curative procedure. This is where palliative stenting may be advantageous, allowing a patient to return to eating sooner, avoiding a prolonged hospitalization, and avoiding the significant recovery from major surgery. On the other hand, the disadvantage of stenting is the possible need for reintervention in individuals with a longer survival. In general, however, in the palliative setting for gastric cancer, it is uncommon for individuals to require reintervention after stenting. This is something that will require re-evaluation, though, as more effective systemic therapies are introduced to practice (Table 3).
Table 3.
Surgery and quality of life in gastric cancer
| Type of surgical therapy | Quality of life summary |
| Resection for cure | DG leads to better QoL |
| TG is superior to PG for proximal cancers | |
| For GEJ tumors, abdominal procedures are associated with better QoL | |
| Laparoscopy is likely associated with quicker attainment of post-operative QoL | |
| Goal is return to baseline QoL | |
| Palliative resection | Non surgical (less invasive) procedures are desirable |
| Surgical resection only in highly selected individuals - excellent performance status and life expectancy | |
| Goal is to improve QoL | |
| Prophylactic gastrectomy | Total gastrectomy is essential |
| Similar post-operative reduction in QoL as for confirmed malignancy | |
| Goal is return to pre-operative baseline | |
| Further studies required |
DG: Distal gastrectomy; TG: Total gastrectomy; QoL: Quality of life; GEJ: Gastroesophageal junction.
GASTRECTOMY FOR ONCOLOGIC PROPHYLAXIS
Up to 3% of gastric cancers arise from a hereditary predisposition syndrome such as HDGC[91,92]. For patients with a significant family history of gastric cancer, genetic testing is recommended, leading to the diagnosis of a germline mutation in the tumor suppressor gene E-cadherin (CDH1) in 25%-30%[92-94]. Carriers of the CDH1 gene have a 70%-80% lifetime risk of gastric cancer. In view of that high risk and because early endoscopic detection of HDGC is difficult, prophylactic gastrectomy is recommended over frequent endoscopic surveillance[93,95-97]. In young, healthy patients who undergo a TG the mortality rate is less than 1%[98].
The majority of literature on QoL after gastrectomy revolves around those with diagnosed malignancy. The impact of QoL in those undergoing prophylactic gastrectomy is largely unexplored. Indeed, as prophylactic gastrectomies are more commonly considered, further data to inform candidates on the effects of the procedure on QoL will become essential. Candidates for prophylactic gastrectomy are in fact considering a life-altering procedure. Not all will have a confirmed genetic predisposition; some will consider the procedure because of a significant family history. In this setting, candidates will be exposed to all the immediate risks (e.g., bleeding, anastomotic leak, etc.) and longer-term complications of TG (e.g., weight loss, diarrhea, dumping syndrome), as well as any less-well understood psychological and emotional effects.
Perhaps the best information that is available is from Worster et al[99], who followed a group of sixty patients undergoing prophylactic gastrectomy. Using validated QoL questionnaires, they found no difference in pre-operative scores between those with a confirmed CDH1 mutation and those without. Similar to patients undergoing gastrectomy for confirmed malignancy, these patients experienced a post-operative reduction in QoL indices, particularly physical and mental functioning scores. These returned to baseline by the one-year mark at the latest. However, a number of symptoms were more persistent, including diarrhea, fatigue, discomfort with eating, reflux and distorted body image[99]. This highlights the importance of pre-operative patient preparation and counseling. The decision-making process that at-risk patients travel through on the way towards prophylactic gastrectomy is complex[100]. Clearly stating post-operative expectations in terms of body image, weight loss and difficulties with eating may enhance the decision-making process and improve post-operative QoL.
It is clear that QoL data are lacking in the area of prophylactic gastrectomy. Patients are likely in a wholly different pre-operative mindset than those with diagnosed malignancy, looking forward to a procedure that will alter their life substantially in order to prevent a malignancy they will likely - but not inevitably - develop. Gaining insight into the post-operative QoL outcomes with further research will no doubt lead to improved QoL through improved patient education.
CONCLUSION
In recent years, there has been significant progress in defining and measuring QoL for patients with gastric cancer and patients undergoing gastric procedures. In general, there has been an improved appreciation of the importance of QoL as an outcome that must be considered in the context of survival and performance status. Further studies will be required to define the QoL cost-benefit ratio in palliative gastric procedures as well as in prophylactic gastrectomies. Moreover, it is likely that, as more effective systemic therapies for gastric cancer are developed, the appropriateness of surgery will have to be constantly re-evaluated. QoL will be a critical outcome measure as these developments unfold.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 14, 2015
First decision: July 14, 2015
Article in press: November 9, 2015
P- Reviewer: Gurkan A, Iizuka T S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39. doi: 10.1097/01.sla.0000149300.28588.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham SC, Kamangar F, Kim MP, Hammoud S, Haque R, Maitra A, Montgomery E, Heitmiller RE, Choti MA, Lillemoe KD, et al. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg. 2005;9:718–725. doi: 10.1016/j.gassur.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 6.Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Crose N, Gennari L. Total versus subtotal gastrectomy: surgical morbidity and mortality rates in a multicenter Italian randomized trial. The Italian Gastrointestinal Tumor Study Group. Ann Surg. 1997;226:613–620. doi: 10.1097/00000658-199711000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170–178. doi: 10.1097/00000658-199908000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottomley A. The cancer patient and quality of life. Oncologist. 2002;7:120–125. doi: 10.1634/theoncologist.7-2-120. [DOI] [PubMed] [Google Scholar]
- 9.Schipper H, Clinch J, Olweny L. Definitions and conceptual issues. In: Spilker B, editor. Quality of Life and pharmacoeconomics in clinical trials. Philadelphia: Lippincott-Raven; 1996. pp. 11–24. [Google Scholar]
- 10.Conroy T, Marchal F, Blazeby JM. Quality of life in patients with oesophageal and gastric cancer: an overview. Oncology. 2006;70:391–402. doi: 10.1159/000099034. [DOI] [PubMed] [Google Scholar]
- 11.Munene G, Francis W, Garland SN, Pelletier G, Mack LA, Bathe OF. The quality of life trajectory of resected gastric cancer. J Surg Oncol. 2012;105:337–341. doi: 10.1002/jso.22139. [DOI] [PubMed] [Google Scholar]
- 12.Kaptein AA, Morita S, Sakamoto J. Quality of life in gastric cancer. World J Gastroenterol. 2005;11:3189–3196. doi: 10.3748/wjg.v11.i21.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 14.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 15.Kemmler G, Holzner B, Kopp M, Dünser M, Margreiter R, Greil R, Sperner-Unterweger B. Comparison of two quality-of-life instruments for cancer patients: the functional assessment of cancer therapy-general and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30. J Clin Oncol. 1999;17:2932–2940. doi: 10.1200/JCO.1999.17.9.2932. [DOI] [PubMed] [Google Scholar]
- 16.Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 17.Troidl H, Kusche J, Vestweber KH, Eypasch E, Koeppen L, Bouillon B. Quality of life: an important endpoint both in surgical practice and research. J Chronic Dis. 1987;40:523–528. doi: 10.1016/0021-9681(87)90009-9. [DOI] [PubMed] [Google Scholar]
- 18.Troidl H, Kusche J, Vestweber KH, Eypasch E, Maul U. Pouch versus esophagojejunostomy after total gastrectomy: a randomized clinical trial. World J Surg. 1987;11:699–712. doi: 10.1007/BF01656592. [DOI] [PubMed] [Google Scholar]
- 19.Thybusch-Bernhardt A, Schmidt C, Küchler T, Schmid A, Henne-Bruns D, Kremer B. Quality of life following radical surgical treatment of gastric carcinoma. World J Surg. 1999;23:503–508. doi: 10.1007/pl00012339. [DOI] [PubMed] [Google Scholar]
- 20.Vickery CW, Blazeby JM, Conroy T, Arraras J, Sezer O, Koller M, Rosemeyer D, Johnson CD, Alderson D. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer. 2001;37:966–971. doi: 10.1016/s0959-8049(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 21.Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O, Moore J, Koller M, Turhal NS, Stuart R, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. 2004;40:2260–2268. doi: 10.1016/j.ejca.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Eremenco S, Cashy J, Webster K, Ohashi G, Locker G, Pelletier G, Cella D. FACT-Gastric: a new international measure of QOL in gastric cancer. J Clin Oncol. 2004;22 Suppl 14:abstract 8123. [Google Scholar]
- 23.Garland SN, Pelletier G, Lawe A, Biagioni BJ, Easaw J, Eliasziw M, Cella D, Bathe OF. Prospective evaluation of the reliability, validity, and minimally important difference of the functional assessment of cancer therapy-gastric (FACT-Ga) quality-of-life instrument. Cancer. 2011;117:1302–1312. doi: 10.1002/cncr.25556. [DOI] [PubMed] [Google Scholar]
- 24.Debb SM, Arnold B, Perez B, Cella D. Validation of the FACT-Gastric cancer quality of life questionnaire for use in Spanish-speaking countries. Psychooncology. 2011;20:19–27. doi: 10.1002/pon.1698. [DOI] [PubMed] [Google Scholar]
- 25.Zhou HJ, So JB, Yong WP, Luo N, Zhu F, Naidoo N, Li SC, Yeoh KG. Validation of the functional assessment of cancer therapy-gastric module for the Chinese population. Health Qual Life Outcomes. 2012;10:145. doi: 10.1186/1477-7525-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura M, Kido Y, Yano M, Hosoya Y. Reliability and validity of a new scale to assess postoperative dysfunction after resection of upper gastrointestinal carcinoma. Surg Today. 2005;35:535–542. doi: 10.1007/s00595-005-2988-5. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura M, Kido Y, Hosoya Y, Yano M, Nagai H, Monden M. Postoperative gastrointestinal dysfunction after 2-field versus 3-field lymph node dissection in patients with esophageal cancer. Surg Today. 2007;37:379–382. doi: 10.1007/s00595-006-3413-4. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura M, Kido Y, Egawa T. Development of a 32-item scale to assess postoperative dysfunction after upper gastrointestinal cancer resection. J Clin Nurs. 2008;17:1440–1449. doi: 10.1111/j.1365-2702.2007.02179.x. [DOI] [PubMed] [Google Scholar]
- 29.Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, Ota M, Iwasaki Y, Uchida N, Kodera Y, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18:407–416. doi: 10.1007/s10120-014-0377-8. [DOI] [PubMed] [Google Scholar]
- 30.Nakada K, Ikeda M, Takahashi M, Kinami S, Yoshida M, Uenosono Y, Kawashima Y, Oshio A, Suzukamo Y, Terashima M, et al. Characteristics and clinical relevance of postgastrectomy syndrome assessment scale (PGSAS)-45: newly developed integrated questionnaires for assessment of living status and quality of life in postgastrectomy patients. Gastric Cancer. 2015;18:147–158. doi: 10.1007/s10120-014-0344-4. [DOI] [PubMed] [Google Scholar]
- 31.Terashima M, Tanabe K, Yoshida M, Kawahira H, Inada T, Okabe H, Urushihara T, Kawashima Y, Fukushima N, Nakada K. Postgastrectomy Syndrome Assessment Scale (PGSAS)-45 and changes in body weight are useful tools for evaluation of reconstruction methods following distal gastrectomy. Ann Surg Oncol. 2014;21 Suppl 3:S370–S378. doi: 10.1245/s10434-014-3583-z. [DOI] [PubMed] [Google Scholar]
- 32.Svedlund J, Sjödin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 33.Karanicolas PJ, Graham D, Gönen M, Strong VE, Brennan MF, Coit DG. Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann Surg. 2013;257:1039–1046. doi: 10.1097/SLA.0b013e31828c4a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avery K, Hughes R, McNair A, Alderson D, Barham P, Blazeby J. Health-related quality of life and survival in the 2 years after surgery for gastric cancer. Eur J Surg Oncol. 2010;36:148–154. doi: 10.1016/j.ejso.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, Bae JM, Yun YH, Kim S. Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg. 2012;256:1008–1013. doi: 10.1097/SLA.0b013e31827661c9. [DOI] [PubMed] [Google Scholar]
- 36.Lee SS, Chung HY, Yu W. Quality of life of long-term survivors after a distal subtotal gastrectomy. Cancer Res Treat. 2010;42:130–134. doi: 10.4143/crt.2010.42.3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korenaga D, Orita H, Okuyama T, Moriguchi S, Maehara Y, Sugimachi K. Quality of life after gastrectomy in patients with carcinoma of the stomach. Br J Surg. 1992;79:248–250. doi: 10.1002/bjs.1800790321. [DOI] [PubMed] [Google Scholar]
- 38.Tyrväinen T, Sand J, Sintonen H, Nordback I. Quality of life in the long-term survivors after total gastrectomy for gastric carcinoma. J Surg Oncol. 2008;97:121–124. doi: 10.1002/jso.20925. [DOI] [PubMed] [Google Scholar]
- 39.Bozzetti F. Total versus subtotal gastrectomy in cancer of the distal stomach: facts and fantasy. Eur J Surg Oncol. 1992;18:572–579. [PubMed] [Google Scholar]
- 40.Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J, Miller G, Martin I. Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg. 1998;22:1048–1055. doi: 10.1007/s002689900515. [DOI] [PubMed] [Google Scholar]
- 41.Gouzi JL, Huguier M, Fagniez PL, Launois B, Flamant Y, Lacaine F, Paquet JC, Hay JM. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg. 1989;209:162–166. doi: 10.1097/00000658-198902000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A. Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg. 2011;35:357–364. doi: 10.1007/s00268-010-0860-2. [DOI] [PubMed] [Google Scholar]
- 43.Rausei S, Mangano A, Galli F, Rovera F, Boni L, Dionigi G, Dionigi R. Quality of life after gastrectomy for cancer evaluated via the EORTC QLQ-C30 and QLQ-STO22 questionnaires: surgical considerations from the analysis of 103 patients. Int J Surg. 2013;11 Suppl 1:S104–S109. doi: 10.1016/S1743-9191(13)60028-X. [DOI] [PubMed] [Google Scholar]
- 44.Park S, Chung HY, Lee SS, Kwon O, Yu W. Serial comparisons of quality of life after distal subtotal or total gastrectomy: what are the rational approaches for quality of life management? J Gastric Cancer. 2014;14:32–38. doi: 10.5230/jgc.2014.14.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen XZ, Zhang WH, Yang K, Hu JK. Digestive tract reconstruction pattern as a determining factor in postgastrectomy quality of life. World J Gastroenterol. 2014;20:330–332. doi: 10.3748/wjg.v20.i1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komatsu S, Ichikawa D, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Konishi H, Morimura R, Murayama Y, Kuriu Y, et al. Clinical outcomes and quality of life according to types of reconstruction following laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Laparosc Endosc Percutan Tech. 2015;25:69–73. doi: 10.1097/SLE.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 47.Nunobe S, Okaro A, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Billroth 1 versus Roux-en-Y reconstructions: a quality-of-life survey at 5 years. Int J Clin Oncol. 2007;12:433–439. doi: 10.1007/s10147-007-0706-6. [DOI] [PubMed] [Google Scholar]
- 48.Lee MS, Ahn SH, Lee JH, Park do J, Lee HJ, Kim HH, Yang HK, Kim N, Lee WW. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539–1547. doi: 10.1007/s00464-011-2064-8. [DOI] [PubMed] [Google Scholar]
- 49.Takiguchi S, Yamamoto K, Hirao M, Imamura H, Fujita J, Yano M, Kobayashi K, Kimura Y, Kurokawa Y, Mori M, et al. A comparison of postoperative quality of life and dysfunction after Billroth I and Roux-en-Y reconstruction following distal gastrectomy for gastric cancer: results from a multi-institutional RCT. Gastric Cancer. 2012;15:198–205. doi: 10.1007/s10120-011-0098-1. [DOI] [PubMed] [Google Scholar]
- 50.Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989;210:596–602. doi: 10.1097/00000658-198911000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T. Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg. 2010;97:558–562. doi: 10.1002/bjs.6944. [DOI] [PubMed] [Google Scholar]
- 52.Khan O, Goh S, Byrne B, Somers S, Mercer S, Toh S. Long-term outcomes of extended proximal gastrectomy for oesophagogastric junctional tumours. World J Surg. 2011;35:2245–2251. doi: 10.1007/s00268-011-1235-z. [DOI] [PubMed] [Google Scholar]
- 53.Harrison LE, Karpeh MS, Brennan MF. Total gastrectomy is not necessary for proximal gastric cancer. Surgery. 1998;123:127–130. [PubMed] [Google Scholar]
- 54.Katai H, Sano T, Fukagawa T, Shinohara H, Sasako M. Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg. 2003;90:850–853. doi: 10.1002/bjs.4106. [DOI] [PubMed] [Google Scholar]
- 55.Wen L, Chen XZ, Wu B, Chen XL, Wang L, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, et al. Total vs. proximal gastrectomy for proximal gastric cancer: a systematic review and meta-analysis. Hepatogastroenterology. 2012;59:633–640. doi: 10.5754/hge11834. [DOI] [PubMed] [Google Scholar]
- 56.An JY, Youn HG, Choi MG, Noh JH, Sohn TS, Kim S. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg. 2008;196:587–591. doi: 10.1016/j.amjsurg.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 57.Ronellenfitsch U, Najmeh S, Andalib A, Perera RM, Rousseau MC, Mulder DS, Ferri LE. Functional outcomes and quality of life after proximal gastrectomy with esophagogastrostomy using a narrow gastric conduit. Ann Surg Oncol. 2015;22:772–779. doi: 10.1245/s10434-014-4078-7. [DOI] [PubMed] [Google Scholar]
- 58.Son MW, Kim YJ, Jeong GA, Cho GS, Lee MS. Long-Term Outcomes of Proximal Gastrectomy versus Total Gastrectomy for Upper-Third Gastric Cancer. J Gastric Cancer. 2014;14:246–251. doi: 10.5230/jgc.2014.14.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–1459. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 60.Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–5339. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 61.Haverkamp L, Ruurda JP, van Leeuwen MS, Siersema PD, van Hillegersberg R. Systematic review of the surgical strategies of adenocarcinomas of the gastroesophageal junction. Surg Oncol. 2014;23:222–228. doi: 10.1016/j.suronc.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Barbour AP, Lagergren P, Hughes R, Alderson D, Barham CP, Blazeby JM. Health-related quality of life among patients with adenocarcinoma of the gastro-oesophageal junction treated by gastrectomy or oesophagectomy. Br J Surg. 2008;95:80–84. doi: 10.1002/bjs.5912. [DOI] [PubMed] [Google Scholar]
- 63.Spector NM, Hicks FD, Pickleman J. Quality of life and symptoms after surgery for gastroesophageal cancer: a pilot study. Gastroenterol Nurs. 2002;25:120–125. doi: 10.1097/00001610-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 66.de Bree E, Charalampakis V, Melissas J, Tsiftsis DD. The extent of lymph node dissection for gastric cancer: a critical appraisal. J Surg Oncol. 2010;102:552–562. doi: 10.1002/jso.21646. [DOI] [PubMed] [Google Scholar]
- 67.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309–315. doi: 10.1016/S1470-2045(06)70623-4. [DOI] [PubMed] [Google Scholar]
- 68.Díaz De Liaño A, Oteiza Martínez F, Ciga MA, Aizcorbe M, Cobo F, Trujillo R. Impact of surgical procedure for gastric cancer on quality of life. Br J Surg. 2003;90:91–94. doi: 10.1002/bjs.4011. [DOI] [PubMed] [Google Scholar]
- 69.Strong VE. Laparoscopic resection for gastric carcinoma: Western experience. Surg Oncol Clin N Am. 2012;21:141–158. doi: 10.1016/j.soc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 72.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 73.Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446–456. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 74.WHO. World Health Organization. 2010. Available from: http://www.who.int. [Google Scholar]
- 75.Cunningham SC, Schulick RD. Palliative management of gastric cancer. Surg Oncol. 2007;16:267–275. doi: 10.1016/j.suronc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Miner TJ, Jaques DP, Karpeh MS, Brennan MF. Defining palliative surgery in patients receiving noncurative resections for gastric cancer. J Am Coll Surg. 2004;198:1013–1021. doi: 10.1016/j.jamcollsurg.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 77.Miner TJ, Karpeh MS. Gastrectomy for gastric cancer: defining critical elements of patient selection and outcome assessment. Surg Oncol Clin N Am. 2004;13:455–466, viii. doi: 10.1016/j.soc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Medina-Franco H, Contreras-Saldívar A, Ramos-De La Medina A, Palacios-Sanchez P, Cortés-González R, Ugarte JA. Surgery for stage IV gastric cancer. Am J Surg. 2004;187:543–546. doi: 10.1016/j.amjsurg.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 79.Samarasam I, Chandran BS, Sitaram V, Perakath B, Nair A, Mathew G. Palliative gastrectomy in advanced gastric cancer: is it worthwhile? ANZ J Surg. 2006;76:60–63. doi: 10.1111/j.1445-2197.2006.03649.x. [DOI] [PubMed] [Google Scholar]
- 80.Lasithiotakis K, Antoniou SA, Antoniou GA, Kaklamanos I, Zoras O. Gastrectomy for stage IV gastric cancer. a systematic review and meta-analysis. Anticancer Res. 2014;34:2079–2085. [PubMed] [Google Scholar]
- 81.Lin SZ, Tong HF, You T, Yu YJ, Wu WJ, Chen C, Zhang W, Ye B, Li CM, Zhen ZQ, et al. Palliative gastrectomy and chemotherapy for stage IV gastric cancer. J Cancer Res Clin Oncol. 2008;134:187–192. doi: 10.1007/s00432-007-0268-z. [DOI] [PubMed] [Google Scholar]
- 82.Chang YR, Han DS, Kong SH, Lee HJ, Kim SH, Kim WH, Yang HK. The value of palliative gastrectomy in gastric cancer with distant metastasis. Ann Surg Oncol. 2012;19:1231–1239. doi: 10.1245/s10434-011-2056-x. [DOI] [PubMed] [Google Scholar]
- 83.Mahar AL, Coburn NG, Karanicolas PJ, Viola R, Helyer LK. Effective palliation and quality of life outcomes in studies of surgery for advanced, non-curative gastric cancer: a systematic review. Gastric Cancer. 2012;15 Suppl 1:S138–S145. doi: 10.1007/s10120-011-0070-0. [DOI] [PubMed] [Google Scholar]
- 84.Miyagaki H, Fujitani K, Tsujinaka T, Hirao M, Yasui M, Kashiwazaki M, Ikenaga M, Miyazaki M, Mishima H, Nakamori S. The significance of gastrectomy in advanced gastric cancer patients with non-curative factors. Anticancer Res. 2008;28:2379–2384. [PubMed] [Google Scholar]
- 85.Kulig P, Sierzega M, Kowalczyk T, Kolodziejczyk P, Kulig J. Non-curative gastrectomy for metastatic gastric cancer: rationale and long-term outcome in multicenter settings. Eur J Surg Oncol. 2012;38:490–496. doi: 10.1016/j.ejso.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 86.Kaminishi M, Yamaguchi H, Shimizu N, Nomura S, Yoshikawa A, Hashimoto M, Sakai S, Oohara T. Stomach-partitioning gastrojejunostomy for unresectable gastric carcinoma. Arch Surg. 1997;132:184–187. doi: 10.1001/archsurg.1997.01430260082018. [DOI] [PubMed] [Google Scholar]
- 87.Kikuchi S, Tsutsumi O, Kobayashi N, Tsukamoto H, Shimao H, Sakakibara Y, Hiki Y, Kakita A. Does gastrojejunostomy for unresectable cancer of the gastric antrum offer satisfactory palliation? Hepatogastroenterology. 1999;46:584–587. [PubMed] [Google Scholar]
- 88.Navarra G, Musolino C, Venneri A, De Marco ML, Bartolotta M. Palliative antecolic isoperistaltic gastrojejunostomy: a randomized controlled trial comparing open and laparoscopic approaches. Surg Endosc. 2006;20:1831–1834. doi: 10.1007/s00464-005-0454-5. [DOI] [PubMed] [Google Scholar]
- 89.Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543–550. doi: 10.1055/s-2004-814434. [DOI] [PubMed] [Google Scholar]
- 90.Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, Kuipers EJ, Siersema PD. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490–499. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 91.Varley JM, McGown G, Thorncroft M, Tricker KJ, Teare MD, Santibanez-Koref MF, Martin J, Birch JM, Evans DG. An extended Li-Fraumeni kindred with gastric carcinoma and a codon 175 mutation in TP53. J Med Genet. 1995;32:942–945. doi: 10.1136/jmg.32.12.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cisco RM, Ford JM, Norton JA. Hereditary diffuse gastric cancer: implications of genetic testing for screening and prophylactic surgery. Cancer. 2008;113:1850–1856. doi: 10.1002/cncr.23650. [DOI] [PubMed] [Google Scholar]
- 93.Pharoah PD, Guilford P, Caldas C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348–1353. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- 94.Kaurah P, MacMillan A, Boyd N, Senz J, De Luca A, Chun N, Suriano G, Zaor S, Van Manen L, Gilpin C, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA. 2007;297:2360–2372. doi: 10.1001/jama.297.21.2360. [DOI] [PubMed] [Google Scholar]
- 95.Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, Chung DC, Norton J, Ragunath K, Van Krieken JH, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47:436–444. doi: 10.1136/jmg.2009.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lim YC, di Pietro M, O’Donovan M, Richardson S, Debiram I, Dwerryhouse S, Hardwick RH, Tischkowitz M, Caldas C, Ragunath K, et al. Prospective cohort study assessing outcomes of patients from families fulfilling criteria for hereditary diffuse gastric cancer undergoing endoscopic surveillance. Gastrointest Endosc. 2014;80:78–87. doi: 10.1016/j.gie.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 97.Pandalai PK, Lauwers GY, Chung DC, Patel D, Yoon SS. Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery. 2011;149:347–355. doi: 10.1016/j.surg.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 98.Blair V, Martin I, Shaw D, Winship I, Kerr D, Arnold J, Harawira P, McLeod M, Parry S, Charlton A, et al. Hereditary diffuse gastric cancer: diagnosis and management. Clin Gastroenterol Hepatol. 2006;4:262–275. doi: 10.1016/j.cgh.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 99.Worster E, Liu X, Richardson S, Hardwick RH, Dwerryhouse S, Caldas C, Fitzgerald RC. The impact of prophylactic total gastrectomy on health-related quality of life: a prospective cohort study. Ann Surg. 2014;260:87–93. doi: 10.1097/SLA.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 100.Garland SN, Lounsberry J, Pelletier G, Bathe OF. “How do you live without a stomach?”: a multiple case study examination of total gastrectomy for palliation or prophylaxis. Palliat Support Care. 2011;9:305–313. doi: 10.1017/S1478951511000253. [DOI] [PubMed] [Google Scholar]


