Abstract
Photodynamic diagnosis based on 5-aminolevulinic acid-induced protoporphyrin IX has been clinically applied in many fields based upon its evidenced efficacy and adequate safety. In order to establish a personalized medicine approach for treating gastric cancer patients, rapid intraoperative detection of malignant lesions has become important. Feasibility of photodynamic diagnosis using 5-aminolevulinic acid for gastric cancer patients has been investigated, especially for the detection of peritoneal dissemination and lymph node metastasis. This method enables intraoperative real-time fluorescence detection of peritoneal dissemination, exhibiting higher sensitivity than white light observation without histopathological examination. The method also enables detection of metastatic foci within excised lymph nodes, exhibiting a diagnostic accuracy comparable to that of a current molecular diagnostics technique. Although several complicating issues still need to be resolved, such as the effect of tissue autofluorescence and the insufficient depth penetration of excitation light, this simple and rapid method has the potential to become a useful diagnostic tool for gastric cancer, as well as urinary bladder cancer and glioma.
Keywords: Gastric cancer, Photodynamic diagnosis, 5-aminolevulinic acid, Protoporphyrin IX, Lymph node metastasis, Personalized medicine, Peritoneal dissemination
Core tip: To perform personalized treatment for gastric cancer, rapid intraoperative detection of malignant lesions is desirable. Photodynamic diagnosis based on 5-aminolevulinic acid-induced protoporphyrin IX may help in this regard. This method enables intraoperative real-time fluorescence detection of peritoneal dissemination in gastric cancer patients, exhibiting higher sensitivity than white light observation without histopathological examination. The method also enables detection of metastatic foci within excised lymph nodes, exhibiting a diagnostic accuracy comparable to a current molecular diagnostics technique. This method has the potential to become a useful diagnostic tool for gastric cancer treatment.
INTRODUCTION
Personalized medicine has become a trending topic in the treatment of gastric cancer[1-3]. In order to apply an individualized therapeutic strategy for each patient with gastric cancer, a precise evaluation of the extent of disease progression is essential[4].
Recent advances in endoscopic technology have enabled the early detection of malignant lesions, allowing a subset of patients with early gastric cancer to undergo a curative endoscopic resection[5]. However, a large percentage of gastric cancer patients still require some sort of surgical intervention. Distal or total gastrectomy with appropriate lymph node dissection is usually performed as a radical procedure, but the quality of life of patients who have undergone radical gastrectomy is diminished to some extent[6]. A less invasive personalized surgery, avoiding unnecessary lymph node dissection and reduced gastric resection, might be possible if lymph node metastases could be detected preoperatively or intraoperatively[7].
On another front, patients with advanced gastric cancer are often excluded from radical gastrectomy because advanced gastric cancer with peritoneal dissemination usually requires chemotherapy rather than surgery[8,9]. Sensitive intraoperative detection of occult peritoneal dissemination might save such patients from unnecessary gastrectomy and lead to early induction of appropriate chemotherapy[10].
Photodynamic diagnostics is considered a potent candidate to fill these demands. Photodynamic diagnosis using 5-aminolevulinic acid (5-ALA-PDD) has been widely used clinically because of its adequate safety and efficacy[11,12]. 5-ALA is an intrinsic amino acid intimately involved with heme synthesis in nucleated cells. Because cancer cells exhibit alterations of enzymatic activity in the heme synthetic pathway, exogenous administration of 5-ALA causes intracellular accumulation of a heme precursor, protoporphyrin IX (PpIX), in cancer cells[13]. Importantly, PpIX emits red light upon excitation with 405-nm blue-violet light, whereas 5-ALA is not fluorescent.
In the field of gastrointestinal cancer treatment, 5-ALA-PDD has been used for the in vivo detection of peritoneal dissemination in patients with advanced gastrointestinal cancer[14-16]. In addition, the feasibility of using 5-ALA-PDD to detect lymph node metastases is now being validated in clinical trials[17,18]. Thus, the availability of 5-ALA-PDD is increasingly anticipated since there are no other cancer-specific fluorophores easily applicable to clinical use.
In this review, we cover recent topics related to the use of 5-ALA-PDD in gastric cancer, focusing on fluorescence diagnostic laparoscopy and intraoperative fluorescence detection of metastatic lymph nodes.
ACCUMULATION OF PPIX IN GASTRIC CANCER CELLS
Although the mechanism of PpIX accumulation in cancer cells is not entirely clear, alterations of enzymatic activities in the heme synthetic pathway, i.e. increased activity of porphobilinogen deaminase and decreased activity of ferrochelatase, are considered to be the primary cause[19]. Porphobilinogen deaminase is induced upon administration of exogenous 5-ALA, and the enzymatic activity is increased accordingly[20]. Ferrochelatase, which acts in the final step of heme synthesis, contains an iron-sulfur cluster that is inhibited by nitric oxide[21,22]. Since the concentration of nitric oxide is often increased in cancer cells by upregulation of the inducible form of nitric oxide synthase, ferrochelatase activity is suppressed in cancer cells[23,24].
In addition, recent in vitro studies with gastric cancer cell lines have revealed other molecules that might regulate 5-ALA-PDD efficacy in gastric cancer. Intracellular uptake of exogenously administered 5-ALA is mediated by a peptide transporter, PEPT-1/2, and extracellular efflux of 5-ALA-induced PpIX is mediated by ATP-binding cassette transporter G2 (ABCG2)[25,26]. Therefore, the expression levels of PEPT-1/2 and ABCG2 are likely to be closely involved in the efficacy of 5-ALA-PDD in gastric cancer.
Furthermore, mitochondrial ATP-binding cassette transporter B6 (ABCB6) was found to mediate cytoplasm-to-mitochondria transport of coproporphyrinogen III, an intermediate metabolite of heme synthesis[27]. The effect of mitochondrial ABCB6 expression on 5-ALA-PDD was shown in an in vitro study with gastric cancer cell lines[28]. The results correlated well with those from a study with excised glioma tissues[29].
Thus, various molecular factors are involved in intracellular PpIX accumulation. A schematic of the 5-ALA metabolic pathway and intracellular PpIX accumulation in gastric cancer cells is shown in Figure 1.
Figure 1.
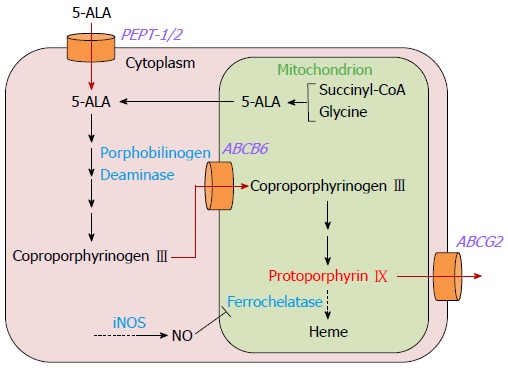
Metabolic pathway of 5-aminolevulinic acid and mechanism of intracellular protoporphyrin IX accumulation in gastric cancer cells.
PPIX FLUORESCENCE IN GASTRIC CANCER CELLS
PpIX emits red light with a peak at 635-nm upon excitation with 405-nm blue-violet light. Adding 5-ALA to the culture medium induces PpIX accumulation in cultured cancer cells. In vitro analysis of various types of gastric cancer cell lines has shown that treatment with 5-ALA and 405-nm excitation light induced the characteristic 635-nm PpIX fluorescence peak in all of the lines, with the fluorescence intensity dependent on the cell’s degree of differentiation (unpublished data) (Figure 2). This result strongly supports the notion that 5-ALA-PDD is clinically applicable to gastric cancer.
Figure 2.
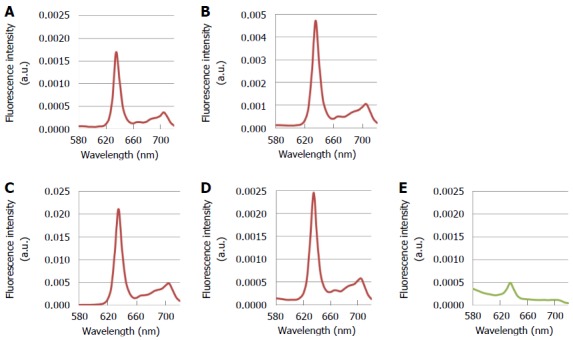
Fluorescence spectra of various kinds of gastric cancer cell lines after treatment with 5-aminolevulinic acid and excitation with 405-nm light. A: MKN-7, derived from a well differentiated gastric cancer; B: MKN-74, derived from a moderately differentiated gastric cancer; C: MKN-45, derived from a poorly differentiated gastric cancer; D: Kato-III, derived from a signet-ring cell carcinoma; E: NIH-3T3, a non-cancerous fibroblast-like cell line. Each cell line was incubated with 1 mmol/L 5-ALA for 30 min. The conditions of spectral acquisition were equivalent among all samples. Spectra were measured with the MCPD-7000 spectral analytical system (Otsuka Electronics, Co., Ltd., Osaka, Japan).
5-ALA ADMINISTRATION TO GASTRIC CANCER PATIENTS AND MANAGEMENT OF 5-ALA-ADMINISTERED PATIENTS
In a clinical setting, 5-ALA is usually administered orally. In order to diminish its characteristic acidic taste, we have prepared and delivered it as a 50% glucose solution. Orally administered 5-ALA is rapidly absorbed in the upper gastrointestinal tract, and the plasma level of 5-ALA reaches a peak within 1 h after oral administration. At first, PpIX accumulation is observed in normal tissues, especially in the gastrointestinal mucosa, at 2-4 h after 5-ALA administration[30]. Thereafter, the accumulated PpIX in normal tissues is gradually metabolized, whereas PpIX accumulation in cancer cells is sustained for a while. After 6 h, the difference in PpIX concentrations between normal tissue and cancer tissue reaches its peak[13]. Thus, the timing of 5-ALA administration should be based on the estimated duration to fluorescence observation.
5-ALA is basically a safe substrate, also present in the human body. However, 5-ALA administration is contraindicated for patients with porphyria[31]. Since 5-ALA is an initial chemical of porphyrin metabolism, high levels of 5-ALA in patients with porphyria lead to abnormal accumulation of various porphyrins, depending on the type of porphyria. This aberrance may cause phototoxic skin disorder, neurological dysfunction, and/or liver damage[32,33].
Gastric cancer patients with accompanying pyloric stenosis should also be excluded from 5-ALA-PDD since efficient absorption of 5-ALA is impossible. In addition, gastric cancer patients receiving certain antacid agents, such as histamine H2 receptor antagonists and proton pump inhibitors, should be excluded because 5-ALA is unstable in high-pH conditions[34,35].
Patients who are administered photosensitizers should avoid unnecessary light exposure to reduce the risk of developing cutaneous photosensitivity. Other available photosensitizers, e.g., porfimer sodium (Photofrin) and talaporfin sodium (Laserphyrin), have a long serum half-life and thus necessitate longer-term protection from light exposure[36]. However, physicians need not be so diligent in the case of 5-ALA, since 5-ALA-induced PpIX is metabolized to heme within a few hours[37].
We have performed 5-ALA-PDD for > 100 patients with gastrointestinal malignancies in clinical trials, and have observed no apparent adverse effects to date, including phototoxic skin disorder or liver dysfunction.
5-ALA-PDD FOR PERITONEAL DISSEMINATION IN GASTRIC CANCER PATIENTS
The efficacy of 5-ALA-PDD for detection of peritoneal dissemination has been demonstrated in a number of studies using animal models and in clinical trials[14,16,38-40]. In the treatment of gastric cancer, precise detection of occult dissemination is certainly important to select patients requiring chemotherapy and to pursue an improved prognosis[9]. Minute dissemination is sometimes undetectable by white light observation, and definitive diagnosis can only be achieved by histopathological examination of the excised specimen. In this regard, fluorescence observation is advantageous because it enables real-time in vivo visualization without the need for time-consuming histological examination.
A fluorescence laparoscopic system capable of providing 405-nm excitation light through a rigid laparoscope is now commercially available, and as a result diagnostic laparoscopy can be performed with minimal invasiveness. Figure 3 shows 5-ALA-PDD images from a patient with advanced gastric cancer suspected to be accompanied by peritoneal dissemination. An intraperitoneal nodule exhibited red fluorescence (Figure 3A), indicating that it is a disseminated nodule, similar to a serosal invasion of the primary lesion (Figure 3B). 5-ALA-PDD also enabled a qualitative diagnosis of superficial liver metastases (Figure 3C), although deeply-seated liver metastases could not be evaluated due to the insufficient penetration depth of the excitation light.
Figure 3.
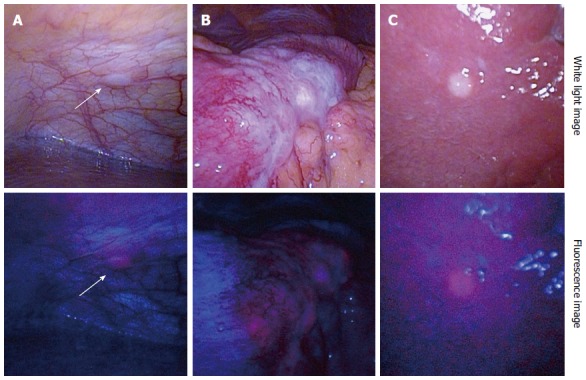
Representative images of 5-aminolevulinic acid - photodynamic diagnosis. Upper column: White light images; Lower column: Fluorescence images. A: Peritoneal dissemination (arrows); B: Serosal invasion of primary gastric cancer; C: Superficial liver metastasis. Excitation: 405-nm; Emission: > 450-nm.
Regarding the diagnostic performance of 5-ALA-PDD for peritoneal dissemination, Almerie et al[38] determined in a systematic review that the ranges of sensitivity and specificity were 83% to 100% and 95% to 100%, respectively[38]. The authors reported that 5-ALA-PDD increased the detection rate of malignant peritoneal nodules by 21% to 34% over white light alone.
Detailed analyses of animal models and clinical trials have indicated satisfactory diagnostic performance of 5-ALA-PDD in gastric cancer[15,16,40]. Kishi et al[40] first validated the efficacy of 5-ALA-PDD by analyzing 729 peritoneal nodules obtained from 8 mice. In that study, the detection rate with 5-ALA-PDD was 72%, which was significantly higher than that for the white light observation (39%). In addition, in a clinical trial for patients with advanced gastric cancer, Murayama et al[16] evaluated the availability of 5-ALA-PDD. The trial consisted of 13 patients, 5 of which were diagnosed with peritoneal dissemination by 5-ALA-PDD. The diagnoses were confirmed by subsequent histopathological examination. Whereas 14 peritoneal nodules with suspected dissemination were detected under white light observation, only 12 nodules showed red fluorescence by 5-ALA-PDD. Histopathological examination indicated that only those 12 nodules were disseminated. Therefore, the diagnostic accuracy of 5-ALA-PDD was concluded to be greater than that of white light imaging[16].
Furthermore, Kishi et al[15] demonstrated the usefulness of adding 5-ALA-PDD to conventional white light laparoscopy in another clinical trial with 52 advanced gastric cancer patients. The authors reported that, using white light observation, 24 of the 52 patients showed no macroscopic evidence of peritoneal dissemination, but when 5-ALA-PDD was used dissemination was detected in 5 of these 24 patients. Thus, the authors concluded that 5-ALA-PDD improved the sensitivity for the detection of peritoneal dissemination[15].
On the basis of these clinical trials, 5-ALA-PDD appears to be a useful and promising procedure for the detection of peritoneal dissemination. Future studies are needed, however, to determine whether the increased sensitivity leads to an improvement in the final outcome for patients who have undergone 5-ALA-PDD.
5-ALA-PDD FOR LYMPH NODE METASTASIS OF GASTRIC CANCER
Limited operations with reduced lymph node dissection based on the sentinel node concept have recently attracted much attention in the field of gastric cancer surgery, similar to that in the field of breast cancer[41-43]. In performing sentinel node navigation surgery for gastric cancer, partial or segmental gastrectomy with limited lymphatic basin dissection is performed, and sustained post-gastrectomy quality of life can be provided[44].
However, in order to perform such procedures with adequate tolerability, a rapid and precise method is required to diagnose the presence or absence of lymph node metastases intraoperatively[45]. The existing intraoperative rapid histopathological diagnosis, which is based on frozen sections, has several liabilities. One is the amount of time required to make a histological section. Another liability is that minute metastases might not be detected due to the limited number of histological sections, resulting in a relatively high proportion of false-negatives[46].
To overcome these issues, we used 5-ALA-PDD for the detection of lymph node metastases of gastric cancer. Previously, Murayama et al[47] reported an excellent diagnostic accuracy (100% sensitivity and 100% specificity) of 5-ALA-PDD for detecting metastatic lymph nodes in a model mouse of rectal cancer. Building upon this finding, our group conducted a clinical trial with 14 patients with advanced gastric cancer, and examined a total of 144 lymph nodes[17]. Figure 4 shows representative 5-ALA-PDD images of metastases in resected lymph nodes. The metastatic lymph nodes exhibit a distinct red fluorescence consistent with the distribution of a metastatic focus. Although some non-metastatic lymph nodes were also fluorescent, these nodes could be distinguished from metastasis-bearing lymph nodes by their characteristic follicular fluorescence pattern. Using a diagnostic algorithm for the fluorescence pattern, we found that 5-ALA-PDD achieved an acceptable diagnostic power, with 92.4% accuracy (133/144).
Figure 4.
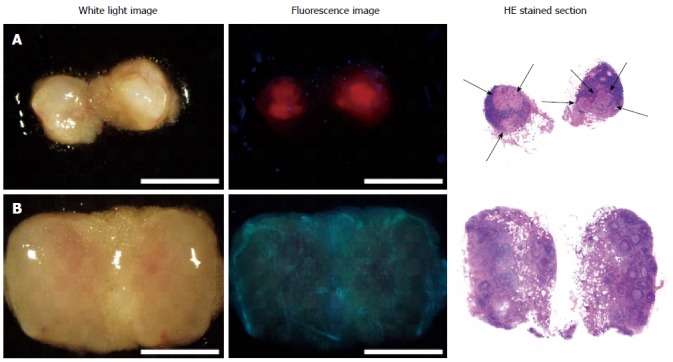
5-aminolevulinic acid - photodynamic diagnostic imaging of metastatic and non-metastatic lymph nodes[17]. Left column: White-light images; Middle column: Fluorescence images; Right column: Hematoxylin-eosin stained sections. A: Metastatic lymph node; B: Non-metastatic lymph node. Arrows indicate metastatic foci. In the metastatic lymph node, red fluorescence aligns with the metastatic foci. White light and fluorescence images were acquired with the SZX-12 stereomicroscope (Olympus, Tokyo, Japan) equipped with the DP71 color charge-coupled digital camera (Olympus). Excitation: 395-nm to 415-nm; Emission: > 430-nm; Scale bars: 3-mm.
Furthermore, we performed a quantitative analysis based on the nodules’ fluorescence intensities. Metastatic lymph nodes were significantly brighter than non-metastatic lymph nodes (P < 0.0001)[17]. Subsequent receiver operating characteristic analysis revealed 70.8% sensitivity and 94.4% specificity, with an area under the curve of 0.832. Although a sustained effort to improve the diagnostic sensitivity is required, future clinical application is strongly anticipated based on clinical results that will gradually accumulate in the near future.
Another innovative diagnostic method, the one-step nucleic acid amplification (OSNA) assay, has already been applied widely in the field of breast cancer[48]. OSNA is a new type of molecular diagnostic tool that is based on amplification of CK19 mRNA, which is expressed in metastasized foci within a lymph node[49]. The ability of OSNA to detect metastatic lymph nodes of gastric cancer has been evaluated; Yaguchi et al[50] reported a diagnostic accuracy of 96.6%. Yet, some obstacles remain: tissue contamination may cause false-positive results, and low CK19 expression may lead to false-negatives[51]. The greatest obstacle, however, is that subsequent histological examination cannot be performed, because OSNA requires homogenized tissue specimens. In this regard, 5-ALA-PDD offers an advantage, since metastatic foci are visualized in non-homogenized nodules.
FUTURE PROSPECTS
Although fluorescence diagnostics have many advantages, the exclusion of background tissue autofluorescence remains a major unresolved issue and needs to be addressed in order to improve diagnostic accuracy. Surrounding connective tissues have their own autofluorescence, which sometimes interferes with the precise detection of PpIX red fluorescence. However, we attained highly sensitive detection of metastasized lymph nodes in patients with colorectal cancer by using the spectral unmixing method[18]. Specifically, we demonstrated an improved diagnostic performance of 5-ALA-PDD, with 88.3% sensitivity, 92.0% specificity, and 87.4% accuracy. The receiver operating characteristic analysis revealed that the spectral unmixing method improved the area under the curve up to 0.95, indicating that the method might be an innovative solution.
Furthermore, we established a novel imaging strategy to specifically detect accumulated PpIX and filed an international patent application (WO/2013/002350). Through such technical advances, we anticipate that we will be able to attain a satisfactory diagnostic accuracy of 5-ALA-PDD in the near feature.
The limited depth penetration of the excitation light is another issue that still needs to be addressed. Although the penetration is sufficient for the detection of peritoneal dissemination, detection of lymph node metastases requires cutting the nodes and performing fluorescence observation at the cut surface. In a study aiming to overcome this disadvantage, Shimoyama et al[52] showed the potential of the lanthanide nanoparticle, a light energy upconverter. The combination of optimal lanthanide nanoparticle and 5-ALA-PDD might allow the use of near-infrared excitation light, which can penetrate deeply into the tissue and result in better diagnostic accuracy.
CONCLUSION
We reviewed the recent advances in the use of 5-ALA-PDD for the treatment of gastric cancer. Fluorescence diagnostics offer real-time visualization with high sensitivity unsurpassed by other diagnostic tools. With further technical development, application of 5-ALA-PDD is expected to become more widespread in gastric cancer treatment.
Footnotes
Conflict-of-interest statement: The following authors disclose financial relationships relevant to this publication: Koizumi N, Harada Y, Otsuji E and Takamatsu T have filed an international patent application (WO/2013/002350) for a novel imaging strategy to specifically detect accumulated protoporphyrin IX, which is cited in this review.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 30, 2015
First decision: September 9, 2015
Article in press: November 9, 2015
P- Reviewer: Chowdhury P S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
References
- 1.Arigami T, Uenosono Y, Yanagita S, Matsushita D, Arima H, Hirata M, Uchikado Y, Nakajo A, Okumura H, Ishigami S, et al. Feasibility of sentinel node navigation surgery after noncurative endoscopic resection for early gastric cancer. J Gastroenterol Hepatol. 2013;28:1343–1347. doi: 10.1111/jgh.12269. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama K, Katai H. Surgical treatment of gastric cancer in Japan, trend from standardization to individualization. Chirurgia (Bucur) 2014;109:722–730. [PubMed] [Google Scholar]
- 3.Miao RL, Wu AW. Towards personalized perioperative treatment for advanced gastric cancer. World J Gastroenterol. 2014;20:11586–11594. doi: 10.3748/wjg.v20.i33.11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merrett ND. Multimodality treatment of potentially curative gastric cancer: geographical variations and future prospects. World J Gastroenterol. 2014;20:12892–12899. doi: 10.3748/wjg.v20.i36.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666–2677. doi: 10.1007/s00464-011-1627-z. [DOI] [PubMed] [Google Scholar]
- 6.Wu CW, Hsieh MC, Lo SS, Lui WY, P’eng FK. Quality of life of patients with gastric adenocarcinoma after curative gastrectomy. World J Surg. 1997;21:777–782. doi: 10.1007/s002689900305. [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa Y, Kitano S, Kubota T, Kumai K, Otani Y, Saikawa Y, Yoshida M, Kitajima M. Minimally invasive surgery for gastric cancer--toward a confluence of two major streams: a review. Gastric Cancer. 2005;8:103–110. doi: 10.1007/s10120-005-0326-7. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Tanabe K, Taomoto J, Yamamoto H, Tokumoto N, Yoshida K, Ohdan H. Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol Lett. 2010;1:743–747. doi: 10.3892/ol_00000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto M, Sakaguchi Y, Matsuyama A, Yoshinaga K, Tsutsui S, Ishida T. Surgery after preoperative chemotherapy for patients with unresectable advanced gastric cancer. Oncology. 2013;85:241–247. doi: 10.1159/000354420. [DOI] [PubMed] [Google Scholar]
- 10.Ishigami S, Uenosono Y, Arigami T, Yanagita S, Okumura H, Uchikado Y, Kita Y, Kurahara H, Kijima Y, Nakajo A, et al. Clinical utility of perioperative staging laparoscopy for advanced gastric cancer. World J Surg Oncol. 2014;12:350. doi: 10.1186/1477-7819-12-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerner SP, Goh A. Novel endoscopic diagnosis for bladder cancer. Cancer. 2015;121:169–178. doi: 10.1002/cncr.28905. [DOI] [PubMed] [Google Scholar]
- 12.Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93:1003–1013. doi: 10.3171/jns.2000.93.6.1003. [DOI] [PubMed] [Google Scholar]
- 13.Bedwell J, MacRobert AJ, Phillips D, Bown SG. Fluorescence distribution and photodynamic effect of ALA-induced PP IX in the DMH rat colonic tumour model. Br J Cancer. 1992;65:818–824. doi: 10.1038/bjc.1992.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo Y, Murayama Y, Konishi H, Morimura R, Komatsu S, Shiozaki A, Kuriu Y, Ikoma H, Kubota T, Nakanishi M, et al. Fluorescent detection of peritoneal metastasis in human colorectal cancer using 5-aminolevulinic acid. Int J Oncol. 2014;45:41–46. doi: 10.3892/ijo.2014.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishi K, Fujiwara Y, Yano M, Motoori M, Sugimura K, Ohue M, Noura S, Marubashi S, Takahashi H, Sakon M. Diagnostic laparoscopy with 5-aminolevulinic-acid-mediated photodynamic diagnosis enhances the detection of peritoneal micrometastases in advanced gastric cancer. Oncology. 2014;87:257–265. doi: 10.1159/000365356. [DOI] [PubMed] [Google Scholar]
- 16.Murayama Y, Ichikawa D, Koizumi N, Komatsu S, Shiozaki A, Kuriu Y, Ikoma H, Kubota T, Nakanishi M, Harada Y, et al. Staging fluorescence laparoscopy for gastric cancer by using 5-aminolevulinic acid. Anticancer Res. 2012;32:5421–5427. [PubMed] [Google Scholar]
- 17.Koizumi N, Harada Y, Murayama Y, Harada K, Beika M, Yamaoka Y, Dai P, Komatsu S, Kubota T, Ichikawa D, et al. Detection of metastatic lymph nodes using 5-aminolevulinic acid in patients with gastric cancer. Ann Surg Oncol. 2013;20:3541–3548. doi: 10.1245/s10434-013-3017-3. [DOI] [PubMed] [Google Scholar]
- 18.Harada K, Harada Y, Beika M, Koizumi N, Inoue K, Murayama Y, Kuriu Y, Nakanishi M, Minamikawa T, Yamaoka Y, et al. Detection of lymph node metastases in human colorectal cancer by using 5-aminolevulinic acid-induced protoporphyrin IX fluorescence with spectral unmixing. Int J Mol Sci. 2013;14:23140–23152. doi: 10.3390/ijms141123140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieg RC, Messmann H, Rauch J, Seeger S, Knuechel R. Metabolic characterization of tumor cell-specific protoporphyrin IX accumulation after exposure to 5-aminolevulinic acid in human colonic cells. Photochem Photobiol. 2002;76:518–525. doi: 10.1562/0031-8655(2002)076<0518:mcotcs>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Gibson SL, Cupriks DJ, Havens JJ, Nguyen ML, Hilf R. A regulatory role for porphobilinogen deaminase (PBGD) in delta-aminolaevulinic acid (delta-ALA)-induced photosensitization? Br J Cancer. 1998;77:235–242. doi: 10.1038/bjc.1998.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dailey HA, Dailey TA, Wu CK, Medlock AE, Wang KF, Rose JP, Wang BC. Ferrochelatase at the millennium: structures, mechanisms and [2Fe-2S] clusters. Cell Mol Life Sci. 2000;57:1909–1926. doi: 10.1007/PL00000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellers VM, Johnson MK, Dailey HA. Function of the [2FE-2S] cluster in mammalian ferrochelatase: a possible role as a nitric oxide sensor. Biochemistry. 1996;35:2699–2704. doi: 10.1021/bi952631p. [DOI] [PubMed] [Google Scholar]
- 23.Inoue K, Karashima T, Kamada M, Shuin T, Kurabayashi A, Furihata M, Fujita H, Utsumi K, Sasaki J. Regulation of 5-aminolevulinic acid-mediated protoporphyrin IX accumulation in human urothelial carcinomas. Pathobiology. 2009;76:303–314. doi: 10.1159/000245896. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto F, Ohgari Y, Yamaki N, Kitajima S, Shimokawa O, Matsui H, Taketani S. The role of nitric oxide in delta-aminolevulinic acid (ALA)-induced photosensitivity of cancerous cells. Biochem Biophys Res Commun. 2007;353:541–546. doi: 10.1016/j.bbrc.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Hagiya Y, Endo Y, Yonemura Y, Takahashi K, Ishizuka M, Abe F, Tanaka T, Okura I, Nakajima M, Ishikawa T, et al. Pivotal roles of peptide transporter PEPT1 and ATP-binding cassette (ABC) transporter ABCG2 in 5-aminolevulinic acid (ALA)-based photocytotoxicity of gastric cancer cells in vitro. Photodiagnosis Photodyn Ther. 2012;9:204–214. doi: 10.1016/j.pdpdt.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Kobuchi H, Moriya K, Ogino T, Fujita H, Inoue K, Shuin T, Yasuda T, Utsumi K, Utsumi T. Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS One. 2012;7:e50082. doi: 10.1371/journal.pone.0050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K, Hagiya Y, Endo Y, Nakajima M, Ishizuka M, Tanaka T, Ogura S. Effects of plasma membrane ABCB6 on 5-aminolevulinic acid (ALA)-induced porphyrin accumulation in vitro: tumor cell response to hypoxia. Photodiagnosis Photodyn Ther. 2015;12:45–51. doi: 10.1016/j.pdpdt.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Zhao SG, Chen XF, Wang LG, Yang G, Han DY, Teng L, Yang MC, Wang DY, Shi C, Liu YH, et al. Increased expression of ABCB6 enhances protoporphyrin IX accumulation and photodynamic effect in human glioma. Ann Surg Oncol. 2013;20:4379–4388. doi: 10.1245/s10434-011-2201-6. [DOI] [PubMed] [Google Scholar]
- 30.Loh CS, Vernon D, MacRobert AJ, Bedwell J, Bown SG, Brown SB. Endogenous porphyrin distribution induced by 5-aminolaevulinic acid in the tissue layers of the gastrointestinal tract. J Photochem Photobiol B. 1993;20:47–54. doi: 10.1016/1011-1344(93)80130-2. [DOI] [PubMed] [Google Scholar]
- 31.Doss MO, Kühnel A, Gross U. Alcohol and porphyrin metabolism. Alcohol Alcohol. 2000;35:109–125. doi: 10.1093/alcalc/35.2.109. [DOI] [PubMed] [Google Scholar]
- 32.Sassa S. Diagnosis and therapy of acute intermittent porphyria. Blood Rev. 1996;10:53–58. doi: 10.1016/s0268-960x(96)90020-x. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda H, Casas A, Batlle A. Aminolevulinic acid: from its unique biological function to its star role in photodynamic therapy. Int J Biochem Cell Biol. 2005;37:272–276. doi: 10.1016/j.biocel.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Elfsson B, Wallin I, Eksborg S, Rudaeus K, Ros AM, Ehrsson H. Stability of 5-aminolevulinic acid in aqueous solution. Eur J Pharm Sci. 1999;7:87–91. doi: 10.1016/s0928-0987(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 35.Gadmar ØB, Moan J, Scheie E, Ma LW, Peng Q. The stability of 5-aminolevulinic acid in solution. J Photochem Photobiol B. 2002;67:187–193. doi: 10.1016/s1011-1344(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 36.Nanashima A, Nagayasu T. Current status of photodynamic therapy in digestive tract carcinoma in Japan. Int J Mol Sci. 2015;16:3434–3440. doi: 10.3390/ijms16023434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bown SG, Rogowska AZ. New photosensitizers for photodynamic therapy in gastroenterology. Can J Gastroenterol. 1999;13:389–392. doi: 10.1155/1999/454789. [DOI] [PubMed] [Google Scholar]
- 38.Almerie MQ, Gossedge G, Wright KE, Jayne DG. Photodynamic diagnosis for detection of peritoneal carcinomatosis. J Surg Res. 2015;195:175–187. doi: 10.1016/j.jss.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Endo Y, Fujita T, Ishibashi H, Nishioka T, Canbay E, Li Y, Ogura S, Yonemura Y. Cytoreductive surgery under aminolevulinic acid-mediated photodynamic diagnosis plus hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from ovarian cancer and primary peritoneal carcinoma: results of a phase I trial. Ann Surg Oncol. 2014;21:4256–4262. doi: 10.1245/s10434-014-3901-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kishi K, Fujiwara Y, Yano M, Inoue M, Miyashiro I, Motoori M, Shingai T, Gotoh K, Takahashi H, Noura S, et al. Staging laparoscopy using ALA-mediated photodynamic diagnosis improves the detection of peritoneal metastases in advanced gastric cancer. J Surg Oncol. 2012;106:294–298. doi: 10.1002/jso.23075. [DOI] [PubMed] [Google Scholar]
- 41.Fujimura T, Fushida S, Tsukada T, Kinoshita J, Oyama K, Miyashita T, Takamura H, Kinami S, Ohta T. A new stage of sentinel node navigation surgery in early gastric cancer. Gastric Cancer. 2015;18:210–217. doi: 10.1007/s10120-014-0446-z. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi H, Kitagawa Y. Sentinel node navigation surgery in patients with early gastric cancer. Dig Surg. 2013;30:104–111. doi: 10.1159/000350875. [DOI] [PubMed] [Google Scholar]
- 43.Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–3710. doi: 10.1200/JCO.2013.50.3789. [DOI] [PubMed] [Google Scholar]
- 44.Mitsumori N, Nimura H, Takahashi N, Kawamura M, Aoki H, Shida A, Omura N, Yanaga K. Sentinel lymph node navigation surgery for early stage gastric cancer. World J Gastroenterol. 2014;20:5685–5693. doi: 10.3748/wjg.v20.i19.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otsubo R, Oikawa M, Hirakawa H, Shibata K, Abe K, Hayashi T, Kinoshita N, Shigematsu K, Hatachi T, Yano H, et al. Novel diagnostic procedure for determining metastasis to sentinel lymph nodes in breast cancer using a semi-dry dot-blot method. Int J Cancer. 2014;134:905–912. doi: 10.1002/ijc.28408. [DOI] [PubMed] [Google Scholar]
- 46.Miyashiro I, Hiratsuka M, Sasako M, Sano T, Mizusawa J, Nakamura K, Nashimoto A, Tsuburaya A, Fukushima N. High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer. 2014;17:316–323. doi: 10.1007/s10120-013-0285-3. [DOI] [PubMed] [Google Scholar]
- 47.Murayama Y, Harada Y, Imaizumi K, Dai P, Nakano K, Okamoto K, Otsuji E, Takamatsu T. Precise detection of lymph node metastases in mouse rectal cancer by using 5-aminolevulinic acid. Int J Cancer. 2009;125:2256–2263. doi: 10.1002/ijc.24707. [DOI] [PubMed] [Google Scholar]
- 48.Tamaki Y, Sato N, Homma K, Takabatake D, Nishimura R, Tsujimoto M, Yoshidome K, Tsuda H, Kinoshita T, Kato H, et al. Routine clinical use of the one-step nucleic acid amplification assay for detection of sentinel lymph node metastases in breast cancer patients: results of a multicenter study in Japan. Cancer. 2012;118:3477–3483. doi: 10.1002/cncr.26683. [DOI] [PubMed] [Google Scholar]
- 49.Tsujimoto M, Nakabayashi K, Yoshidome K, Kaneko T, Iwase T, Akiyama F, Kato Y, Tsuda H, Ueda S, Sato K, et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res. 2007;13:4807–4816. doi: 10.1158/1078-0432.CCR-06-2512. [DOI] [PubMed] [Google Scholar]
- 50.Yaguchi Y, Sugasawa H, Tsujimoto H, Takata H, Nakabayashi K, Ichikura T, Ono S, Hiraki S, Sakamoto N, Horio T, et al. One-step nucleic acid amplification (OSNA) for the application of sentinel node concept in gastric cancer. Ann Surg Oncol. 2011;18:2289–2296. doi: 10.1245/s10434-011-1591-9. [DOI] [PubMed] [Google Scholar]
- 51.Kumagai K, Yamamoto N, Miyashiro I, Tomita Y, Katai H, Kushima R, Tsuda H, Kitagawa Y, Takeuchi H, Mukai M, et al. Multicenter study evaluating the clinical performance of the OSNA assay for the molecular detection of lymph node metastases in gastric cancer patients. Gastric Cancer. 2014;17:273–280. doi: 10.1007/s10120-013-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimoyama A, Watase H, Liu Y, Ogura S, Hagiya Y, Takahashi K, Inoue K, Tanaka T, Murayama Y, Otsuji E, et al. Access to a novel near-infrared photodynamic therapy through the combined use of 5-aminolevulinic acid and lanthanide nanoparticles. Photodiagnosis Photodyn Ther. 2013;10:607–614. doi: 10.1016/j.pdpdt.2013.07.005. [DOI] [PubMed] [Google Scholar]


