Abstract
AIM: To identify definitions of cytomegalovirus (CMV) infection and intestinal disease, in inflammatory bowel disease (IBD), to determine the prevalence associated with these definitions.
METHODS: We conducted a systematic review and interrogated PubMed, EMBASE and Cochrane for literature on prevalence and diagnostics of CMV infection and intestinal disease in IBD patients. As medical headings we used “cytomegalovirus” OR “CMV” OR “cytomegalo virus” AND “inflammatory bowel disease” OR “IBD” OR “ulcerative colitis” OR “colitis ulcerosa” OR “Crohn’s disease”. Both MeSH-terms and free searches were performed. We included all types of English-language (clinical) trials concerning diagnostics and prevalence of CMV in IBD.
RESULTS: The search strategy identified 924 citations, and 52 articles were eligible for inclusion. We identified 21 different definitions for CMV infection, 8 definitions for CMV intestinal disease and 3 definitions for CMV reactivation. Prevalence numbers depend on used definition, studied population and region. The highest prevalence for CMV infection was found when using positive serum PCR as a definition, whereas for CMV intestinal disease this applies to the use of tissue PCR > 10 copies/mg tissue. Most patients with CMV infection and intestinal disease had steroid refractory disease and came from East Asia.
CONCLUSION: We detected multiple different definitions used for CMV infection and intestinal disease in IBD patients, which has an effect on prevalence numbers and eventually on outcome in different trials.
Keywords: Inflammatory bowel disease, Cytomegalovirus, Ulcerative colitis, Crohn’s disease, Systematic review
Core tip: The use of different definitions for cytomegalovirus (CMV) infection and CMV intestinal disease in inflammatory bowel disease (IBD) patients has great impact on the reported prevalence rates (definitions with highest prevalence: positive serum PCR (CMV infection) and tissue PCR > 10 copies/mg tissue (CMV intestinal disease)). In addition, prevalence rates depend on the studied population (highest prevalence: steroid refractory disease), and region (highest prevalence: East Asia). Our key message is not to develop more sensitive diagnostics to find CMV, but to organise a global consensus meeting on CMV in IBD to formulate one unified definition for clinically relevant CMV (intestinal) disease in IBD.
INTRODUCTION
Cytomegalovirus (CMV) is a member of human herpesvirus family[1]. CMV infection is common and mostly asymptomatic in healthy people. Seroprevalence data differ depending on the healthcare circumstances or immunocompetency of the host, but most studies give a seroprevalence of 40%-100% in adults[2-4], with the highest prevalence in non-western countries[5,6]. In immunocompromised patients CMV infection may follow a complicated course resulting in retinitis, pneumonia and intestinal disease (mostly colitis) or other organ specific disease[7-9]. The role of cytomegalovirus (CMV) in inflammatory bowel disease (IBD) is under scrutiny. Here, the discussion mainly focuses on either the association or causation of CMV infection and intestinal disease in adverse outcomes in IBD patients. The applied definitions for both CMV infection and CMV intestinal disease, are crucial in this issue, and the answer to the above key question can only be given when there is a unified gold standard definition of clinical relevant CMV infection and intestinal disease, which is lacking at this moment. Our aim was to perform a systematic review of the literature to identify the different definitions for CMV infection and intestinal disease in IBD patients, and to connect the used definitions to the reported prevalence. In addition we summarize the different diagnostic strategies for CMV infection and intestinal disease.
MATERIALS AND METHODS
Search strategy and study selection
A search of the medical literature was conducted using MEDLINE, EMBASE and the Cochrane central register of controlled trials, until December 2014, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[10,11]. The study was registered in the PROSPERO database registry (PROSPERO registration number CRD 42015017451). The search strategy was designed to identify English-language (clinical) trials concerning diagnostics and prevalence of CMV in IBD. The following medical search headings were used: “cytomegalovirus” OR “CMV” OR “cytomegalo virus” AND “Inflammatory Bowel Disease” OR “IBD” OR “ulcerative colitis” OR “colitis ulcerosa” OR “Crohn’s Disease”. Both MeSH-terms and free searches were performed. Studies were eligible if they reported on prevalence and/or diagnostic criteria to define CMV infection or CMV intestinal disease in IBD (Table 1).
Table 1.
Eligibility criteria for inclusion
| Adults |
| Diagnosis of IBD, according to international guidelines |
| English language |
| Prevalence and/or diagnostics CMV infection or intestinal disease reported |
| Minimal sample size 10 patients |
IBD: Inflammatory bowel disease; CMV: Cytomegalovirus.
Because of the paucity of prospective, randomized, controlled trials on this subject, we aimed to consider all types of evidence available. To this end, we also included observational studies, case-control studies and retrospective studies. We excluded non-English literature, case reports, case series with n < 10 cases and review articles. We also excluded studies that concerned CMV and pouchitis, or studies that concerned the causative relationship of CMV and IBD.
Two independent reviewers (Römkens TEH, Bulte GJ) performed the first selection, and screened title and abstract of the papers identified by electronic search. They completed an inclusion form for eligible studies. Additional articles were obtained through citation snowballing to locate primary sources. When no abstract was available, the article was always screened by full text. Three investigators read all full text papers, and disagreements were resolved by consensus (Römkens TEH, Bulte GJ and Nissen LHC).
Outcome assessment
The primary outcomes were: (1) a list with all used definitions of CMV infection or CMV intestinal disease in IBD patients; and (2) prevalence numbers to assess the effect that the applied definition has on the reported prevalence of CMV in IBD patients. Secondary outcomes were: (1) prevalence of CMV in subpopulations as ulcerative colitis (UC), Crohn’s disease (CD), steroid refractory disease; and (2) prevalence of CMV in different regions of the world. We also summarized different diagnostic strategies for CMV infection in IBD patients.
Data extraction and assessment of risk of bias
The data extraction process was carried out using a pre-established protocol. Full papers were reviewed using a purpose-designed database. The following clinical data were extracted for each trial: single- or multicenter, country of origin, duration of follow up, study period, sample size, characteristics of the studied population, medication at baseline, used diagnostics for CMV infection, criteria used to define CMV infection, criteria used to define CMV intestinal disease, CMV prevalence and authors conclusion. Assessment of risk of bias was performed independently by three investigators, with disagreements resolved by discussion. Risk of bias was assessed by recording of study type, retrospective or prospective design, sample size, study period and follow up, whether or not randomization or blinding was used, and recording specific population characteristics that pose a risk for selection bias (type and severity of IBD, medication use, exclusion criteria).
Statistical analysis
All abstracted data were entered into a structured database. We displayed the results for both CMV infection and intestinal disease in IBD patients separately, in line with the fact that these are two different entities. In addition to detailed tables, we provide the main results in scatterplots in order to give more insight in sample size, median and range of the various definitions. Next to this, we provide scatterplots of prevalence by definition in different subgroups as UC, CD, steroid refractory IBD, and IBD patients in different regions of the world. Kappa statistics were used to analyze agreement among the reviewers. Evidence-tables were provided for all characteristics of the included studies. We could not carry out a meta-analysis because head-to-head comparison of the included studies was impossible due to heterogeneity.
RESULTS
Selected studies
Our search strategy generated a total of 924 citations, of which 190 (168 + 22) published articles appeared to be relevant, and were retrieved for further assessment (Figure 1). Of these, 138 (118 + 20) articles were excluded for various reasons, leaving 52 eligible articles. The main reasons for exclusion were case reports; reviews; non-IBD patients; no information on CMV (intestinal) infection and non-English literature. Agreement in this step between reviewers was 88% (Cohen’s Kappa 0.73) before consensus meeting. Study (risk of bias) variables are shown in Appendix 1. Most of the included studies have small sample sizes. None of the included studies was randomized and most of the study designs were single centre (n = 42/50) with more than half of them using a retrospective design (26/48). Some used blinding for the reviewing pathologist (6/43) or endoscopist (2/43).
Figure 1.
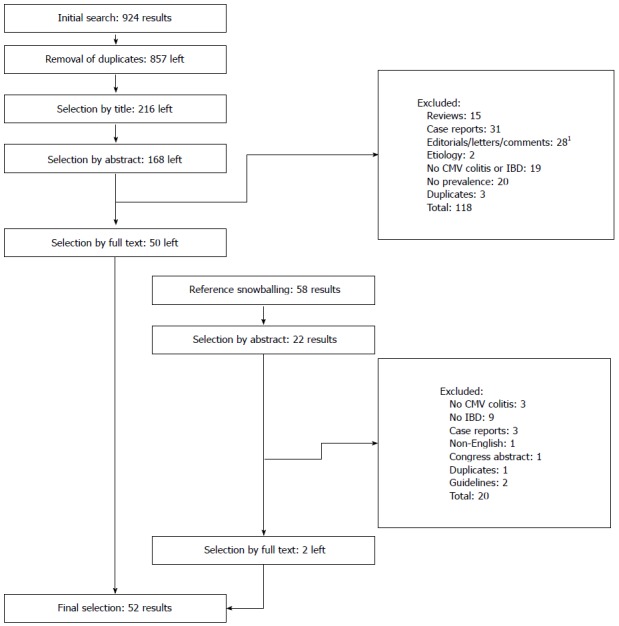
Algorithm of inclusion. 1Letters/comments presenting original data were included in the review.
Definitions and prevalence
We identified 21 different definitions for CMV infection, and 8 definitions for CMV intestinal disease (Tables 2 and 3). Three definitions for reactivation of CMV are listed in Table 4. Some studies do not mention or define CMV infection, but report prevalence numbers of “mucosal expression”[12], or “presence of CMV”[13,14] and some provide a prevalence rate without defining CMV infection[15-62].
Table 2.
Prevalence by definition of cytomegalovirus-infection
| Definition | Studies, n | Median | Range |
| Antigenemia[17-21] | 5 | 32% | 6%-34% |
| Tissue PCR[22-25] | 4 | 11% | 1%-32% |
| IHC1[26-29] | 4 | 13% | 9%-23% |
| HE[30-32] | 3 | 17% | 5%-36% |
| HE or IHC[33,34] | 2 | 8% | 5%-11% |
| HE and IHC[35,36] | 2 | 19% | 12%-27% |
| IgM or HE or IHC2[37,38] | 2 | 9% | 5%-13% |
| Antigenemia or Tissue PCR[39] | 1 | NA | NA |
| Serum PCR[40] | 1 | 84% | - |
| (HE and IHC) or tissue PCR[41] | 1 | 4% | - |
| Tissue PCR 10 copies/µg; OR histology OR Antigenemia[42] | 1 | 54% | - |
| Antigenemia (2 tests: C7-HRP OR C10/C11) OR histology[43] | 1 | 9% | - |
| Antigenemia or blood PCRquantitative[44] | 1 | 36% | - |
| IgM or serum PCR or HE[45] | 1 | 78% | - |
| IgM or tissue PCRqualitative or HE[46] | 1 | 16% | - |
| IgG and (blood culture, antigenemia or histology or IgM or urine culture)[47] | 1 | 6% | - |
| IgM or serum PCRqualitative or feces PCR[48] | 1 | 5% | - |
| Inclusions: HE[49] | 1 | 13% | - |
| Active infection: tissue PCR[50] | 1 | 13% | - |
| Active replication: (HE or IHC) and antigenemia[51] | 1 | 36% | - |
| Blood dissemination: (viremia, antigenemia, RNAemia) or (viremia or tissue culture)[52] | 1 | 16% | - |
| Total: 21 | Total: 36 |
1One study defined a clinically relevant infection as > 10 IHC + cells/section but also recognized “scattered” positivity as 1-9 cells per section[29]; 2Once mentioned as “acute infection”. PCR: Polymerase chain reaction; HE: Hematoxylin and eosin staining; IHC: Immunohistochemistry; IgM: Immunoglobulin M; IgG: immunoglobulin G; NA: Not applicable.
Table 3.
Prevalence by definition of cytomegalovirus-intestinal disease
| Definition | Studies, n | Median | Range |
| HE or IHC1,2[38,44,53,54] | 4 | 6% | 2%-29% |
| HE[20,55] | 2 | 9% | 0%-17% |
| Tissue PCRquantitative > 10 copies/mg[56,57] | 2 | 34% | 30%-38% |
| Serology and (IHC or antigenemia or serum PCR or tissue PCR)[58] | 1 | 6% | - |
| IHC[59] | 1 | 0% | - |
| HE or IHC or tissue PCR[51] | 1 | 33% | - |
| (Pp65 antigenemia or tissue PCRquantitative) and IHC and intestinal symptoms[39] | 1 | NA3 | - |
| IHC positive when inflammation present[52] | 1 | 1% | - |
| Total: 8 | Total: 13 |
1One study tested both before and after iv-steroid administration; 2No data on prevalence: definition is used as the gold standard to compare other diagnostics;
No prevalence is reported in this study. PCR: Polymerase chain reaction; HE: Hematoxylin and eosin staining; IHC: Immunohistochemistry.
Table 4.
Prevalence by definition of cytomegalovirus-reactivation
| Definition | Studies, n | Median | Range |
| IgM or HE or PCR1[60] | 1 | 10% | - |
| Serum PCR in IgG positive patients[61] | 1 | 0% | - |
| Antigenemia or plasma PCR[62] | 1 | 36% | - |
| Total: 3 | Total: 3 |
1Not specified what material is used for PCR testing. PCR: Polymerase chain reaction; HE: Hematoxylin and eosin staining; IgM: Immunoglobulin M; IgG: Immunoglobulin G.
Prevalence data for definitions used more than once for respectively CMV infection and intestinal disease are listed in a scatter plot (Figures 2 and 3).
Figure 2.
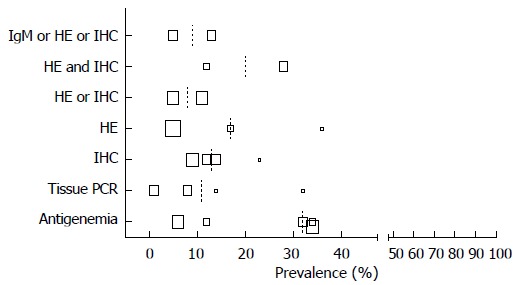
Prevalence cytomegalovirus infection by authors definition. Size of the squares corresponds with the population size in 5 categories (n = 0-25, n = 25-50, n = 50-100, n = 100-250, n > 250). Median depicted as dotted line.
Figure 3.
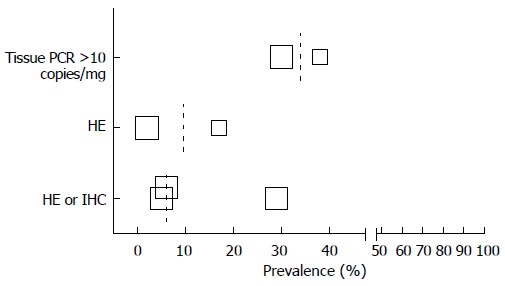
Prevalence cytomegalovirus intestinal disease by authors definition. Size of the squares corresponds with the population size in 5 categories (n = 0-25, n = 25-50, n = 50-100, n = 100-250, n > 250). Median depicted as dotted line.
By using the authors” definition of either CMV infection or intestinal disease, we found different prevalence numbers in various regions of the world and in different subpopulations, which are depicted in scatterplots for CMV infection in Figures 4 and 5. If available CMV seroprevalence data are depicted in Figure 6. More detailed information on this topic and prevalence numbers for CMV intestinal disease are given in supplementary files (Appendix 2 A, B and Appendix 3 A, B). In general, the prevalence is negligible in healthy controls. We found a higher prevalence in UC than in CD both for CMV infection (median 14% vs 2.5%) and CMV intestinal disease (median 19.5% vs 11%). The prevalence is highest in steroid refractory disease for CMV infection (median 32.5%) and intestinal disease (median 32.5%).
Figure 4.
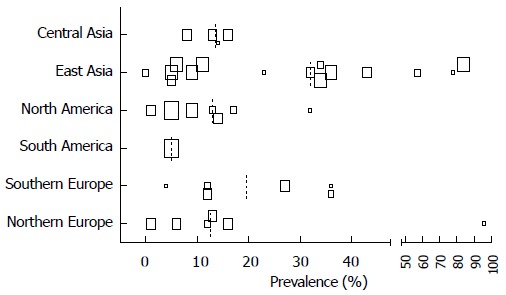
Prevalence cytomegalovirus infection in different global regions by authors definition. Size of the squares corresponds with the population size in 5 categories (n = 0-25, n = 25-50, n = 50-100, n = 100-250, n > 250). Median depicted as dotted line.
Figure 5.
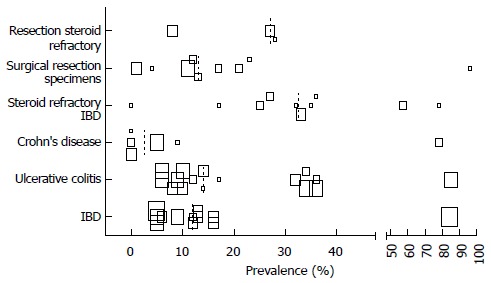
Prevalence cytomegalovirus infection in different sub populations, by authors definition. Size of the squares corresponds with the population size in 5 categories (n = 0-25, n = 25-50, n = 50-100, n = 100-250, n > 250). Median depicted as dotted line.
Figure 6.
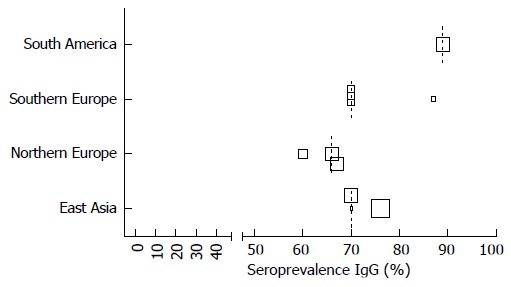
Cytomegalovirus seroprevalence data in different regions of the world. Size of the squares corresponds with the population size in 5 categories (n = 0-25, n = 25-50, n = 50-100, n = 100-250, n > 250). Median depicted as dotted line.
Diagnostic tests for CMV
More than 10 different tests were used to diagnose CMV (active) infection and intestinal disease, which can be found in the definition Tables 2 and 3. Histology, with or without immunohistochemistry (IHC), is most widely used to diagnose CMV disease (infection and/or intestinal disease; n = 30/48). PCR on tissue is used in almost one third of the included studies (14/48). In Table 5 we present an overview of the test characteristics as described earlier in literature[24,63-70].
Table 5.
Test characteristics different diagnostic tools for cytomegalovirus
| Test | Pro | Con | Sens | Spec |
| Serology | Fast, quantification possible | Systemic, not proving intestinal disease; | 98%-100% | 96%-99% |
| Antigenemia | Fast, quantification possible | Systemic, not proving intestinal disease labor intensive | 60%-100% | 83%-100% |
| Serum PCR | Fast, quantification possible | Systemic, not proving intestinal disease | 65%-100% | 40%-94% |
| HE Histology (gold standard?) | Specific, proofs intestinal disease | Slow; low sensitivity | 10%-87% | 92%-100% |
| Histology with IHC | Specific, proofs intestinal disease | Slow | 93% | 92%-100% |
| Tissue PCR | Quantification possible | Cut-off point unclear, uncertain clinical significance | 65%-100% | 40%-100% |
| Stool PCR | Quantification possible | Little experience | 83%% | 93% |
| Viral Culture | Very specific | Very slow | 45%-78% | 89%-100% |
| Rapid Vial culture | Very specific | Little experience | 68%-100% | 89%-100% |
Sens: Sensitivity; Spec: Specificity; PCR: Polymerase chain reaction; HE: Hematoxylin and eosin staining; IHC: Immunohistochemistry.
DISCUSSION
This systematic review maps the prevalence of both CMV infection and intestinal disease in IBD patients. We found that the prevalence greatly depends on the used definition. Studies reporting the highest prevalence of CMV infection use positive serum PCR as test, while studies that use positive antigenemia follow suit. In case of diagnosing CMV intestinal disease, the highest prevalence applies for tissue PCR (> 10 copies/mg tissue). IBD patients with steroid refractory disease and those coming from East Asia have the highest prevalence of CMV infection and/or intestinal disease. In general CMV infection or intestinal disease is seen more frequently in UC compared to CD.
The difference of CMV infection and clinically relevant intestinal disease has been outlined earlier in literature. There, CMV infection is described as (incidental) finding of positive PCR or detection of CMV antigens or antibodies in serum, whereas CMV disease is a clinical syndrome where CMV infection is accompanied by manifest clinical symptoms[67,69,71,72]. Subclinical reactivation of CMV, without symptoms, is seen in approximately 50% of active UC cases on immunosuppressive therapy[62]. In practice however, the different entities described above, are used interchangeably. Likewise, no unified definitions have been used in clinical trials, and our study documents that the terms CMV infection and CMV intestinal disease are used reciprocally. This makes it difficult to compare data from different studies and to draw conclusions for outcome.
Interestingly, by using the author’s definition, we found the highest prevalence of CMV infection and intestinal disease in East Asia. Population based CMV seroprevalence studies are lacking, but a review on this topic found that seroprevalence tended to be highest in South America, Africa and Asia, and was also higher in parts of Europe and the Middle East[6]. The most likely explanation is the use of different diagnostic methods for CMV infection in different regions of the world. We show high prevalence numbers for CMV infection when antigenemia is used as test (Table 2), where 80% of these studies appear to come from Japan. CMV infection and more importantly CMV colitis is unusual in the healthy population[13,28,29,54] as described before in a systematic review[71]. Only one study reported presence of CMV DNA in 29% of asymptomatic control samples, but robust replication studies to confirm this unusual finding are lacking[14]. We found lower prevalence of CMV infection (and to a lesser extent in intestinal disease) for CD compared to UC[13,34] (Appendix 3). The most frequently studied population in relation to CMV is those with steroid resistant IBD or refractory UC. Apropos it should be noted that these studies use different definitions for steroid resistant IBD or refractory UC which may affect the prevalence[71,73]. We found the highest prevalence of CMV infection and CMV intestinal disease in steroid refractory disease, for each definition of CMV used[54] (Appendix 3). Whether or not CMV reactivation has a role in the process of steroid resistance, is still under debate[57,73-75].
The surveyed literature contains 29 different methods to diagnose CMV infection and/or intestinal disease, probably mainly caused by the fact that still no single gold standard exists for (clinically relevant) CMV infection in IBD. In general, the literature recommends to process biopsies for HE and IHC[33,63,71,76] and/or if available by CMV DNA real-time PCR, with a cut off value that is yet to be identified[76]. The recent ECCO guideline[72] mentions that different techniques for diagnosis of CMV infection are available, but stops short of defining a gold standard. The guideline refers to histopathology combined with IHC (using monoclonal antibodies) as highly specific and sensitive for verifying CMV infection in tissue. In addition the guideline describes quantitative PCR in tissue and in blood as the most commonly used and advantageous technique for diagnosis of CMV infection. Quantitative PCR has a low sensitivity for diagnosing CMV colitis in patients with moderate to severe UC, and cannot substitute histopathology diagnosis[44]. Already in the nineties consensus meetings were held to formulate the definition for CMV infection in general and for all organ specific involvements in transplant recipients. These definitions were updated again in 2002[67]. CMV infection was defined as isolation of the CMV virus or detection of viral proteins or nucleic acid in any body fluid or tissue specimen. There CMV (gastro)intestinal disease was defined by identification of a combination of (1) clinical symptoms; (2) findings of macroscopic mucosal lesions on endoscopy; and (3) demonstration of CMV infection (by culture, histopathology, IHC, or in situ hybridization) in a (gastro)intestinal tract biopsy specimen. According to this guideline detection of CMV by PCR alone is insufficient for diagnosis of CMV gastrointestinal disease. Applying these criteria to IBD patients can be more complicated, since ulceration can also be caused by the underlying IBD. Since then, multiple more sensitive diagnostic tests have been developed to detect CMV, but a threshold or definition of clinical relevant CMV infection or intestinal disease remains to be established. We suggest that a global multidisciplinary consensus meeting on CMV and IBD should be held, in order to develop one unified definition of clinically relevant CMV intestinal disease. In this context, clinically relevant describes the situation that symptoms appear, clinical deterioration occurs, and (antiviral) treatment should be initiated. This single definition could be used in clinical practice, but also in future trials, to serve as a gold standard in the discussion on outcome and optimal (antiviral) treatment of CMV in IBD patients.
Our study has several strengths. First of all, we show that the use of different definitions of CMV infection impacts reported prevalence rates. Second, we provide CMV prevalence data for several important sub populations, as UC, CD, steroid refractory disease, and patients in different continents of the world. Third, two independent reviewers reviewed all eligible studies, and three reviewers read all full text papers, with good agreement scores based on the presented kappa value. Our study comes with some limitations. None of the included studies were randomized, most of them had a retrospective design and only some used blinding of endoscopists or pathologists. The heterogeneity of the design of included studies and wide variations in used definitions for both CMV infection and intestinal disease made it impossible to perform a meta-analysis on these data.
In conclusion we find a wide variety in definitions used for CMV infection and intestinal disease in IBD patients, which impacts corresponding prevalence rates and eventually the outcome in different trials. A global consensus meeting is pivotal to develop one unified gold standard definition for clinically relevant CMV, to be used in clinical practice as well as in future trials.
COMMENTS
Background
Cytomegalo virus (CMV) infection is common, but mostly asymptomatic in the general population. In immunocompromised patients CMV infection may result in complications and organ-specific disease such as colitis. The exact role of CMV in clinical deterioration in inflammatory bowel disease (IBD) patients under debate. There is no global consensus on definitions of clinically relevant CMV disease in IBD patients.
Research frontiers
The optimal treatment strategy of refractory IBD, involving CMV infection, remains to be determined. Various treatment options, as the use of antiretroviral agents or intensified immunosuppressive therapy are being studied. It is most likely that the outcomes of the different trials will be influenced by the used definition of CMV infection and intestinal disease. Therefore one unified definition is essential.
Innovations and breakthroughs
Multiple diagnostic tests circulate to diagnose CMV infection. As a result, more than 20 definitions are used in clinical practice and for research purposes. This review is the first to address this challenging issue and the problems resulting from like the interpretation of study outcomes on this topic.
Applications
A global consensus meeting is necessary to determine one unified definition for CMV infection and intestinal disease in IBD to be used in clinical practice and future research.
Terminology
CMV-infection: systemic presence of viral antigens or replication markers. CMV-intestinal disease: evidence of viral presence in intestinal tissue. Steroid refractory disease: IBD not responding to treatment with high dose intravenous corticosteroids.
Peer-review
This is an interesting and timely manuscript addressing a significant discrepancy between the different definitions of CMV disease in IBD.
Footnotes
Conflict-of-interest statement: All the authors declare that they have no competing interests.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 5, 2015
First decision: August 26, 2015
Article in press: November 30, 2015
P- Reviewer: Kopylov U S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Britt WJ, Boppana S. Human cytomegalovirus virion proteins. Hum Immunol. 2004;65:395–402. doi: 10.1016/j.humimm.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119:924–935. doi: 10.7326/0003-4819-119-9-199311010-00010. [DOI] [PubMed] [Google Scholar]
- 3.de Jong MD, Galasso GJ, Gazzard B, Griffiths PD, Jabs DA, Kern ER, Spector SA. Summary of the II International Symposium on Cytomegalovirus. Antiviral Res. 1998;39:141–162. doi: 10.1016/s0166-3542(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 4.Korndewal MJ, Mollema L, Tcherniaeva I, van der Klis F, Kroes AC, Oudesluys-Murphy AM, Vossen AC, de Melker HE. Cytomegalovirus infection in the Netherlands: seroprevalence, risk factors, and implications. J Clin Virol. 2015;63:53–58. doi: 10.1016/j.jcv.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 5.Pembrey L, Raynor P, Griffiths P, Chaytor S, Wright J, Hall AJ. Seroprevalence of cytomegalovirus, Epstein Barr virus and varicella zoster virus among pregnant women in Bradford: a cohort study. PLoS One. 2013;8:e81881. doi: 10.1371/journal.pone.0081881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 7.Vilibic-Cavlek T, Kolaric B, Ljubin-Sternak S, Kos M, Kaic B, Mlinaric-Galinovic G. Prevalence and dynamics of cytomegalovirus infection among patients undergoing chronic hemodialysis. Indian J Nephrol. 2015;25:95–98. doi: 10.4103/0971-4065.139488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos CA, Brennan DC, Fraser VJ, Olsen MA. Incidence, risk factors, and outcomes of delayed-onset cytomegalovirus disease in a large, retrospective cohort of heart transplant recipients. Transplant Proc. 2014;46:3585–3592. doi: 10.1016/j.transproceed.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heiden D, Tun N, Maningding E, Heiden M, Rose-Nussbaumer J, Chan KN, Khizniak T, Yakubenko A, Lewallen S, Keenan JD, et al. Training clinicians treating HIV to diagnose cytomegalovirus retinitis. Bull World Health Organ. 2014;92:903–908. doi: 10.2471/BLT.14.142372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Sipponen T, Turunen U, Lautenschlager I, Nieminen U, Arola J, Halme L. Human herpesvirus 6 and cytomegalovirus in ileocolonic mucosa in inflammatory bowel disease. Scand J Gastroenterol. 2011;46:1324–1333. doi: 10.3109/00365521.2011.605466. [DOI] [PubMed] [Google Scholar]
- 13.Knösel T, Schewe C, Petersen N, Dietel M, Petersen I. Prevalence of infectious pathogens in Crohn’s disease. Pathol Res Pract. 2009;205:223–230. doi: 10.1016/j.prp.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Wakefield AJ, Fox JD, Sawyerr AM, Taylor JE, Sweenie CH, Smith M, Emery VC, Hudson M, Tedder RS, Pounder RE. Detection of herpesvirus DNA in the large intestine of patients with ulcerative colitis and Crohn’s disease using the nested polymerase chain reaction. J Med Virol. 1992;38:183–190. doi: 10.1002/jmv.1890380306. [DOI] [PubMed] [Google Scholar]
- 15.Nakase H, Yoshino T, Honzawa Y, Chiba T. Low prevalence of CMV infection in patients with Crohn’s disease in comparison with ulcerative colitis: effect of different immune response on prevalence of CMV infection. Dig Dis Sci. 2010;55:1498–1499. doi: 10.1007/s10620-010-1162-0. [DOI] [PubMed] [Google Scholar]
- 16.Hirata I, Murano M. Endoscopic diagnosis of refractory ulcerative colitis. Inflammopharmacology. 2007;15:22–25. doi: 10.1007/s10787-006-1555-z. [DOI] [PubMed] [Google Scholar]
- 17.Iida T, Ikeya K, Watanabe F, Abe J, Maruyama Y, Ohata A, Teruyuki S, Sugimoto K, Hanai H. Looking for endoscopic features of cytomegalovirus colitis: a study of 187 patients with active ulcerative colitis, positive and negative for cytomegalovirus. Inflamm Bowel Dis. 2013;19:1156–1163. doi: 10.1097/MIB.0b013e31828075ce. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Kato J, Kuriyama M, Hiraoka S, Kuwaki K, Yamamoto K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World J Gastroenterol. 2010;16:1245–1251. doi: 10.3748/wjg.v16.i10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada Y, Matsui T, Matake H, Sakurai T, Yamamoto J, Kikuchi Y, Yorioka M, Tsuda S, Yao T, Yao S, et al. Intractable ulcerative colitis caused by cytomegalovirus infection: a prospective study on prevalence, diagnosis, and treatment. Dis Colon Rectum. 2003;46:S59–S65. doi: 10.1097/01.DCR.0000087486.21981.C6. [DOI] [PubMed] [Google Scholar]
- 20.Criscuoli V, Casà A, Orlando A, Pecoraro G, Oliva L, Traina M, Rizzo A, Cottone M. Severe acute colitis associated with CMV: a prevalence study. Dig Liver Dis. 2004;36:818–820. doi: 10.1016/j.dld.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Inokuchi T, Kato J, Hiraoka S, Suzuki H, Nakarai A, Hirakawa T, Akita M, Takahashi S, Harada K, Okada H, et al. Long-term follow-up of ulcerative colitis patients treated on the basis of their cytomegalovirus antigen status. World J Gastroenterol. 2014;20:509–517. doi: 10.3748/wjg.v20.i2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee D, Deb R, Dar L, Mirdha BR, Pati SK, Thareja S, Falodia S, Ahuja V. High frequency of parasitic and viral stool pathogens in patients with active ulcerative colitis: report from a tropical country. Scand J Gastroenterol. 2009;44:325–331. doi: 10.1080/00365520802556809. [DOI] [PubMed] [Google Scholar]
- 23.Van Kruiningen HJ, Poulin M, Garmendia AE, Desreumaux P, Colombel JF, De Hertogh G, Geboes K, Vermeire S, Tsongalis GJ. Search for evidence of recurring or persistent viruses in Crohn’s disease. APMIS. 2007;115:962–968. doi: 10.1111/j.1600-0463.2007.apm_564.x. [DOI] [PubMed] [Google Scholar]
- 24.Herfarth HH, Long MD, Rubinas TC, Sandridge M, Miller MB. Evaluation of a non-invasive method to detect cytomegalovirus (CMV)-DNA in stool samples of patients with inflammatory bowel disease (IBD): a pilot study. Dig Dis Sci. 2010;55:1053–1058. doi: 10.1007/s10620-010-1146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mokhtari M, Tavakkoli H, Rafiee A, Dibaj R. Assessment of relationship between active ulcerative colitis and cytomegalovirus infection among Iranian patients. Adv Biomed Res. 2012;1:19. doi: 10.4103/2277-9175.98118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JJ, Simpson N, Klipfel N, Debose R, Barr N, Laine L. Cytomegalovirus infection in patients with active inflammatory bowel disease. Dig Dis Sci. 2010;55:1059–1065. doi: 10.1007/s10620-010-1126-4. [DOI] [PubMed] [Google Scholar]
- 27.Dimitroulia E, Spanakis N, Konstantinidou AE, Legakis NJ, Tsakris A. Frequent detection of cytomegalovirus in the intestine of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:879–884. doi: 10.1097/01.mib.0000231576.11678.57. [DOI] [PubMed] [Google Scholar]
- 28.Kambham N, Vij R, Cartwright CA, Longacre T. Cytomegalovirus infection in steroid-refractory ulcerative colitis: a case-control study. Am J Surg Pathol. 2004;28:365–373. doi: 10.1097/00000478-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Kuwabara A, Okamoto H, Suda T, Ajioka Y, Hatakeyama K. Clinicopathologic characteristics of clinically relevant cytomegalovirus infection in inflammatory bowel disease. J Gastroenterol. 2007;42:823–829. doi: 10.1007/s00535-007-2103-3. [DOI] [PubMed] [Google Scholar]
- 30.Barahona-Garrido J, Martínez-Benítez B, Espinosa-Cárdenas E, Sarti HM, Gutiérrez-Manjarrez JI, Aguirre-Gutiérrez R, Tellez-Avila FI, Coss-Adame E, García-Juárez I, Yamamoto-Furusho JK. Cytomegalovirus infection in patients who required colectomy for toxic megacolon or severe steroid-refractory ulcerative colitis. Dig Dis Sci. 2010;55:867–868. doi: 10.1007/s10620-009-1109-5. [DOI] [PubMed] [Google Scholar]
- 31.Cottone M, Pietrosi G, Martorana G, Casà A, Pecoraro G, Oliva L, Orlando A, Rosselli M, Rizzo A, Pagliaro L. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol. 2001;96:773–775. doi: 10.1111/j.1572-0241.2001.03620.x. [DOI] [PubMed] [Google Scholar]
- 32.Al-Zafiri R, Gologan A, Galiatsatos P, Szilagyi A. Cytomegalovirus complicating inflammatory bowel disease: a 10-year experience in a community-based, university-affiliated hospital. Gastroenterol Hepatol (N Y) 2012;8:230–239. [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima T, Watanabe T, Hata K, Shinozaki M, Yokoyama T, Nagawa H. Cytomegalovirus infection in ulcerative colitis. Scand J Gastroenterol. 2006;41:706–711. doi: 10.1080/00365520500408584. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi Y, Tange T. Prevalence of cytomegalovirus infection in inflammatory bowel disease patients. Dis Colon Rectum. 2004;47:722–726. doi: 10.1007/s10350-003-0117-3. [DOI] [PubMed] [Google Scholar]
- 35.Eyre-Brook IA, Dundas S. Incidence and clinical significance of colonic cytomegalovirus infection in idiopathic inflammatory bowel disease requiring colectomy. Gut. 1986;27:1419–1425. doi: 10.1136/gut.27.12.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maconi G, Colombo E, Zerbi P, Sampietro GM, Fociani P, Bosani M, Cassinotti A, Casini V, Russo A, Ardizzone S, et al. Prevalence, detection rate and outcome of cytomegalovirus infection in ulcerative colitis patients requiring colonic resection. Dig Liver Dis. 2005;37:418–423. doi: 10.1016/j.dld.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Maher MM, Nassar MI. Acute cytomegalovirus infection is a risk factor in refractory and complicated inflammatory bowel disease. Dig Dis Sci. 2009;54:2456–2462. doi: 10.1007/s10620-008-0639-6. [DOI] [PubMed] [Google Scholar]
- 38.Kim YS, Kim YH, Kim JS, Cheon JH, Ye BD, Jung SA, Park YS, Choi CH, Jang BI, Han DS, et al. Cytomegalovirus infection in patients with new onset ulcerative colitis: a prospective study. Hepatogastroenterology. 2012;59:1098–1101. doi: 10.5754/hge10217. [DOI] [PubMed] [Google Scholar]
- 39.D’Ovidio V, Vernia P, Gentile G, Capobianchi A, Marcheggiano A, Viscido A, Martino P, Caprilli R. Cytomegalovirus infection in inflammatory bowel disease patients undergoing anti-TNFalpha therapy. J Clin Virol. 2008;43:180–183. doi: 10.1016/j.jcv.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Yi F, Zhao J, Luckheeram RV, Lei Y, Wang C, Huang S, Song L, Wang W, Xia B. The prevalence and risk factors of cytomegalovirus infection in inflammatory bowel disease in Wuhan, Central China. Virol J. 2013;10:43. doi: 10.1186/1743-422X-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavagna A, Bergallo M, Daperno M, Sostegni R, Ravarino N, Crocellà L, Ramella A, Rocca R, Torchio B, Cavallo R, et al. The hazardous burden of Herpesviridae in inflammatory bowel disease: the case of refractory severe ulcerative colitis. Dig Liver Dis. 2006;38:887–893. doi: 10.1016/j.dld.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Yoshino T, Nakase H, Ueno S, Uza N, Inoue S, Mikami S, Matsuura M, Ohmori K, Sakurai T, Nagayama S, et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007;13:1516–1521. doi: 10.1002/ibd.20253. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto S, Yoshida Y. What are the factors that affect hospitalization and surgery for aggravation of ulcerative colitis? Eur J Gastroenterol Hepatol. 2014;26:282–287. doi: 10.1097/MEG.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 44.Kim JW, Boo SJ, Ye BD, Kim CL, Yang SK, Kim J, Kim SA, Park SH, Park SK, Yang DH, et al. Clinical utility of cytomegalovirus antigenemia assay and blood cytomegalovirus DNA PCR for cytomegaloviral colitis patients with moderate to severe ulcerative colitis. J Crohns Colitis. 2014;8:693–701. doi: 10.1016/j.crohns.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Minami M, Ohta M, Ohkura T, Ando T, Ohmiya N, Niwa Y, Goto H. Cytomegalovirus infection in severe ulcerative colitis patients undergoing continuous intravenous cyclosporine treatment in Japan. World J Gastroenterol. 2007;13:754–760. doi: 10.3748/wjg.v13.i5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kishore J, Ghoshal U, Ghoshal UC, Krishnani N, Kumar S, Singh M, Ayyagari A. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol. 2004;53:1155–1160. doi: 10.1099/jmm.0.45629-0. [DOI] [PubMed] [Google Scholar]
- 47.de Saussure P, Lavergne-Slove A, Mazeron MC, Alain S, Matuchansky C, Bouhnik Y. A prospective assessment of cytomegalovirus infection in active inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:1323–1327. doi: 10.1111/j.1365-2036.2004.02273.x. [DOI] [PubMed] [Google Scholar]
- 48.do Carmo AM, Santos FM, Ortiz-Agostinho CL, Nishitokukado I, Frota CS, Gomes FU, Leite AZ, Pannuti CS, Boas LS, Teixeira MG, et al. Cytomegalovirus infection in inflammatory bowel disease is not associated with worsening of intestinal inflammatory activity. PLoS One. 2014;9:e111574. doi: 10.1371/journal.pone.0111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper HS, Raffensperger EC, Jonas L, Fitts WT. Cytomegalovirus inclusions in patients with ulcerative colitis and toxic dilation requiring colonic resection. Gastroenterology. 1977;72:1253–1256. [PubMed] [Google Scholar]
- 50.Lévêque N, Brixi-Benmansour H, Reig T, Renois F, Talmud D, Brodard V, Coste JF, De Champs C, Andréoletti L, Diebold MD. Low frequency of cytomegalovirus infection during exacerbations of inflammatory bowel diseases. J Med Virol. 2010;82:1694–1700. doi: 10.1002/jmv.21877. [DOI] [PubMed] [Google Scholar]
- 51.Criscuoli V, Rizzuto MR, Montalbano L, Gallo E, Cottone M. Natural history of cytomegalovirus infection in a series of patients diagnosed with moderate-severe ulcerative colitis. World J Gastroenterol. 2011;17:633–638. doi: 10.3748/wjg.v17.i5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alain S, Ducancelle A, Le Pors MJ, Mazeron MC, de Saussure P, Bouhnik Y, Lavergne A. Cytomegalovirus infection in patients with active inflammatory bowel disease. J Clin Virol. 2005;33:180–182. doi: 10.1016/j.jcv.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Kim YS, Kim YH, Kim JS, Cheon JH, Ye BD, Jung SA, Park YS, Choi CH, Jang BI, Han DS, et al. The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: a prospective multicenter study. J Clin Gastroenterol. 2012;46:51–56. doi: 10.1097/MCG.0b013e3182160c9c. [DOI] [PubMed] [Google Scholar]
- 54.Domènech E, Vega R, Ojanguren I, Hernández A, Garcia-Planella E, Bernal I, Rosinach M, Boix J, Cabré E, Gassull MA. Cytomegalovirus infection in ulcerative colitis: a prospective, comparative study on prevalence and diagnostic strategy. Inflamm Bowel Dis. 2008;14:1373–1379. doi: 10.1002/ibd.20498. [DOI] [PubMed] [Google Scholar]
- 55.Iyer VH, Augustine J, Pulimood AB, Ajjampur SS, Ramakrishna BS. Correlation between coinfection with parasites, cytomegalovirus, and Clostridium difficile and disease severity in patients with ulcerative colitis. Indian J Gastroenterol. 2013;32:115–118. doi: 10.1007/s12664-012-0302-1. [DOI] [PubMed] [Google Scholar]
- 56.Roblin X, Pillet S, Berthelot P, Del Tedesco E, Phelip JM, Chambonnière ML, Peyrin-Biroulet L, Pozzetto B. Prevalence of cytomegalovirus infection in steroid-refractory Crohn’s disease. Inflamm Bowel Dis. 2012;18:E1396–E1397. doi: 10.1002/ibd.21907. [DOI] [PubMed] [Google Scholar]
- 57.Roblin X, Pillet S, Oussalah A, Berthelot P, Del Tedesco E, Phelip JM, Chambonnière ML, Garraud O, Peyrin-Biroulet L, Pozzetto B. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106:2001–2008. doi: 10.1038/ajg.2011.202. [DOI] [PubMed] [Google Scholar]
- 58.Antonelli E, Baldoni M, Giovenali P, Villanacci V, Essatari M, Bassotti G. Intestinal superinfections in patients with inflammatory bowel diseases. J Crohns Colitis. 2012;6:154–159. doi: 10.1016/j.crohns.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Aarnio MT, Böhm JP, Nuorva KP, Pitkänen RI, Kuopio TH, Voutilainen ME. Absence of cytomegalovirus from the gastrointestinal tract of patients with active Crohn’s disease. In Vivo. 2012;26:151–155. [PubMed] [Google Scholar]
- 60.Kim YS, Kim YH, Kim JS, Jeong SY, Park SJ, Cheon JH, Ye BD, Jung SA, Park YS, Choi CH, et al. Long-term outcomes of cytomegalovirus reactivation in patients with moderate to severe ulcerative colitis: a multicenter study. Gut Liver. 2014;8:643–647. doi: 10.5009/gnl13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lavagna A, Bergallo M, Daperno M, Sostegni R, Costa C, Leto R, Crocellà L, Molinaro G, Rocca R, Cavallo R, et al. Infliximab and the risk of latent viruses reactivation in active Crohn’s disease. Inflamm Bowel Dis. 2007;13:896–902. doi: 10.1002/ibd.20131. [DOI] [PubMed] [Google Scholar]
- 62.Matsuoka K, Iwao Y, Mori T, Sakuraba A, Yajima T, Hisamatsu T, Okamoto S, Morohoshi Y, Izumiya M, Ichikawa H, et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331–337. doi: 10.1111/j.1572-0241.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 63.Nakase H, Matsumura K, Yoshino T, Chiba T. Systematic review: cytomegalovirus infection in inflammatory bowel disease. J Gastroenterol. 2008;43:735–740. doi: 10.1007/s00535-008-2246-x. [DOI] [PubMed] [Google Scholar]
- 64.Nakase H, Honzawa Y, Toyonaga T, Yamada S, Minami N, Yoshino T, Matsuura M. Diagnosis and treatment of ulcerative colitis with cytomegalovirus infection: importance of controlling mucosal inflammation to prevent cytomegalovirus reactivation. Intest Res. 2014;12:5–11. doi: 10.5217/ir.2014.12.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langner C, Magro F, Driessen A, Ensari A, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, Fries W, et al. The histopathological approach to inflammatory bowel disease: a practice guide. Virchows Arch. 2014;464:511–527. doi: 10.1007/s00428-014-1543-4. [DOI] [PubMed] [Google Scholar]
- 66.Boom R, Sol C, Weel J, Lettinga K, Gerrits Y, van Breda A, Wertheim-Van Dillen P. Detection and quantitation of human cytomegalovirus DNA in faeces. J Virol Methods. 2000;84:1–14. doi: 10.1016/s0166-0934(99)00127-5. [DOI] [PubMed] [Google Scholar]
- 67.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 68.Rahier JF, Ben-Horin S, Chowers Y, Conlon C, De Munter P, D’Haens G, Domènech E, Eliakim R, Eser A, Frater J, et al. European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009;3:47–91. doi: 10.1016/j.crohns.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101:2857–2865. doi: 10.1111/j.1572-0241.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 70.Boivin G, Handfield J, Toma E, Murray G, Lalonde R, Tevere VJ, Sun R, Bergeron MG. Evaluation of the AMPLICOR cytomegalovirus test with specimens from human immunodeficiency virus-infected subjects. J Clin Microbiol. 1998;36:2509–2513. doi: 10.1128/jcm.36.9.2509-2513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ayre K, Warren BF, Jeffery K, Travis SP. The role of CMV in steroid-resistant ulcerative colitis: A systematic review. J Crohns Colitis. 2009;3:141–148. doi: 10.1016/j.crohns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 73.Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis. 2010;16:1620–1627. doi: 10.1002/ibd.21275. [DOI] [PubMed] [Google Scholar]
- 74.Park SH, Yang SK, Hong SM, Park SK, Kim JW, Lee HJ, Yang DH, Jung KW, Kim KJ, Ye BD, et al. Severe disease activity and cytomegalovirus colitis are predictive of a nonresponse to infliximab in patients with ulcerative colitis. Dig Dis Sci. 2013;58:3592–3599. doi: 10.1007/s10620-013-2828-1. [DOI] [PubMed] [Google Scholar]
- 75.Xue M, Chen SJ, Wang LJ, Du Y, Si JM. Cytomegalovirus: a probable cause of steroid-refractory ulcerative colitis. J Dig Dis. 2013;14:160–165. doi: 10.1111/1751-2980.12037. [DOI] [PubMed] [Google Scholar]
- 76.Sager K, Alam S, Bond A, Chinnappan L, Probert CS. Review article: cytomegalovirus and inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:725–733. doi: 10.1111/apt.13124. [DOI] [PubMed] [Google Scholar]
- 77.Rahbar A, Boström L, Lagerstedt U, Magnusson I, Söderberg-Naucler C, Sundqvist VA. Evidence of active cytomegalovirus infection and increased production of IL-6 in tissue specimens obtained from patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2003;9:154–161. doi: 10.1097/00054725-200305000-00002. [DOI] [PubMed] [Google Scholar]


