Abstract
Surgical resection is the only option of cure for patients with metastatic colorectal cancer (CRC). However, the risk of recurrence within 18 mo after metastasectomy is around 75% and the liver is the most frequent site of relapse. The current international guidelines recommend an adjuvant therapy after surgical resection of CRC metastases despite the lower level of evidence (based on the quality of studies in this setting). However, there is still no standard treatment and the effective role of an adjuvant therapy remains controversial. The aim of this review is to report the state-of-art of systemic chemotherapy and regional chemotherapy with hepatic arterial infusion in the management of patients after resection of metastases from CRC, with a literature review and meta-analysis of the relevant randomized controlled trials.
Keywords: Liver metastases, Adjuvant chemotherapy, Metastasectomy, Colorectal cancer, Adjuvant hepatic artery infusion
Core tip: Surgical resection is the only option of cure for patients with metastatic colorectal cancer (CRC). The risk of recurrence within 18 mo after metastasectomy is about 75% and the liver is the main organ involved. However, there is still no standard treatment and the effective role of adjuvant therapy remains controversial. The aim of this review is to summarize current knowledge on the role of systemic chemotherapy and regional chemotherapy with hepatic arterial infusion in the management of patients after resection of metastases from CRC.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer worldwide and is responsible for 8% of cancer-related deaths in men and 9% in women[1].
About 80% of patients with CRC have localized and resectable disease at diagnosis and, depending on the pathological stage, the 5-year survival rate is 90% in stage I, 70%-80% in stage II and 40%-65% in stage III. The risk of recurrence also depends on the pathological stage of the primary tumor (30% in stage II and 50% in stage III) and is higher within the first two years after surgery[2]. The most frequent sites of CRC recurrence are liver, abdominal lymph nodes, peritoneum and lung. In 30%-40% of patients with advanced CRC, the liver represents the only site of metastases: 25% of these patients present synchronous liver metastases at diagnosis, while 45%-50% of patients with stage II-III develop liver metastases within two years after the primary resection[3-5]. The current management of unresectable metastatic CRC consists of systemic chemotherapy involving various agents, alone or in combination. The choice of therapy is based on several factors, namely the performance status (PS) of patients and the goals of treatment.
When feasible, surgical resection is the treatment of choice for patients with liver or lung metastases with a survival rate ranging from 25% to 50%[6]. The management of patients with resectable metastatic CRC is a typical example of a multidisciplinary task involving both oncologists and surgeons. In recent years, the availability of even more effective therapeutic regimens together with the improvement of surgical techniques have significantly improved the chance of survival for patients with resectable stage IV CRC. However, around 75% of patients undergoing metastasectomy develop recurrence within 18 mo after the surgery and the liver is the most frequent site of relapse[7]. Therefore, effective therapeutic strategies to reduce the risk of relapse in this subgroup of patients are urgently needed.
To date, no standard treatments have been established and the effective role of adjuvant therapy remains controversial. In addition, tumor clonal heterogeneity, a hallmark of most human cancers, may complicate the choice of the best adjuvant treatment for CRC[8]. Indeed, the optimal adjuvant therapy for the primary tumor may not be the best treatment for metastases, given that the biological tumor background may significantly differ between primary and metastastic sites[9,10].
The aim of this review is to report the state-of-art on the role of systemic chemotherapy and regional chemotherapy with hepatic arterial infusion (HAI) in the management of patients after resection of metastases from CRC.
SYSTEMIC CHEMOTHERAPY IN CRC
Strategy of systemic treatment in metastatic disease as the backbone of adjuvant therapy
To date, the systemic treatment of advanced CRC has been based on four main cytotoxic agents: fluoropyrimidine [intravenous 5-fluorouracil/leucovorin (5-FU/LV) and oral fluoropyrimidine capecitabine], oxaliplatin and irinotecan. More recently, new biological targeted agents (bevacizumab, cetuximab, panitumumab, regorafenib and aflibercept) have been added to the chemotherapy armamentarium[3,5].
In patients with good PS and without contraindications, a combination therapy is recommended, whereas a monotherapy should be preferred for elderly patients or those with significant comorbidities or in poor clinical condition. The efficacy of all these agents, alone or in combination, has been supported by studies showing an improvement in overall survival (OS) and response rate (RR) in patients who received systemic treatment. Based on these results, the current international guidelines (European Society for Medical Oncology-ESMO guidelines and National Comprehensive Cancer Network-NCCN guidelines) have recognized at least three lines of therapy, using these agents in various combinations and schedules[3,5]. Several clinical trials have directly compared these treatments, but the large number of potential combinations makes it impossible to define the best therapeutic strategy. However, some key points can help the oncologist select the best chemotherapy for patients with metastatic CRC.
Treatments with 5-FU/LV, oxaliplatin and irinotecan (used sequentially or together upfront) have demonstrated a better outcome in terms of objective response and survival. Some authors showed an increased median OS and a longer progression-free survival (PFS) in patients treated with combinations of 5-FU/LV plus oxaliplatin (FOLFOX) or irinotecan (FOLFIRI), compared with those who received FU/LV alone[11-13]. Subsequently, Grothey et al[14] analyzed data from 11 published phase III trials to assess the effectiveness of these three chemotherapeutic agents in advanced CRC. They found that the median OS reported in these trials was significantly correlated with the percentage of patients receiving all three drugs in the course of their treatment. A similar analysis also comprising the targeted therapy is currently lacking. Finally, a randomized trial has shown that the combination of infusional 5-FU/LV, oxaliplatin and irinotecan (FOLFOXIRI) improves the RR, PFS, and OS compared with FOLFIRI. Due to the greater but manageable toxicity, the use of this chemotherapy regime can be limited to a small group of patients based on their PS and the absence of contraindications[15].
The second key point is that oral capecitabine can be used as an alternative to intravenous 5-FU/LV, as demonstrated in some randomized trials[16]. The combination of capecitabine and oxaliplatin (XELOX) may be used as an alternative to 5-FU/LV/oxaliplatin, with similar efficacy and safety[17,18]. The association of capecitabine and irinotecan (XELIRI) has also demonstrated similar results, but it is burdened with greater toxicity than 5-FU/LV/irinotecan and therefore is less used[19].
For biological targeted agents, the presence of activating mutations of KRAS/NRAS/BRAF genes is now considered the main predictor of response to anti-epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab, because these mutations confer resistance to these drugs and in some cases may also be associated with a detrimental effect[20-22]. Therefore, mutational analysis of the KRAS/NRAS/BRAF line is important in patients with advanced CRC and the use of cetuximab and panitumumab should be limited to patients in whom a RAS gene mutation is excluded. A recent meta-analysis and the TRIBE trial showed that the association of bevacizumab with different chemotherapy regimens in patients with advanced CRC (compared with patients receiving systemic chemotherapy alone) leads to an improved PFS and OS that could exceed 30 mo[23,24]. Therefore, in the absence of contraindications, bevacizumab plus chemotherapy can be used in both first line and second line regimens in patients not previously treated with anti-angiogenic drugs.
Another key point is that the anti-EGFR drugs should not be combined with bevacizumab, as shown by two randomized trials in which the concomitant use of bevacizumab and cetuximab resulted in a detrimental effect in the combination arm[25,26].
Finally, the treatment strategy for patients with advanced CRC should consider if the disease is potentially curable with a combination of chemotherapy and surgery or merely defensive when the goal of the treatment is only increased survival. In the first case, a combination of multiple drugs (chemotherapy +/- target therapy), referred to as “conversion therapy”, is needed to shrink the metastatic tumor mass until it is resectable.
Adjuvant chemotherapy in resectable primary CRC
About 80% of patients with CRC have resectable disease at diagnosis and surgery is the main treatment option with curative intent in these patients[2]. As the risk of recurrence depends on the pathological stage of the primary tumor (30% in stage II and 50% in stage III), the rationale of adjuvant therapy is generally to reduce this risk, improving the survival rate[2].
Adjuvant systemic chemotherapy is currently recommended for stage III CRC patients and for high risk stage II, who present at least one of the following negative prognostic factors: contiguity infiltration of neighboring organs (T4b), grading G3, inadequate number of lymph nodes analyzed (12), vascular, lymphatic and/or perineural invasion, clinical presentation with perforation or occlusion[5,27]. In these patients, adjuvant treatment reduces the risk of recurrence by 5% (78.2% vs 72.9% of 3-year DFS), with a total gain of 1% survival (87.7% vs 86.6% of 3-year OS)[2,28]. The adjuvant chemotherapy regimens are based on therapies that have proven their effectiveness in the advanced setting (Table 1). Conversely, not all treatments commonly used in metastatic disease have maintained their effectiveness when used in the adjuvant setting.
Table 1.
Adjuvant systemic chemotherapy after primary resectable colorectal cancer
| Trials | No. of patients | Schedules | DFS | P value | OS | P value |
| Oxaliplatin-based regimes | ||||||
| X-ACT | 1987 | CAPECITABINE (1004) | 64.2% | 0.12 | 81.3% | 0.07 |
| (Twelves et al[32], 2005) | 5FU/LV (983) | 60.6% | 77.6% | |||
| NSABP C-07 | 2407 | 5FU/LV (1207) | 67.0% | 0.0034 | - | - |
| (Kuebler et al[30], 2007) | FLOX (1200) | 73.2% | ||||
| MOSAIC | 2246 | 5FU/LV (1123) | 67.4% | 0.003 | 68.7% | 0.46 |
| (André et al[29], 2009) | FOLFOX4 (1123) | 73.3% | 78.5% | |||
| XELOXA | 1886 | XELOX (944) | 70.9% | 0.045 | 77.6% | 0.15 |
| (Haller et al[31], 2011) | 5FU/LV (942) | 66.5% | 74.2% | |||
| Irinotecan-based regimes | ||||||
| CALGB-89803 | 1264 | Irinotecan + 5FU/LV (635) | 59.0% | 0.85 | 64.0% | 0.74 |
| (Saltz et al[35], 2007) | 5FU/LV (629) | 61.0% | 67.0% | |||
| PETACC-3 | 2982 | Irinotecan + 5FU/LV (1485) | 56.7% | 0.106 | 73.6% | 0.094 |
| (Van Cutsem et al[36], 2009) | 5FU/LV (1497) | 54.3% | 71.3% | |||
| Bevacizumab + chemotherapy | ||||||
| NSABP C-08 | 2710 | FOLFOX6 + Bevacizumab (1354) | 77.4% | 0.15 | - | - |
| (Allegra et al[37], 2011) | FOLFOX6 (1356) | 75.5% | ||||
| AVANT | 2867 | FOLFOX4 + Bevacizumab (960) | 73.0% | 0.07 | 81.0% | 0.02 |
| (de Gramont et al[38], 2012) | FOLFOX4 (955) | 76.0% | 85.0% | |||
| XELOX + Bevacizumab (952) | 75.0% | 0.44 | 82.0% | 0.21 | ||
| Cetuximab + chemotherapy | ||||||
| NCCTG NO147 | 2686 | FOLFOX6 + Cetuximab (1349) | 71.5% | 0.08 | 72.5% | 0.03 |
| (Alberts et al[41], 2012) | FOLFOX6 (1337) | 74.6% | 86.2% | |||
| PETACC-8 | 337 | FOLFOX4 + Cetuximab (169) | 60.45 | 0.60 | 46.0% | 0.064 |
| (Taieb et al[42], 2012) | FOLFOX4 (168) | 60.7% | 36.0% | |||
| Oral flupropyrimidine in monotherapy | ||||||
| JCOG02051 | 1092 | 5-FU/LV (550) | 74.3% | 0.0236 | - | - |
| (Shimada et al[33], 2014) | UFT/LV (551) | 73.6% | ||||
| ACT-CC1 | 1518 | S-1 (758) | 75.5% | < 0.01 | - | - |
| (Yoshida et al[34], 2014) | UFT/LV (760) | 72.5% | ||||
These trials were randomized, controlled non-inferiority studies. 5-FU/LV: 5-fluorouracil/leucovorin; FOLFOX: 5-FU/LV plus oxaliplatin; DFS: Disease-free survival; OS: Overall survival; UFT: Tegafur-uracil.
The combination of fluoropyrimidine (5-FU/LV or capecitabine) and oxaliplatin is the adjuvant treatment recommended by the current international guidelines[5,27]. The MOSAIC study randomly assigned 1123 patients to receive 5-FU/LV or FOLFOX4 in the post-operative adjuvant setting[28]. After a 6-year follow-up, the advantage in DFS for patients treated with FOLFOX4 was 73.3% (vs 67.4%) with an improvement of OS for patients in stage III (72.9% vs 68.7%, compared with 5-FU/LV alone treatment)[29]. Similar results were observed in a phase III trial (NSABP C-07) evaluating FLOX (bolus of 5-FU/LV plus oxaliplatin) vs 5-FU/LV alone[30]. The XELOXA phase III study compared XELOX (capecitabine plus oxaliplatin) with bolus 5-FU/LV in stage III patients: the 3-year DFS rates were 70.9% and 66.5%, respectively, but after 5 years of follow-up the OS had not yet reached statistical significance (P = 0.14)[31]. In patients with non-optimal PS, monotherapy with fluoropyrimidine can be considered a viable alternative to the doublet chemotherapy and capecitabine has shown a similar efficacy and a better tolerability than intravenous 5-FU/LV[32]. Recently, two Japanese phase III trials (JCOG0205, ACTS-CC) showed the safety and efficacy of other oral fluoropyrimidines as adjuvant treatments for patients with resectable CRC[33,34]. The authors demonstrated the non-inferiority of tegafur-uracil/leucovorin (UFT/LV) and S-1 (tegafur-gimeracil-oteracil) to 5-FU/LV in terms of DFS.
According to the efficacy demonstrated in patients with metastatic CRC, the irinotecan-based regimes were also assessed in the adjuvant setting, but the results failed to demonstrate any advantage. Two randomized trials (CALGB-89803, PETACC-3) comparing bolus 5-FU/LV plus irinotecan to only 5-FU/LV did not find differences in terms of DFS and OS[35,36].
Bevacizumab has also reached negative results in the adjuvant setting as in the NSABP C-08 trial in which 2710 patients were randomized to receive FOLFOX6 plus Bevacizumab or FOLFOX6 alone[37]. The AVANT study also showed the negative effect of bevacizumab in the adjuvant setting, comparing the outcome of patients treated with FOLFOX4, FOLFOX4 plus bevacizumab and bevacizumab plus XELOX after surgery[38].
Similarly, while cetuximab plus FOLFOX are associated with an increased objective response rate and an improvement of PFS in metastatic CRC compared with the cytotoxic doublets alone[39,40], both NCCTG NO147[41] and PETACC-8 trials[42] demonstrated a detrimental effect of cetuximab in the adjuvant setting. Therefore, both irinotecan-based regime combinations and biological targeted agents should be ruled out in the adjuvant setting of primary CRC.
SURGICAL RESECTION OF METASTASES
Radical surgical resection (R0) is the only option of cure for patients with isolated liver or lung metastases[7]. The median OS of patients after radical surgical resection of liver metastases, upfront or previously treated with a preoperative chemotherapy, ranges from 22 mo to 5 years, with a survival rate of 70% at one year, 36% in 3 years and 25% at 5 years[4,43-48]. However, around 75% of patients develop recurrence within 18 mo after the first resection of CRC metastases and the liver is the most frequent site of relapse[7]. Recent studies have shown that the survival of patients undergoing repeated hepatic resections is comparable to that of the first metastasectomy[49,50], and, in a small case series, the survival benefit of a third hepatectomy seems to be similar to that achieved by the first and second surgery[51,52]. Finally, it has been shown that highly selected metastatic CRC patients can achieve longer survival even after third metastasectomy, compared with patients treated with medical therapy alone[53].
Therefore, over the years, the gain in OS of metastatic CRC patients has been mainly thanks to the improvement of surgical techniques that have revised the definition of respectability, no longer limited by number or size of metastases, improvements to imaging techniques and the integration of pre- and post-operative chemotherapy with more active agents. In recent years, several prognostic scores have been proposed for a better selection of those patients who may benefit most from the integration of surgery with systemic treatments[54-59].
Some prognostic factors are shared by all scoring scales. In particular, extrahepatic disease, node-positive primary disease, the size and number of hepatic metastases, an interval less than 2 years from primary tumor to metastases and high pre-operative CEA levels have proved unfavorable prognostic factors. However, the predictive value of all these scores has not been assessed in the specific group of patients receiving neoadjuvant chemotherapy before resection of metastases, and recent studies have shown that none of these factors are reliable prognostic tools[60-62]. Even in the era of modern chemotherapy, negative surgical margins remain an important determinant of survival for patients undergoing hepatectomy for CRC liver metastases, but most reports claim the width of a negative surgical margin does not affect outcome[63]. Although there is still no consensus on the definition of R1, the width of surgical margins has been gradually reduced to 0.1 mm. A recent French study showed that in multivariate analysis positive surgical margins (R1 defined as resection below 1 mm) did not constitute a negative prognostic factor of survival per se, but may be related to more aggressive disease[64]. Conversely, other studies confirmed the role of resection margin status as an important determinant of OS. Angelsen et al[65] reported that resection margins below 5 mm may increase the risk for local recurrence and shorten the time to recurrence. A United States study showed a better 5-year OS in patients who underwent R0 liver resection (tumor-free margin ≥ 1 mm) compared with R1 resection (< 1 mm)[66]. A more recent analysis by Sadot et al[67] compared 2368 patients who underwent R1 (0 mm) or R0 hepatic resection (divided into three groups: 0.1-0.9 mm, 1-9 mm, ≥ 10 mm) for CRC liver metastases and demonstrated that all margin widths, including sub-mm, correlated with improved OS compared with R1 resection (P = 0.05), whereas there was no significant difference in OS between 1-9 mm and ≥ 10 mm groups.
Interestingly, wedge resection and anatomic resection yield similar positive surgical margin and recurrence rates, recurrence patterns, and 5-year OS rates, therefore both approaches are considered equivalent for patients with CRC liver metastases[68,69].
In recent years, accumulating evidence on the role of surgery also for lung CRC metastases has shown that, in well-selected patients, resection of solitary liver and lung metastases may provide long-term survival[70,71]. A recent Japanese retrospective study evaluated the clinical outcome of patients undergoing surgical resection of lung metastases, showing a 5-year OS of 65.7% and a 5-year DFS of 35.3%[72]. The main prognostic factors affecting the long-term outcome were negative surgical margins, the absence of mediastinal and hilar lymph node involvement, and a solitary metastasis. Moreover, the 5-year OS may be influenced by the histologic characteristics of the primary tumor or metastases[73-75]. Taken together all these results show that resection of lung metastases may improve the survival rate in well-selected patients with metastatic CRC.
On the basis of all the results outlined above, it is well established that the selection of patients with metastastic CRC eligible for surgery is mandatory to identify those patients with limited and resectable or potentially resectable disease representing the subset of patients who could really benefit from surgery. In the case of patients with upfront resectable disease, the indication for neo-adjuvant chemotherapy is still debated as no OS difference has been found with the addition of peri-operative chemotherapy compared with surgery alone for patients with resectable CRC liver metastases[76]. Patients with potentially resectable disease should be referred to intensive systemic treatments, defined as “conversion therapies”, associated with a higher disease RR. However, the impact of pre-operative chemotherapy on the long-term outcome of radically resected metastatic CRC patients is still undefined and neither the type of conventional regimen nor the combined use of targeted agents seems to independently influence outcome following resection[77]. It is noteworthy that pre-operative chemotherapy can induce regimen-specific liver damage, increasing the risk of mortality after liver resection. A retrospective study by Vauthey et al[78] evaluated the postoperative outcome of 406 patients after metastasectomy with or without pre-operative chemotherapy (5-FU/LV alone, oxaliplatin + 5-FU/LV, or irinotecan + 5-FU/LV). In pre-operative chemotherapy group, oxaliplatin was associated with sinusoidal injury and irinotecan with steatohepatitis, but only irinotecan-based regimes also increased the 90-d mortality rate compared with surgery alone. These data were confirmed by Pawlik et al[79], who found regimen-specific hepatic injury in about 20%-30% of their patients treated with pre-operative chemotherapy.
New surgical techniques have recently been considered to treat patients with a small future liver remnant. Portal vein embolization and two-stage hepatectomy is based on hypertrophy of the future liver remnant caused by contra-lateral portal vein occlusion. The functional reserve of the liver grows within 2-4 wk and the patients may be subjected to subsequent metastasectomy[80,81]. Instead, associating liver partition and portal vein occlusion for staged hepatectomy (ALPPS) combined portal vein ligation with in situ parenchymal transaction, reducing the risk of tumor progression during the period of liver regeneration and increasing the resectability rate[82]. A multicenter Italian study showed no significant difference in feasibility between these two surgical techniques, but the overall complication rate was higher in the ALPPS group[83]. Consequently, ALPPS should be proposed with caution in patients with CRC liver metastases and small functional liver reserve.
In addition to surgical techniques, ablative therapies [such as radiofrequency ablation (RFA), cryosurgery or microwave] can be used as potentially curative treatments for CRC liver metastases. In several studies, the 5-year OS ranged between 20%-30% in patients with advanced CRC who underwent RFA[84,85]. Pawlik et al[86]’s study was the first to evaluate the outcome of a large series of patients treated with combined hepatic resection and RFA. More recently, Eltawil et al[87] estimated the recurrence rate of 174 patients with CRC liver metastases (24 undergoing liver resection with RFA and 150 undergoing surgery alone). The median OS were 38 mo vs 52 mo and the median RFS were 7.4 and 13 mo, without statistically significant differences (P = 0.95 and P = 0.08, respectively). These studies suggested that RFA combined with liver resection may enhance long-term survival in a select group of patients.
To date, no randomized trials have compared RFA and surgery. A recent Cochrane review included 18 studies comparing RFA and any other treatment (10 observational, 7 clinical controlled trials and 1 randomized clinical trial)[88]. These data did not allow any definitive conclusion to be reached and are insufficient to recommend RFA as a radical treatment for CRC liver metastases.
Cryotherapy (in which liquid nitrogen or argon gas is delivered to the liver tumor) is another local ablative technique used to treat patients unsuitable for liver resection, alone or in combination with surgery. A retrospective United States study analyzed 158 patients with CRC liver metastases treated with surgery and/or ablation treatment. The ablation techniques were performed by radiofrequency ablation, cryotherapy and microwave ablation (total: 315 treated tumors). The local recurrence rate in the cryotherapy group was statistically significantly higher than in the RFA group both in univariate and multivariate analysis (P = 0.03 and P = 0.018, respectively)[89].
POST-METASTASECTOMY ADJUVANT SYSTEMIC CHEMOTHERAPY AND META-ANALYSIS
Adjuvant systemic chemotherapy after metastasectomy
The management of CRC patients after surgical resection of metastases is still debated. In these patients, the current international guidelines recommend an adjuvant strategy for 6 mo: postoperative adjuvant chemotherapy or peri-operative chemotherapy (3 mo before surgery and 3 mo after surgery)[3,5]. However, there is no standard treatment and the effective role of systemic adjuvant chemotherapy remains controversial.
The rationale for adjuvant chemotherapy post-metastasectomy is based on several studies (Table 2). The first studies comparing treatment with only controls had several dropouts and low accrual ratios. Langer et al[90] studied a group of CRC patients who underwent surgical resection of liver metastases and for the first time they compared metastasectomy alone vs metastasectomy followed by systemic 5-FU/LV treatment. DFS and OS were better in the adjuvant chemotherapy arm vs the surgery alone arm (4-year DFS was 45%, vs 35%, and 4-year OS was 57%, vs 47%) but the trial was prematurely closed due to slow accrual and statistical significance was not reached either for OS or PFS (P = 0.35 and P = 0.39, respectively). In another multicenter trial, Portier et al[91] randomized 171 patients after hepatic resection of metastases from CRC to control alone or to adjuvant systemic chemotherapy with 5-FU/LV. The authors observed an improvement in DFS for patients treated with 5-FU/LV compared with the control group (24.4 mo vs 17.6 mo, respectively, P = 0.028) but no statistically significant difference in OS was observed (P = 0.13). This trial was also stopped because of the slow accrual. A pooled analyses of these two trials showed a marginal statistical significance in favor of adjuvant chemotherapy 5-FU/LV-based regime, independently associated with both PFS and OS[92].
Table 2.
Systemic adjuvant chemotherapy studies after metastasectomy
| Ref. | No. of patients | Setting | Randomized study | Regimes of chemotherapy |
Outcomes |
||
| DFS | PFS | OS | |||||
| Controlled studies | |||||||
| Langer et al[90], 2002 | arm2 = 55 vs arm1 = 52 | Phase III | YES | 5-FU/LV vs surgery + 5-FU/LV (arm2 vs arm1) | 4-yr DFS:35% vs 45%(P = 0.35) HR = 1.28 (95%CI: 0.76-2.14) | - | 4-yr OS:47% vs 57% (P = 0.39)HR = 1.30 (95%CI: 0.71-2.36) |
| Portier et al[91], 2006 | 171 (86 vs 85) | Phase III | YES | 5-FU/LV vs surgery alone | 5-yr DFS: 33.5% vs 26.7% (P = 0.028) HR = 0.66 (95%CI: 0.46-0.96) | - | 5-yr OS:51.1% vs 41.9% (P = 0.13) HR = 0.73 (95%CI: 0.48-1.10) |
| Mitry et al[92], 2008 | 278 (138 vs 140) | Pooled analysis of two phase III studies | YES | 5-FU/LV vs surgery alone | Median DFS: 27.9 mo vs 18.8 mo (P = 0.058) 5-yr DFS: 36.7% vs 27.7% HR = 0.76 (95%CI: 0.57-1.01) | - | Median OS: 62.2 vs 47.3 mo (P = 0.095) 5-yr OS: 52.8% 39.6%HR = 0.76 (95%CI: 0.55-1.05) |
| Kanemitsu et al[94], 2009 | 300 | Phase II/III | YES | FOLFOX6 vs surgery alone | In progress (results not yet available) | ||
| Ychou et al[99], 2009 | 306 (153 vs 153) | Phase III | YES | FOLFIRI vs 5-FU/LV | 2-yr DFS: 50.7% vs 46.2% (P = 0.44) HR = 0.89 (95%CI: 0.66-1.19) | - | 3-yr OS: 72.7% vs 71.6% (P = 0.69) HR = 1.09 (95%CI: 0.72-1.64) |
| Kim et al[98], 2009 | 156 [58 + 48 + 50] | Retrospective | NO | Oxaliplatin regimes (group I); Irinotecanregimes (group II) orFluoropyrimidine alone(group III) | Median DFS:23.4, 14.1 and 16.3 mo(respectively, P = 0.088)HR group1 vs 3: 0.63 (95%CI: 0.39-1.03) HR group2 vs 3: 0.98 (95%CI: 0.61-1.56) | - | Median OS:51.2, 47.9 and 60 mo(respectively, P = 0.219) |
| Liu et al[100], 2010 | 50 [31 (17 + 14) vs 19] | Retrospective | NO | FOLFOX/FOLFIRI vs 5-FU/LV | 3-yr DFS: 50.8% vs 21.1% (P = 0.022) HR = 0.37 (95%CI: 0.15-0.94) | - | 3-yr OS: 85.7% vs 51.8% (P = 0.027)5-yr OS: 54.0% vs 34.6% (P = 0.027) HR = 0.27 (95%CI: 0.083-0.86) |
| Snoeren et al[106], 2010 | CAPOX + Bevacizumab vs CAPOX alone | In progress (results not yet available) | |||||
| Kemeny et al[104], 2011 | 73 (35 vs 38) | Phase II | YES | HAI/systemic therapy + BEVA vs HAI/systemic therapy alone | 4-yr DFS: 71% vs 83% (P = 0.4) | - | 4-yr OS: 81% vs 85% (P = 0.5) |
| Brandi et al[101], 2013 | 151 (78 vs 73) | Cohort study | NO | Oxaliplatinregimes or Irinotecan regimes vs surgery alone | Median DFS: 16 vs 9.7 mo(P = 0.014) 5-yr DFS: 17.4% vs 10.5% (P = 0.82) HR = 0.64 (95%CI: 0.46-0.90) | - | Median OS: 42 vs 39 mo (P = 0.8) |
| Turan et al[105], 2013 | 204 (87 vs 117) | Cohort study | NO | Irinotecan regimes or oxaliplatin regimes + bevacizumab vs chemotherapy alone | Median DFS: 14 vs 18 mo (P = 0.37) | - | Median OS: 43 vs 54 mo (P = 0.25) |
| Nordlinger et al[93], 20131 | 364 (171 vs 152) | Phase III study | YES | Peri-operative FOLFOX4 vs surgery alone | - | 3-yr PFS: 38.2% vs 30.3% (P = 0.0068) HR = 0.81 (95%CI: 0.64-1.02) | 5-yr OS:51.2% vs 47.8% (P = 0.3) HR = 0.88 (95%CI: 0.68-1.14) |
| Primrose et al[107], 20141 | 236 (119 vs 117) | Phase III | YES | FOLFOX/CAPOX + cetuximab vs FOLFOX/CAPOX alone | - | Median PFS: 14.1 vs 20.5 mo(P = 0.03) HR = 1.48 (95%CI: 1.04-2.12) | Median OS: 39.1 vs 32 mo (P = 0.16) HR = 1.49 (95%CI: 0.86-2.60) |
| Kobayashi et al[102], 2014 | 177 (88 vs 89) | Phase III | YES | UFT/LV vs surgery alone | - | 3-yr PFS: 38.6% vs 32.3% (P = 0.003) HR = 0.56 (95%CI: 0.38-0.83) | 3-yr OS: 82.8% vs 81.6% (P = 0.41) HR = 0.80 (95%CI: 0.48-1.35) |
| Non controlled studies | |||||||
| Kim et al[97], 2011 | 60 | Single armed | NO | FOLFOX6 | Median DFS: 32.8 mo (95%CI: 5.8-59.6)5-yr DFS: 39.20% | - | Median OS: 62.8 mo (95%CI: 44.1-81.3)5-yr OS: 55.5% |
| Kato et al[103], 2015 | 60 | Single armed | NO | S-1 | 1-yr DFS: 68.30%3-yr DFS: 47.40% | - | 1-yr OS: 96.70%3-yr OS: 80% |
| Nakayama et al[95], 2015 | 88 | Single armed | NO | Oxaliplatin regimes | 3-yr DFS: 54% | - | - |
| Katayose et al[96], 2015 | 49 | Phase II single armed | NO | mFOLFOX6 | 2-yr DFS: 59.20% | - | - |
The primary end-point was PFS because these studies evaluated the role of peri-operative chemotherapy, but some patients became ineligible for surgical treatment during the study. 5-FU/LV: 5-fluorouracil/leucovorin; FOLFOX: 5-FU/LV plus oxaliplatin; DFS: Disease-free survival; PFS: Progression-free survival; OS: Overall survival; UFT: Tegafur-uracil.
Cytotoxic doublets have also been studied in the adjuvant setting. Nordlinger et al[76] randomized 364 patients with resectable liver metastases from CRC. Comparing the combination of surgery and perioperative FOLFOX-4 treatment (6 cycles before and 6 cycles after surgery) with liver resection alone, they showed that the 3-year PFS was better in the chemotherapy group compared with controls. However, the gain in PFS did not affect the long-term OS: at a follow-up of 8.5 years, the median 5-year OS was 51.2% in the peri-operative chemotherapy group vs 47.8% in the surgery only group, without a significant difference between the two[93]. Several Japanese studies have examined the efficacy and safety of oxaliplatin-based adjuvant treatments. In a randomized, controlled phase II/III trial, Kanemitsu et al[94] compared hepatectomy followed by m-FOLFOX-6 adjuvant chemotherapy with surgery alone, but the final results are not yet available. Another two studies (a retrospective cohort study and a phase II non-controlled clinical trial) suggested that adjuvant chemotherapy after metastasectomy provides a benefit in DFS[95,96]. The same comparison was evaluated by Kim et al[97] in an uncontrolled study analyzing 60 patients who underwent oxaliplatin-regimen postoperative chemotherapy. In another study, the same authors compared the clinical outcomes of 156 patients treated with different chemotherapeutic regimes after metastasectomy from CRC: oxaliplatin/fluoropyrimidine (group I), irinotecan/fluoropyrimidine (group II) and fluoropyrimidine alone (group III). The median DFS was 23.4 mo in group I, 14.1 mo in group II and 16.3 mo in group III (P = 0.03). Therefore, oxaliplatin-based adjuvant chemotherapy seems to show a better DFS than the other two chemotherapeutic regimes, confirming the inefficacy or detrimental effect of irinotecan in the adjuvant setting post-metastasectomy, as already observed in the adjuvant setting after primary resection[98].
Other studies have evaluated the use of adjuvant irinotecan-based chemotherapy after hepatic resection of liver metastases from CRC. In a phase III study, Ychou et al[99] studied 306 patients treated with two different adjuvant chemotherapy regimens: FOLFIRI vs 5-FU/LV. Although the median DFS was 24.7 mo in the FOLFIRI group (vs 21.6 mo), this difference was not statistically significant (P = 0.44). Their study showed that the use of FOLFIRI after R0 resection added no benefit compared with only 5-FU/LV. Conversely, a retrospective study by Liu et al[100] showed that FOLFOX/FOLFIRI chemotherapy was associated with an improvement in DFS and OS compared with 5-FU/LV treatment alone. The median DFS was 34.3 mo and the median OS was 57.7 mo for patients treated with FOLFOX/FOLFIRI, vs 14.2 mo and 49 mo in the control group.
A recent study by our group analyzed 151 patients from two Italian centers, who underwent R0 resection of CRC liver or lung metastases (131 and 20 patients respectively): 78 patients received adjuvant chemotherapy for 6 mo after surgery and 73 underwent observation alone. The median DFS was 16 mo for patients treated with adjuvant chemotherapy, vs 9.7 mo for patients who underwent observation alone (P = 0.014). However, there were no differences in OS between the two groups of patients, probably due to the small sample size of the study[101].
Some recent studies have suggested the potential efficacy of other oral fluoropyrimidines also in the adjuvant setting post-metastasectomy. A phase III trial (UFT/LV trial) randomized 180 patients after metastasectomy to receive adjuvant UFT/LV chemotherapy or surgery alone. The 3-year DFS was 38.6% in UFT/LV group and 32.3% in surgery group (P = 0.003), while a not yet significant difference in the 3-year OS was observed (82.8% vs 81.6% respectively, P = 0.41)[102]. N-SOG 01 was an uncontrolled single-arm study reporting the outcome of 60 patients treated with adjuvant S-1 chemotherapy after resection of CRC liver metastases: the 1-year and 3-year DFS were 68.3% and 47.4%, respectively, and 1-year and 3-year OS were 96.7% and 80%[103].
A combination of biological targeted agents and chemotherapy improved the outcomes of metastatic CRC, whereas there is no evidence supporting their use in the adjuvant setting after metastasectomy. Kemeny et al[104] randomized 73 patients who underwent liver resection to adjuvant HAI plus systemic therapy with bevacizumab (BEV) or without bevacizumab (NoBEV). With a median follow-up of 30 mo, 4-year survival was 81% in patients treated with BEV vs 85% in the NoBEV group (P = 0.5). Therefore, the addition of BEV to HAI plus systemic chemotherapy does not improve survival, while the combination seems to be associated with an increased biliary toxicity. In a retrospective analysis by Turan et al[105], 204 patients who underwent resection of liver metastases were treated with fluoropyrimidine-based, irinotecan-based or oxaliplatin-based regimes, combined with or without BEV. The median OS and the median recurrence-free survival rates were similar in the BEV and NoBEV groups (P = 0.25 and P = 0.37, respectively). This study showed that there was no survival benefit of adding BEV to chemotherapy, and no difference between the various chemotherapy regimens. More recently, a randomized (still in progress) phase III trial compared the combination of BEV plus capecitabine + oxaliplatin (CAPOX) vs CAPOX alone as adjuvant treatment post-radical resection of liver metastases[106].
Finally, a phase III clinical trial randomized 236 WT-KRAS patients to receive chemotherapy with or without cetuximab before and after liver resection. PFS was 14.1 mo in the chemotherapy plus cetuximab group and 20.5 mo in the chemotherapy alone group, similarly to what happens in the adjuvant setting of primary CRC surgery. These results confirm the detrimental effect of cetuximab in the adjuvant post-metastasectomy setting, being associated with a shorter PFS[107].
Meta-analysis of randomized controlled studies
To better understand the role of adjuvant systemic therapy, we used Stata 12 SE (Stata Corporation, Texas, TX, United States) to perform a meta-analysis based on the only three randomized controlled trials. We pooled data judged to be homogeneous based on type of treatment, type of study, regime of chemotherapy and control group. The results were presented separately for types of outcome. In addition, we tested for statistical heterogeneity by means of the χ2 test. We considered a P-value less than 0.10 to indicate whether there was a problem with heterogeneity. Moreover, we quantified the degree of heterogeneity using the I2 statistic, where an I2 value of 25% to 50% indicated a low degree of heterogeneity, 50%-75% a moderate degree of heterogeneity, and ≥ 75% a high degree of heterogeneity[108]. Individual studies were pooled if sufficient data were available. When studies were statistically heterogeneous, they were combined using a random-effects model; otherwise, a fixed-effect model was used. The effect size was expressed as HR along with the 95%CI for all estimates (Figure 1). Although the analysis did not reach statistical significance (HR = 0.83: 95%CI: 0.68-1.02, P = 0.07), these data demonstrated a benefit of adjuvant therapy post-metastasectomy compared to surgery alone. Further studies are needed to confirm these findings.
Figure 1.
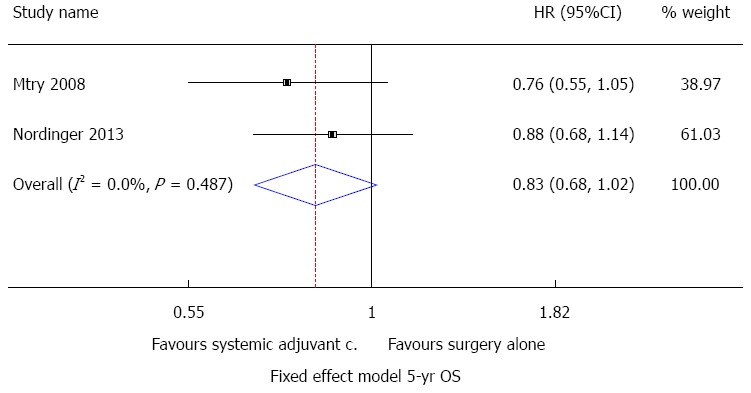
Meta-analysis of the effects of systemic adjuvant chemotherapy studies after metastasectomy vs surgery alone (outcome, 5-year OS). The tegafur-uracil/leucovorin trial is not included in the meta-analysis because the follow-up period is not yet completed. Fixed effect model.
POST-METASTASECTOMY ADJUVANT HAI
Several studies have evaluated the role of HAI in the adjuvant treatment of liver metastases from CRC after curative resections. The rationale for HAI is that the normal liver parenchyma receives blood from the hepatic vein, while the blood flow to tumors derives from the branches of the hepatic artery. Moreover, the direct infusion of chemotherapy into the liver minimizes the side-effects of the chemotherapy and allows high doses to be administered[109]. Floxuridine (FUDR), a derivate of 5-FU, plus dexamethasone are the chemotherapeutic agents most frequently used in HAI and different systemic chemotherapies have been administered with HAI in the adjuvant setting (Table 3)[110].
Table 3.
Hepatic arterial infusion plus systemic chemotherapy in the adjuvant setting
| Ref. | Numbers of patients | Setting | Randomized study | Regimes of therapy |
Outcomes |
||
| DFS | PFS | OS/DSS | |||||
| Controlled studies | |||||||
| Ota et al[115], 1999 | 84 (37 vs 47) | Cohort study | NO | HAI/5-FU vs control group | 5-yr DFS: 72.6% vs 29.8% (P = 0.0005) | - | 5-yr OS:61.4% vs 28% (P = 0.0069) |
| Kemeny et al[112], 2005 | 156 (74 vs 82) | Phase III | YES | HAI/FUDR plus systemic 5-FU ± LV vs systemic 5-FU ± LV alone | - | Median PFS: 31.3 vs 17.2 mo (P = 0.02) | Median OS:68.4 vs 58.8 mo (P = 0.10) |
| House et al[113], 2011 | 250 (125 vs 125) | Cohort study | NO | HAI/FUDR plus systemic chemotherapy (5FU/LV + irinotecan or oxaliplatin) vs systemic chemotherapy alone | 5-yr DFS: 48% vs 25% (P < 0.01)HR = 0.71 (95%CI: 0.48-0.96) | - | 5-yr DSS:75% vs 55% (P < 0.01) HR = 0.39 (95%CI: 0.23-0.68) |
| Goéré et al[116], 2013 | 98 (44 vs 54) | Cohort study | NO | HAI/oxaliplatin plus systemic 5-FU/LV vs systemic irinotecan regimes or oxaliplatin regimes alone | 3-yr DFS: 33% vs 5%(P < 0.0001) HR = 0.37 (95%CI: 0.23-0.60) | - | 3-yr OS:75% vs 62% (P = 0.17) 5-yr OS:54% vs 52% (P = 0.34) |
| Non controlled studies | |||||||
| Alberts et al[114], 2010 | 55 | Phase II single armed | NO | HAI/FUDR plus systemic capecitabine + oxaliplatin | 2-yr DFS:59.7% Median DFS: 32.7 mo | - | 2-yr OS:89.10% |
DFS: Disease-free survival; PFS: Progression-free survival; OS: Overall survival; DSS: Disease-specific survival.
In a randomized phase II trial, Kemeny et al[111] studied 156 patients who underwent resection of hepatic metastases: 72 received HAI-FUDR plus systemic chemotherapy (5-FU with or without LV) and 82 received systemic chemotherapy alone. The median OS was 72.2 mo in the HAI group vs 59.3 mo in monotherapy group, with survival rates at 2 years of 86% and 72% respectively. The 2-year actuarial rates of overall PFS were 57% in the combined therapy group and 42% in the chemotherapy alone group. Recently, the same authors re-analyzed these results after a median follow-up of 10 years and showed that the PFS of patients treated with combined-therapy was 31.3 mo compared with 17.2 mo in the group treated with systemic chemotherapy alone. However, no statistically significant difference was observed for median OS (68.4 mo vs 58.8 mo respectively, P = 0.10)[112]. A retrospective study compared the outcome in patients receiving oxaliplatin-based or irinotecan-based chemotherapy (5-FU/LV + oxaliplatin or 5-FU/LV + irinotecan) with or without HAI-FUDR after metastasectomy. The findings showed that HAI plus systemic chemotherapy was associated with an improvement in both DFS and disease-specific survival (DSS) rates: 5-year DFS was 48% (vs 25% in chemotherapy alone group) and DSS was 76% (vs 55%)[113]. Moreover, a recent phase II trial assessed the potential benefit of HAI-FUDR combined with systemic oxaliplatin and capecitabine, showing a median DFS of 32.7 mo[114], but these findings need to be confirmed by phase III studies.
Besides FUDR, other chemotherapeutic agents have been used in the context of HAI. Ota et al[115] studied 84 patients who underwent surgical resection of liver metastasis and were then treated with arterial infusion of 5-FU. The 5-year liver DFS was 72.6% in the HAI group (vs 29.8% in the control group; P = 0.0005) and the 5-year survival ratio was 61.4% (vs 28.0%; P = 0.0069). More recently, Goéré et al[116] demonstrated a better 3-year DFS in patients who received postoperative HAI with oxaliplatin plus systemic 5-FU therapy in comparison with patients who received systemic chemotherapy alone (33% vs 5%, respectively). After a median follow-up of 60 mo, 3-year OS was also higher in the HAI group, but no statistically significant difference was observed (75% vs 62%, P = 0.17). A Cochrane review of 7 randomized controlled trials showed no significant advantage for adjuvant HAI compared with systemic therapy alone in a pool of 592 patients who underwent metastasectomy[2].
To date, the use of HAI in the adjuvant setting has not demonstrated a significant difference in term of OS, also due to the increasing efficacy of the new systemic chemotherapy regimens. HAI, however, could be employed only to achieve a better DFS.
CONCLUSION
The decision to implement an adjuvant treatment after resection of metastases from CRC is becoming a major challenge in oncology because the positive role of metastasectomy has been definitely ascertained in patients with advanced CRC in the last decade and the number of these patients is increasing. An ideal study would compare the putative most effective adjuvant therapy post-metastasectomy vs surgery alone, stratifying resected patients also on the basis of the risk of recurrence. However, this study is currently unlikely due to the high dropout rate it would incur.
Nonetheless, the data obtained from controlled studies (cohort or randomized studies) on systemic treatment allow us to draw some important conclusions: (1) a systemic chemotherapy with 5-FU +/- oxaliplatin seems to confer an advantage in terms of survival, also supported by the meta-analysis presented in this paper; and (2) not all active drugs in advanced disease appear to be effective in the adjuvant setting. In particular, studies that have used irinotecan-based regimes were negative. However, this aspect should be confirmed in larger series, taking into account the biological heterogeneity between primary tumors and their metastases.
Ultimately, on the basis of all the available data, adjuvant chemotherapy post-metastasectomy should be recommended.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest and no financial support to declare.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 3, 2015
First decision: September 11, 2015
Article in press: November 13, 2015
P- Reviewer: Berkane S, Han SY, Hashimoto N, Kadiyska TK S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sargent D, Sobrero A, Grothey A, O’Connell MJ, Buyse M, Andre T, Zheng Y, Green E, Labianca R, O’Callaghan C, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii1–iii9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D’Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN clinical practice in oncology: colon cancer. NCCN.org 2015, version 2. Available from: http://guide.medlive.cn/guideline/7015.
- 6.Biasco G, Derenzini E, Grazi G, Ercolani G, Ravaioli M, Pantaleo MA, Brandi G. Treatment of hepatic metastases from colorectal cancer: many doubts, some certainties. Cancer Treat Rev. 2006;32:214–228. doi: 10.1016/j.ctrv.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Penna C, Nordlinger B. Colorectal metastasis (liver and lung) Surg Clin North Am. 2002;82:1075–1090, x-xi. doi: 10.1016/s0039-6109(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 8.Yates LR, Campbell PJ. Evolution of the cancer genome. Nat Rev Genet. 2012;13:795–806. doi: 10.1038/nrg3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–364. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–345. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- 11.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 12.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 13.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 14.Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol. 2005;23:9441–9442. doi: 10.1200/JCO.2005.04.4792. [DOI] [PubMed] [Google Scholar]
- 15.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem E, Hoff PM, Harper P, Bukowski RM, Cunningham D, Dufour P, Graeven U, Lokich J, Madajewicz S, Maroun JA, et al. Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer. 2004;90:1190–1197. doi: 10.1038/sj.bjc.6601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Díaz-Rubio E, Tabernero J, Gómez-España A, Massutí B, Sastre J, Chaves M, Abad A, Carrato A, Queralt B, Reina JJ, et al. Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol. 2007;25:4224–4230. doi: 10.1200/JCO.2006.09.8467. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 20.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 21.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 22.Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS, Go WY. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240–2247. doi: 10.1200/JCO.2013.53.2473. [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz HI, Tebbutt NC, Kabbinavar F, Giantonio BJ, Guan ZZ, Mitchell L, Waterkamp D, Tabernero J. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. 2013;18:1004–1012. doi: 10.1634/theoncologist.2013-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cremolini C, Loupakis F, Falcone A. FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2015;372:291–292. doi: 10.1056/NEJMc1413996. [DOI] [PubMed] [Google Scholar]
- 25.Tol J, Koopman M, Rodenburg CJ, Cats A, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, Mol L, Antonini NF, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol. 2008;19:734–738. doi: 10.1093/annonc/mdm607. [DOI] [PubMed] [Google Scholar]
- 26.Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 27.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, Arnold D; ESMO Guidelines Working Group. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi64–vi72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 28.Adjuvant chemotherapy with oxaliplatin, in combination with fluorouracil plus leucovorin prolongs disease-free survival, but causes more adverse events in people with stage II or III colon cancer Abstracted from: Andre T, Boni C, Mounedji-Boudiaf L, et al. Multicenter international study of oxaliplatin/5-fluorouracil/leucovorin in the adjuvant treatment of colon cancer (MOSAIC) investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343-51. Cancer Treat Rev. 2004;30:711–713. doi: 10.1016/j.ctrv.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 29.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 30.Kuebler JP, Wieand HS, O’Connell MJ, Smith RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE, Atkins JN, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 31.Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 32.Twelves C, Wong A, Nowacki MP, Abt M, Burris H, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 33.Shimada Y, Hamaguchi T, Mizusawa J, Saito N, Kanemitsu Y, Takiguchi N, Ohue M, Kato T, Takii Y, Sato T, et al. Randomised phase III trial of adjuvant chemotherapy with oral uracil and tegafur plus leucovorin versus intravenous fluorouracil and levofolinate in patients with stage III colorectal cancer who have undergone Japanese D2/D3 lymph node dissection: final results of JCOG0205. Eur J Cancer. 2014;50:2231–2240. doi: 10.1016/j.ejca.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida M, Ishiguro M, Ikejiri K, Mochizuki I, Nakamoto Y, Kinugasa Y, Takagane A, Endo T, Shinozaki H, Takii Y, et al. S-1 as adjuvant chemotherapy for stage III colon cancer: a randomized phase III study (ACTS-CC trial) Ann Oncol. 2014;25:1743–1749. doi: 10.1093/annonc/mdu232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, Fields AL, Mayer RJ. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25:3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 36.Van Cutsem E, Labianca R, Bodoky G, Barone C, Aranda E, Nordlinger B, Topham C, Tabernero J, André T, Sobrero AF, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27:3117–3125. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 37.Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, Atkins JN, Seay TE, Fehrenbacher L, Goldberg RM, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225–1233. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 39.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 40.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 41.Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383–1393. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taieb J, Tabernero J, Mini E, Subtil F, Folprecht G, van Laethem J, Thaler J, Bridgewater JA, Van Cutsem E, Rougier P, et al. Bouchè O, Lapage C, Girault C, Emile J, Laurent-Puig P, Bedenne L. Adjuvant FOLFOX-4 with or without cetuximab (CTX) in patients (PTS) with resected stage III colon cancer: DFS and OS results and subgroup analyses of the PETACC-8 Intergroup phase III trial. Ann Oncol. 2012;23(suppl 9):abstract # LBA4. [Google Scholar]
- 43.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 44.D’Angelica M, Brennan MF, Fortner JG, Cohen AM, Blumgart LH, Fong Y. Ninety-six five-year survivors after liver resection for metastatic colorectal cancer. J Am Coll Surg. 1997;185:554–559. doi: 10.1016/s1072-7515(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 45.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 46.Steele G, Bleday R, Mayer RJ, Lindblad A, Petrelli N, Weaver D. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumor Study Group Protocol 6584. J Clin Oncol. 1991;9:1105–1112. doi: 10.1200/JCO.1991.9.7.1105. [DOI] [PubMed] [Google Scholar]
- 47.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 49.Adam R, Bismuth H, Castaing D, Waechter F, Navarro F, Abascal A, Majno P, Engerran L. Repeat hepatectomy for colorectal liver metastases. Ann Surg. 1997;225:51–60; discussion 60-62. doi: 10.1097/00000658-199701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrowsky H, Gonen M, Jarnagin W, Lorenz M, DeMatteo R, Heinrich S, Encke A, Blumgart L, Fong Y. Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg. 2002;235:863–871. doi: 10.1097/00000658-200206000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871–873; discussion 883-884. doi: 10.1097/01.sla.0000098112.04758.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morise Z, Sugioka A, Fujita J, Hoshimoto S, Kato T, Hasumi A, Suda T, Negi H, Hattori Y, Sato H, et al. Does repeated surgery improve the prognosis of colorectal liver metastases? J Gastrointest Surg. 2006;10:6–11. doi: 10.1016/j.gassur.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Brandi G, Corbelli J, de Rosa F, Di Girolamo S, Longobardi C, Agostini V, Garajová I, La Rovere S, Ercolani G, Grazi GL, et al. Second surgery or chemotherapy for relapse after radical resection of colorectal cancer metastases. Langenbecks Arch Surg. 2012;397:1069–1077. doi: 10.1007/s00423-012-0974-0. [DOI] [PubMed] [Google Scholar]
- 54.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 55.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318; discussion 318-321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, Geller DA, Gayowski TJ, Fung JJ, Starzl TE. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagashima I, Takada T, Matsuda K, Adachi M, Nagawa H, Muto T, Okinaga K. A new scoring system to classify patients with colorectal liver metastases: proposal of criteria to select candidates for hepatic resection. J Hepatobiliary Pancreat Surg. 2004;11:79–83. doi: 10.1007/s00534-002-0778-7. [DOI] [PubMed] [Google Scholar]
- 58.Konopke R, Kersting S, Distler M, Dietrich J, Gastmeier J, Heller A, Kulisch E, Saeger HD. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009;29:89–102. doi: 10.1111/j.1478-3231.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 59.Schindl M, Wigmore SJ, Currie EJ, Laengle F, Garden OJ. Prognostic scoring in colorectal cancer liver metastases: development and validation. Arch Surg. 2005;140:183–189. doi: 10.1001/archsurg.140.2.183. [DOI] [PubMed] [Google Scholar]
- 60.Gomez D, Cameron IC. Prognostic scores for colorectal liver metastasis: clinically important or an academic exercise? HPB (Oxford) 2010;12:227–238. doi: 10.1111/j.1477-2574.2010.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayez N, Lalmahomed ZS, van der Pool AE, Vergouwe Y, van Montfort K, de Jonge J, Eggermont AM, Ijzermans JN, Verhoef C. Is the clinical risk score for patients with colorectal liver metastases still useable in the era of effective neoadjuvant chemotherapy? Ann Surg Oncol. 2011;18:2757–2763. doi: 10.1245/s10434-011-1819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schreckenbach T, Malkomes P, Bechstein WO, Woeste G, Schnitzbauer AA, Ulrich F. The clinical relevance of the Fong and the Nordlinger scores in the era of effective neoadjuvant chemotherapy for colorectal liver metastasis. Surg Today. 2015;45:1527–1534. doi: 10.1007/s00595-014-1108-9. [DOI] [PubMed] [Google Scholar]
- 63.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722, discussion 722-724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Truant S, Séquier C, Leteurtre E, Boleslawski E, Elamrani M, Huet G, Duhamel A, Hebbar M, Pruvot FR. Tumour biology of colorectal liver metastasis is a more important factor in survival than surgical margin clearance in the era of modern chemotherapy regimens. HPB (Oxford) 2015;17:176–184. doi: 10.1111/hpb.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Angelsen JH, Horn A, Eide GE, Viste A. Surgery for colorectal liver metastases: the impact of resection margins on recurrence and overall survival. World J Surg Oncol. 2014;12:127. doi: 10.1186/1477-7819-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andreou A, Aloia TA, Brouquet A, Dickson PV, Zimmitti G, Maru DM, Kopetz S, Loyer EM, Curley SA, Abdalla EK, et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257:1079–1088. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sadot E, Groot Koerkamp B, Leal JN, Shia J, Gonen M, Allen PJ, DeMatteo RP, Kingham TP, Kemeny N, Blumgart LH, et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg. 2015;262:476–485; discussion 483-485. doi: 10.1097/SLA.0000000000001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zorzi D, Mullen JT, Abdalla EK, Pawlik TM, Andres A, Muratore A, Curley SA, Mentha G, Capussotti L, Vauthey JN. Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg. 2006;10:86–94. doi: 10.1016/j.gassur.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 69.Guzzetti E, Pulitanò C, Catena M, Arru M, Ratti F, Finazzi R, Aldrighetti L, Ferla G. Impact of type of liver resection on the outcome of colorectal liver metastases: a case-matched analysis. J Surg Oncol. 2008;97:503–507. doi: 10.1002/jso.20979. [DOI] [PubMed] [Google Scholar]
- 70.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84:324–338. doi: 10.1016/j.athoracsur.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 71.Kim HK, Cho JH, Lee HY, Lee J, Kim J. Pulmonary metastasectomy for colorectal cancer: how many nodules, how many times? World J Gastroenterol. 2014;20:6133–6145. doi: 10.3748/wjg.v20.i20.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hishida T, Okumura T, Boku N, Y O. Surgical outcome for pulmonary metastasis of colorectal cancer in the modern chemotherapy era: Results of a retrospective Japanese multicenter study. J Clin Oncol. 2014;32(suppl):abstr 3528. [Google Scholar]
- 73.Shiono S, Ishii G, Nagai K, Yoshida J, Nishimura M, Murata Y, Tsuta K, Nishiwaki Y, Kodama T, Ochiai A. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg. 2005;79:278–282; discussion 283. doi: 10.1016/j.athoracsur.2004.06.096. [DOI] [PubMed] [Google Scholar]
- 74.Vogelsang H, Haas S, Hierholzer C, Berger U, Siewert JR, Präuer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:1066–1071. doi: 10.1002/bjs.4602. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe I, Arai T, Ono M, Sugito M, Kawashima K, Ito M, Nagai K, Saito N. Prognostic factors in resection of pulmonary metastasis from colorectal cancer. Br J Surg. 2003;90:1436–1440. doi: 10.1002/bjs.4331. [DOI] [PubMed] [Google Scholar]
- 76.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adam R, Barroso E, Laurent C, Nuzzo G, Hubert C, Mentha G, Ijzermans J, Capussotti L, Lopezben S, Mirza D, et al. The LiverMetSurvey Centers. Impact of the type and modalities of the type and modalties of preoperative chemotherapy on the outcome of liver resection for colorectal metastases. J Clin Oncol. 2011;29:(abstr 3519). [Google Scholar]
- 78.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 79.Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860–868. doi: 10.1007/s11605-007-0149-4. [DOI] [PubMed] [Google Scholar]
- 80.Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049; discussion 1049-1051. doi: 10.1097/01.sla.0000145965.86383.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adam R, Miller R, Pitombo M, Wicherts DA, de Haas RJ, Bitsakou G, Aloia T. Two-stage hepatectomy approach for initially unresectable colorectal hepatic metastases. Surg Oncol Clin N Am. 2007;16:525–536, viii. doi: 10.1016/j.soc.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 82.Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 83.Ratti F, Schadde E, Masetti M, Massani M, Zanello M, Serenari M, Cipriani F, Bonariol L, Bassi N, Aldrighetti L, et al. Strategies to Increase the Resectability of Patients with Colorectal Liver Metastases: A Multi-center Case-Match Analysis of ALPPS and Conventional Two-Stage Hepatectomy. Ann Surg Oncol. 2015;22:1933–1942. doi: 10.1245/s10434-014-4291-4. [DOI] [PubMed] [Google Scholar]
- 84.Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg. 2007;246:559–565; discussion 565-567. doi: 10.1097/SLA.0b013e318155a7b6. [DOI] [PubMed] [Google Scholar]
- 85.Gleisner AL, Choti MA, Assumpcao L, Nathan H, Schulick RD, Pawlik TM. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg. 2008;143:1204–1212. doi: 10.1001/archsurg.143.12.1204. [DOI] [PubMed] [Google Scholar]
- 86.Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10:1059–1069. doi: 10.1245/ASO.2003.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eltawil KM, Boame N, Mimeault R, Shabana W, Balaa FK, Jonker DJ, Asmis TR, Martel G. Patterns of recurrence following selective intraoperative radiofrequency ablation as an adjunct to hepatic resection for colorectal liver metastases. J Surg Oncol. 2014;110:734–738. doi: 10.1002/jso.23689. [DOI] [PubMed] [Google Scholar]
- 88.Cirocchi R, Trastulli S, Boselli C, Montedori A, Cavaliere D, Parisi A, Noya G, Abraha I. Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst Rev. 2012;6:CD006317. doi: 10.1002/14651858.CD006317.pub3. [DOI] [PubMed] [Google Scholar]
- 89.Kingham TP, Tanoue M, Eaton A, Rocha FG, Do R, Allen P, De Matteo RP, D’Angelica M, Fong Y, Jarnagin WR. Patterns of recurrence after ablation of colorectal cancer liver metastases. Ann Surg Oncol. 2012;19:834–841. doi: 10.1245/s10434-011-2048-x. [DOI] [PubMed] [Google Scholar]
- 90.Langer B, Bleiberg H, Labianca R, Shepherd L, Nitti D, Marsoni S, Tu D, Sargeant AM, Fields A. Fluorouracil (FU) plus leucovorin (I-LV) versus observation after potentially curative resection of liver or lung metastases from colorectal cancer (CRC): results of the ENG (EORTC/NCIC/CTG/GIVIO) randomized trial. Proc Am Clin Oncol. 2002;21:abstract 592. [Google Scholar]
- 91.Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 92.Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, Nitti D, Torri V, Elias D, O’Callaghan C, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 93.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 94.Kanemitsu Y, Kato T, Shimizu Y, Inaba Y, Shimada Y, Nakamura K, Sato A, Moriya Y; Colorectal Cancer Study Group (CCSG) of Japan Clinical Oncology Group. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol. 2009;39:406–409. doi: 10.1093/jjco/hyp035. [DOI] [PubMed] [Google Scholar]
- 95.Nakayama I, Suenaga M, Wakatsuki T, Ichimura T, Ozaka M, Takahari D, Shinozaki E, Chin K, Ueno M, Mizunuma N, et al. Safety, tolerability, and efficacy of oxaliplatin-based adjuvant chemotherapy after curative resection of hepatic or extrahepatic metastases of Stage IV colorectal cancer. Cancer Chemother Pharmacol. 2015;76:133–139. doi: 10.1007/s00280-015-2780-1. [DOI] [PubMed] [Google Scholar]
- 96.Katayose Y, Yamamoto K, Nakagawal K, Takemura S, Takahashi M, Nakamura R, Shimamura H, Rikiyama T, Egawa S, Yoshda H, et al. Feasibility Assessment of Modified FOLFOX-6 as adjuvant treatment after resection of liver metastases from colorectal cancer: analyses of a multicenter phase II clinical trial (Miyagi-HBPCOG Trial-001) Hepatogastroenterology. 2015;62:303–308. [PubMed] [Google Scholar]
- 97.Kim HR, Min BS, Kim JS, Shin SJ, Ahn JB, Rho JK, Kim NK, Rha SY. Efficacy of oxaliplatin-based chemotherapy in curatively resected colorectal cancer with liver metastasis. Oncology. 2011;81:175–183. doi: 10.1159/000333440. [DOI] [PubMed] [Google Scholar]
- 98.Kim SY, Kim HJ, Hong YS, Jung KH, Park JW, Choi HS, Oh JH, Park SJ, Kim SH, Nam BH, et al. Resected colorectal liver metastases: does the survival differ according to postoperative chemotherapy regimen? J Surg Oncol. 2009;100:713–718. doi: 10.1002/jso.21403. [DOI] [PubMed] [Google Scholar]
- 99.Ychou M, Hohenberger W, Thezenas S, Navarro M, Maurel J, Bokemeyer C, Shacham-Shmueli E, Rivera F, Kwok-Keung Choi C, Santoro A. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol. 2009;20:1964–1970. doi: 10.1093/annonc/mdp236. [DOI] [PubMed] [Google Scholar]
- 100.Liu JH, Hsieh YY, Chen WS, Hsu YN, Chau GY, Teng HW, King KL, Lin TC, Tzeng CH, Lin JK. Adjuvant oxaliplatin- or irinotecan-containing chemotherapy improves overall survival following resection of metachronous colorectal liver metastases. Int J Colorectal Dis. 2010;25:1243–1249. doi: 10.1007/s00384-010-0996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brandi G, Derenzini E, Falcone A, Masi G, Loupakis F, Pietrabissa A, Pinna AD, Ercolani G, Pantaleo MA, Di Girolamo S, et al. Adjuvant systemic chemotherapy after putative curative resection of colorectal liver and lung metastases. Clin Colorectal Cancer. 2013;12:188–194. doi: 10.1016/j.clcc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi A, Hasegawa K, Saiura A, Takayama T, Miyagawa S, Yamamoto J, Bandai Y, Teruya M, Yoshimi F, Kawasaki S, et al. A randomized controlled trial evaluating efficacy of adjuvant oral uracil-tegafur (UFT) with leucovorin (LV) after resection of colorectal cancer liver metastases; the UFT/LV study. J Clin Oncol. 2014;32:5 suppl (abstr 3584). [Google Scholar]
- 103.Kato T, Uehara K, Maeda A, Sakamoto E, Hiramatsu K, Takeuchi E, Goto H, Tojima Y, Yatsuya H, Nagino M; Nagoya Surgical Oncology Group. Phase II multicenter study of adjuvant S-1 for colorectal liver metastasis: survival analysis of N-SOG 01 trial. Cancer Chemother Pharmacol. 2015;75:1281–1288. doi: 10.1007/s00280-015-2752-5. [DOI] [PubMed] [Google Scholar]
- 104.Kemeny NE, Jarnagin WR, Capanu M, Fong Y, Gewirtz AN, Dematteo RP, D’Angelica MI. Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. J Clin Oncol. 2011;29:884–889. doi: 10.1200/JCO.2010.32.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turan N, Benekli M, Koca D, Ustaalioglu BO, Dane F, Ozdemir N, Ulas A, Oztop I, Gumus M, Ozturk MA, et al. Adjuvant systemic chemotherapy with or without bevacizumab in patients with resected liver metastases from colorectal cancer. Oncology. 2013;84:14–21. doi: 10.1159/000342429. [DOI] [PubMed] [Google Scholar]
- 106.Snoeren N, Voest EE, Bergman AM, Dalesio O, Verheul HM, Tollenaar RA, van der Sijp JR, Schouten SB, Rinkes IH, van Hillegersberg R. A randomized two arm phase III study in patients post radical resection of liver metastases of colorectal cancer to investigate bevacizumab in combination with capecitabine plus oxaliplatin (CAPOX) vs CAPOX alone as adjuvant treatment. BMC Cancer. 2010;10:545. doi: 10.1186/1471-2407-10-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601–611. doi: 10.1016/S1470-2045(14)70105-6. [DOI] [PubMed] [Google Scholar]
- 108.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xing M, Kooby DA, El-Rayes BF, Kokabi N, Camacho JC, Kim HS. Locoregional therapies for metastatic colorectal carcinoma to the liver--an evidence-based review. J Surg Oncol. 2014;110:182–196. doi: 10.1002/jso.23619. [DOI] [PubMed] [Google Scholar]
- 110.Kingham TP, D’Angelica M, Kemeny NE. Role of intra-arterial hepatic chemotherapy in the treatment of colorectal cancer metastases. J Surg Oncol. 2010;102:988–995. doi: 10.1002/jso.21753. [DOI] [PubMed] [Google Scholar]
- 111.Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, Bertino JR, Turnbull AD, Sullivan D, Stockman J, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 112.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med. 2005;352:734–735. doi: 10.1056/NEJM200502173520723. [DOI] [PubMed] [Google Scholar]
- 113.House MG, Kemeny NE, Gönen M, Fong Y, Allen PJ, Paty PB, DeMatteo RP, Blumgart LH, Jarnagin WR, D’Angelica MI. Comparison of adjuvant systemic chemotherapy with or without hepatic arterial infusional chemotherapy after hepatic resection for metastatic colorectal cancer. Ann Surg. 2011;254:851–856. doi: 10.1097/SLA.0b013e31822f4f88. [DOI] [PubMed] [Google Scholar]
- 114.Alberts SR, Roh MS, Mahoney MR, O’Connell MJ, Nagorney DM, Wagman L, Smyrk TC, Weiland TL, Lai LL, Schwarz RE, et al. Alternating systemic and hepatic artery infusion therapy for resected liver metastases from colorectal cancer: a North Central Cancer Treatment Group (NCCTG)/ National Surgical Adjuvant Breast and Bowel Project (NSABP) phase II intergroup trial, N9945/CI-66. J Clin Oncol. 2010;28:853–858. doi: 10.1200/JCO.2009.24.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ota M, Masui H, Tanaka K, Ichikawa Y, Yamaguchi S, Togo S, Ike H, Oki S, Shimada H. [Efficacy of adjuvant hepatic arterial infusion chemotherapy following resection of colorectal liver metastases] Gan To Kagaku Ryoho. 1999;26:1698–1701. [PubMed] [Google Scholar]
- 116.Goéré D, Benhaim L, Bonnet S, Malka D, Faron M, Elias D, Lefèvre JH, Deschamps F, Dromain C, Boige V, et al. Adjuvant chemotherapy after resection of colorectal liver metastases in patients at high risk of hepatic recurrence: a comparative study between hepatic arterial infusion of oxaliplatin and modern systemic chemotherapy. Ann Surg. 2013;257:114–120. doi: 10.1097/SLA.0b013e31827b9005. [DOI] [PubMed] [Google Scholar]


