Abstract
Duodenal polyps or lesions are uncommonly found on upper endoscopy. Duodenal lesions can be categorized as subepithelial or mucosally-based, and the type of lesion often dictates the work-up and possible therapeutic options. Subepithelial lesions that can arise in the duodenum include lipomas, gastrointestinal stromal tumors, and carcinoids. Endoscopic ultrasonography with fine needle aspiration is useful in the characterization and diagnosis of subepithelial lesions. Duodenal gastrointestinal stromal tumors and large or multifocal carcinoids are best managed by surgical resection. Brunner’s gland tumors, solitary Peutz-Jeghers polyps, and non-ampullary and ampullary adenomas are mucosally-based duodenal lesions, which can require removal and are typically amenable to endoscopic resection. Several anatomic characteristics of the duodenum make endoscopic resection of duodenal lesions challenging. However, advanced endoscopic techniques exist that enable the resection of large mucosally-based duodenal lesions. Endoscopic papillectomy is not without risk, but this procedure can effectively resect ampullary adenomas and allows patients to avoid surgery, which typically involves pancreaticoduodenectomy. Endoscopic mucosal resection and its variations (such as cap-assisted, cap-band-assisted, and underwater techniques) enable the safe and effective resection of most duodenal adenomas. Endoscopic submucosal dissection is possible but very difficult to safely perform in the duodenum.
Keywords: Duodenum, Polyp, Subepithelial, Lesion, Ampulla, Adenoma, Papillectomy, Endoscopic mucosal resection, Underwater, Endoscopic submucosal dissection
Core tip: Duodenal lesions can be categorized as subepithelial or mucosally-based. Endoscopic ultrasonography with fine-needle aspiration aides in the diagnosis of subepithelial lesions. Duodenal gastrointestinal stromal tumors and large or multifocal carcinoids are subepithelial lesions that should undergo surgical resection. Non-ampullary and ampullary adenomas and other mucosally-based duodenal lesions are amenable to endoscopic resection. Endoscopic papillectomy is effective at resecting ampullary adenomas but is not without risk. Various forms of endoscopic mucosal resection (cap-assisted, cap-band-assisted, and underwater) enable the resection of most duodenal adenomas. Endoscopic submucosal dissection is possible but very difficult to safely perform in the duodenum.
INTRODUCTION
The prevalence of finding a duodenal polyp in a patient on upper endoscopy is low, with studies reporting rates from less than 1% to 5%[1,2]. Most duodenal polyps or lesions are found incidentally on esophagogastroduodenoscopy (EGD) that is performed for other reasons. Symptoms that have been attributed to duodenal polyps include dyspepsia, abdominal pain, overt gastrointestinal bleeding, intussusception, obstruction, and anemia. In this review, we will describe commonly encountered duodenal lesions and the approach to the endoscopic resection of these lesions, if applicable, when they are found by endoscopy.
There are certain anatomic characteristics of the duodenum that make endoscopic resection of duodenal lesions challenging. These factors include (1) a narrow lumen; (2) a “C-loop” that makes maintaining endoscope position difficult; (3) Brunner’s glands in the submucosal layer that stiffen the wall and make mucosal lifting difficult; (4) a thin deep muscle layer that results in a higher rate of perforation; and (5) difficult access if emergency or salvage surgery becomes necessary[3-5]. Additionally, the duodenum has an extensive vascular network supplied by the gastroduodenal artery that increases the risk of bleeding, which can be severe and potentially life-threatening.
TYPES OF DUODENAL POLYPS OR LESIONS
Several different lesions can be found on endoscopy in the duodenum. These polyps or lesions can be distinguished by endoscopic, endosonographic, and histologic[6] characteristics, and can be divided into subepithelial and mucosally-based lesions (Table 1).
Table 1.
Types and characteristics of various duodenal lesions that can be encountered on upper endoscopy
| Type of duodenal lesion | Duodenal wall layer | Malignant potential | Requires resection | Amenable to endoscopic resection |
| Lipoma | Subepithelial | No | No | Possible |
| Gastrointestinal stromal tumors | Subepithelial | Yes | Yes | Possible |
| (for small lesions); often requires surgery | ||||
| Carcinoids | Subepithelial | Yes | Yes | Possible |
| (for rare, isolated lesions); often requires surgery | ||||
| Brunner’s gland “adenomas” or hamartomas | Mucosal | No | If patients are symptomatic | Yes |
| Solitary Peutz-Jeghers polyps | Mucosal | Yes | Yes | Yes |
| Adenoma | Mucosal | Yes | Yes | Yes |
Subepithelial lesions
Lipomas: Duodenal lipomas are uncommon, usually asymptomatic, benign, subepithelial, adipose tumors. Characteristic findings on endoscopy include a round lesion with a yellowish hue (Figure 1A) and a positive “pillow” sign (i.e., a visible indentation upon endoscopic palpation with closed biopsy forceps or other devices, Figure 1B). Lipomas are typically non-malignant and only removed when they are symptomatic, or in rare instances when there might be suspicion for a liposarcoma. The fat content of lipomas enables these subepithelial lesions to be reliably identified by using computed tomography (CT) or by endoscopic ultrasonography (EUS) (Figure 1C). If indicated, conventional endoscopic removal is possible when the lipoma is small or pedunculated[7], and larger sessile lesions might be amenable to endoscopic mucosal resection (EMR) or resection with the assistance of an endoloop. While endoscopic submucosal dissection (ESD) has been described in the small bowel, this technique is rarely used in the duodenum given increased rates of complications. However, as most lipomas will not cause any symptoms, endoscopic or surgical resection is typically not required.
Figure 1.
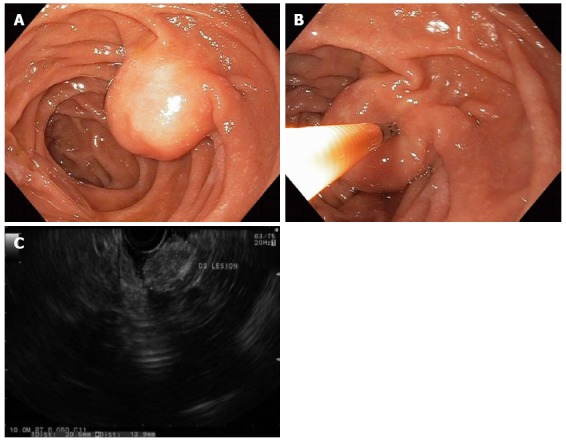
Duodenal lipomas are benign, subepithelial, adipose tumors that rarely cause symptoms. A round subepithelial lesion was found in the second-portion of the duodenum (A); which had a positive “pillow sign” suggesting a lipoma (B); endoscopic ultrasonography found a 2.1-cm hyperechoic lesion arising from the submucosal layer, which was diagnostic of a lipoma (C). As such, endoscopic therapy was not required.
Gastrointestinal stromal tumor: Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the gastrointestinal (GI) tract with the potential for malignant transformation[8] (Figure 2A). GISTs are believed to originate from interstitial cells of Cajal[8], and these submucosal, or better termed - subepithelial - lesions typically arise from the muscularis propria (MP, the deep muscle layer of the GI tract; layer 4 on EUS) and cannot be distinguished from benign leiomyomas by endoscopy, even with routine mucosal biopsies or with EUS alone (Figure 2B). EUS with fine needle aspiration (FNA) is required for definitive diagnosis of a GIST (Figure 2C). While duodenal GISTs are less common than gastric GISTs, they more often produce symptoms in patients[9].
Figure 2.

Gastrointestinal stromal tumor. A: A large subepithelial lesion with a central “dimple” or depression was seen in the duodenum by white light endoscopy; B: Linear endoscopic ultrasonography (EUS) identified a 2.2-cm hypoechoic lesion arising from the deep muscle layer, which suggested a GI stromal tumor (GIST) or leiomyoma; C: EUS with fine needle aspiration enabled cytopathologic confirmation of a GIST; and this tumor was later removed by surgical excision.
Historically, the only role for endoscopy in the management of GISTs, including those of the duodenum, was purely diagnostic, and GISTs that grew or had high mitotic indices on sampling by EUS with FNA were removed by surgical excision. However, with the advent of ESD and by using techniques derived from per-oral endoscopic myotomy (POEM), GISTs that arise from the esophagus and stomach can now be removed endoscopically in specialized centers[8]. However, the submucosal tunneling endoscopic resection (STER)[10] and ESD[8] techniques for removing GISTs and other subepithelial tumors in the esophagus or proximal stomach are not typically employed in the duodenum, due to the challenging anatomic and endoscopic features described above. A suck-ligate-unroof-biopsy technique for the enucleation of subepithelial tumors has been described by using detachable endoloops, but this technique is not widely practiced[11].
At this time, when a GIST is diagnosed in the duodenum or small bowel, except in very usual circumstances, we recommend referral to a surgical oncologist or GI surgeon for operative resection.
Carcinoids: While carcinoids are the most common neuroendocrine tumors of the GI tract, duodenal carcinoid tumors are overall rare, and account for less than 5% of all neuroendocrine tumors[12]. Carcinoids are subepithelial lesions that are typically firm on palpation with closed biopsy forceps and often present as a white or yellow lesion that can be glimpsed through the mucosa on conventional white-light endoscopy (Figure 3A). While EUS with FNA can be utilized for cytopathological diagnosis of carcinoids, deep “bite-on-bite” endoscopic biopsies can sometimes obtain a diagnostic histopathological specimen (Figure 3B and C). Rarely, duodenal carcinoids may arise from the ampulla[13] and these lesions can be mistaken for ampullary adenomas (Figure 4).
Figure 3.
Non-ampullary duodenal carcinoid tumors. A 1-cm duodenal nodule was seen with a white lesion visible in or just below the mucosa, which was suspicious for a carcinoid tumor (A); A larger 1.5-cm subepithelial lesion was found elsewhere in the duodenum of the same patient (B); Bite-on-bite biopsies through the mucosal layer revealed white tissue that was able to be pathologically sampled confirming the diagnosis of a duodenal carcinoid (C).
Figure 4.
A 55-year-old woman was found to have an ampullary mass on upper endoscopy for abdominal pain. Magnetic resonance imaging showed an ampullary tumor with no locoregional invasion or hepatic metastases. Mucosal biopsies showed no pathologic abnormality. The patient was referred for endoscopic ultrasonography (EUS) and possible ampullectomy. On duodenoscopy, a large, irregular ampulla was seen with a whitish hue at the ampullary os (A); EUS demonstrated a 1.6-cm, round, hypoechoic ampullary mass that did not invade into the muscularis propria or into the pancreas (B); On attempted hot-snare resection (C), the lesion was very hard and the snare slipped off the lesion resecting only the surface mucosa, which revealed a white ampullary carcinoid tumor. Ampullectomy was aborted, a biliary sphincterotomy was performed, and a prophylactic pancreatic duct stent was placed (D); Histopathology showed nests of neoplastic cells that stained strongly with antibodies to chromogranin (immunoperoxidase × 20) that extend from the surface epithelium (top and to the right), which confirmed a carcinoid tumor (E). The patient underwent pancreaticoduodenectomy with a curative (R0) resection and no lymph node metastases.
Overall data on the safety of endoscopic resection of small bowel carcinoids are somewhat limited. Endoscopic resection, typically by EMR techniques, has been described as safe for isolated lesions, 10-mm or smaller in size, that are limited to the submucosa and without lymph node metastasis[14]. EUS is required to ensure that the lesion is limited to the submucosa (and does not invade the MP), prior to attempted endoscopic resection. While most GI carcinoids arise from the submucosa (EUS wall layer 3), they can in fact arise from any layer of the wall of the luminal GI tract. As most carcinoids are subepithelial, the risk of perforation and bleeding from attempted endoscopic resection is greater than that from resecting a mucosally-based duodenal lesion. Thus, it is recommended that endoscopic resection of duodenal carcinoids be performed in high-volume centers by expert endoscopists.
In many instances duodenal carcinoids do not present in isolation, and often multiple small bowel carcinoids may exist, some of which can be difficult to see on endoscopic evaluation with EGD or enteroscopy. A more extensive workup including cross-sectional imaging with CT or magnetic resonance imaging (MRI) scans, and possibly functional imaging with somatostatin receptor scintigraphy (“Octreotide scan”), in addition to serum testing for vasoactive peptides, should be considered. In situations of multifocal small bowel carcinoids, surgery will be required.
Mucosally-based lesions
Brunner’s gland “adenomas” or hamartomas: Brunner’s gland “adenomas” or hamartomas account for up to 1% of all duodenal polyps[15]. The majority of patients with this lesion are asymptomatic. Lesions smaller than 10 mm have been described as Brunner’s gland hyperplasia, while “adenomas,” which might be better described as “hamartomas,” are usually pedunculated polyps measuring 1-2 cm or greater in size that arise in the posterior duodenal bulb[16]. These lesions are nearly always benign, though cases of malignancies arising from Brunner’s gland tumors have been reported[17] (Figure 5). Endoscopic removal should be considered for Brunner’s gland tumors that are large in order to provide a definitive diagnosis and to prevent complications from polyp growth, such as bleeding or obstruction[18]. Frequently, these lesions are pedunculated and can be removed by using a snare with or without an endoloop (Figure 6); but for Brunner’s gland tumors that are sessile, sometimes advanced mucosectomy techniques may be required. Surgical excision is more invasive, less cost-effective, and often unnecessary.
Figure 5.
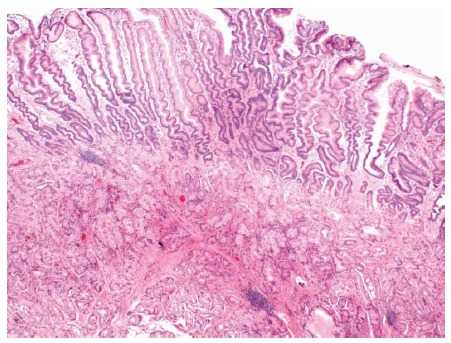
Brunner’s gland hyperplasia was found on histopathology from a sessile polyp that was endoscopically resected from the duodenal bulb. Brunner’s glands extend to the base of the sample. The surface is reactive with extensive gastric metaplasia (HE × 20).
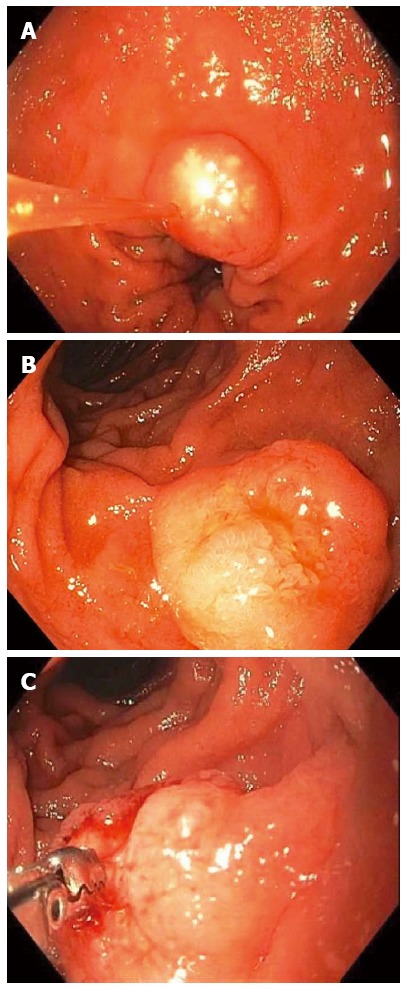
Figure 6.
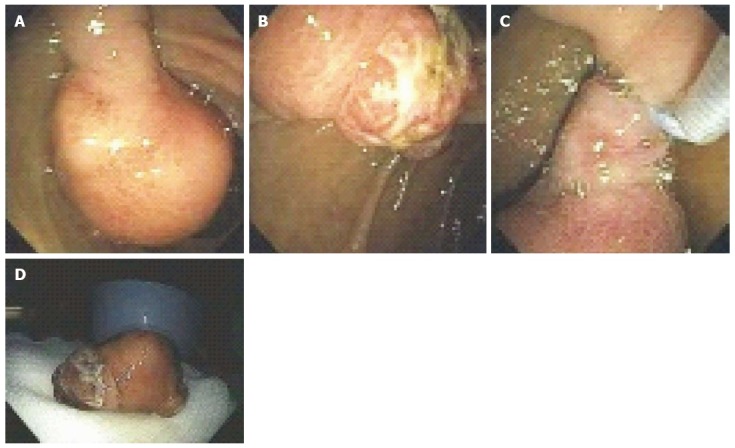
A 53-year-old man presented with exertional dyspnea and 2 wk of melena. For symptomatic anemia, he received 8 units of packed red blood cells over several days. Upper endoscopy showed a 3.5-cm pedunculated polyp on a wide stalk in the posterior duodenal bulb (A, B); An endoloop was placed using a duodenoscope (C) followed by en to snare resection; The resected polyp was so large the bite block had to be removed in order to pull the polyp out of the mouth (D). Pathology showed a Brunner’s gland “adenoma”.
Solitary Peutz-Jeghers polyp: Solitary Peutz-Jeghers (SPJ) polyps in the duodenum are rare and are found in patients without family history of Peutz-Jeghers syndrome or the characteristic skin findings[19]. SPJ polyps are hamartomas that have an irregular lobular or nodular surface on endoscopy (Figure 7). Polypectomy is recommended in order to achieve an accurate diagnosis and, more importantly, because these polyps have a risk of malignant transformation[19]. Because SPJ polyps are rare, if a suspected SPJ polyp is found in the upper GI tract, a more extensive workup to exclude Peutz-Jeghers syndrome (that might include small bowel imaging, colonoscopy, and potentially genetic testing) is reasonable.
Figure 7.
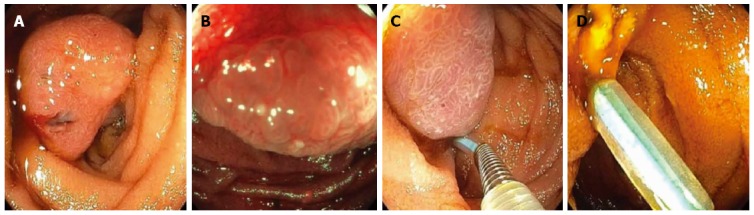
A patient presented for endoscopy because of gastrointestinal bleeding. A solitary Peutz-Jeghers hamartomatous polyp was seen in the duodenum on white light (A) and narrow-band imaging (B); This large polyp was removed endoscopically by first using an endoloop to constrict the broad stalk of the polyp (C); followed by hot-snare resection (D).
Adenomas: Duodenal adenomas should be categorized as being ampullary or non-ampullary, and as sporadic or arising in the context of a genetic syndrome such as familial adenomatous polyposis (FAP). This type of classification is important because of differences in malignancy risk, as well as differences in diagnostic and therapeutic strategies employed in managing these lesions that arise from the mucosa.
Sporadic duodenal adenomas account for up to 7% of duodenal polyps biopsied during upper endoscopy[2]. Most of these lesions are asymptomatic and diagnosed incidentally in older patients (60 to 80 years of age). Sporadic duodenal adenomas are usually solitary, sessile, and they are predominantly found in the second part of the duodenum[12,20,21] (Figure 8). There are currently no well-accepted guidelines for the surveillance of sporadic adenomas, particularly after successful endoscopic resection, though many would advocate repeat upper endoscopy within 1 year to detect and treat residual or recurrent adenoma and to survey for other metachronous adenomas[15]. The need for more extensive and periodic examination of the deeper small bowel by capsule endoscopy or various types of radiographic enterography for other synchronous or metachronous adenomas is unclear.
Figure 8.
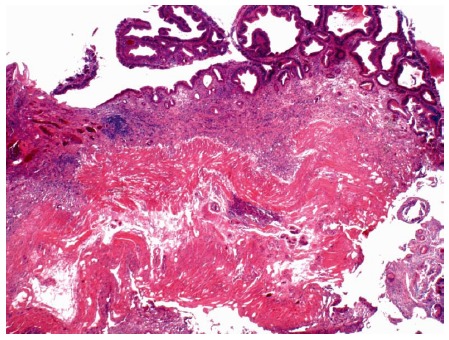
Photomicrograph of a large tubular adenoma that was removed in piecemeal fashion by underwater endoscopic mucosal resection from the second-portion of the duodenum. Dark, adenomatous epithelium lines the surface of the mucosa (top). The muscularis mucosa is cauterized at the base (HE × 20).
In contrast, duodenal adenomas occur in up to 90% of patients with FAP, and most commonly involve the ampulla or the distal duodenum[22] (Figure 9). The lifetime risk of duodenal adenocarcinoma in patients with FAP is estimated to be 3% to 5%. A staging system developed by Spigelman et al[23] has been used to assess the severity of the duodenal adenomatous polyp burden and to predict the risk of developing duodenal cancer so as to determine which patients might require endoscopic resection or even surgical intervention.
Figure 9.
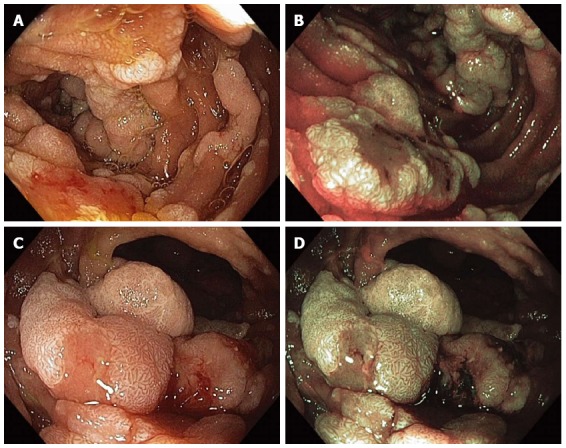
Multiple sessile adenomatous polyps were found in various portions of the duodenum in a patient with familial adenomatous polyposis. Duodenal polyps were visualized with high-definition white-light (WL) endoscopy (A) and narrow-band imaging (NBI) endoscopy (B); The largest adenomas were carefully examined under WL (C) and NBI (D) as they have an increased risk for harboring malignancy.
One retrospective study analyzed the differences between patients with sporadic duodenal adenomas and those with duodenal adenomas associated with FAP with regards to the development of duodenal adenocarcinoma[4]. The mean age of diagnosis of patients with a cancer arising from a sporadic duodenal adenoma was older than for those with cancer arising from adenomas in the setting of FAP (mean age of 65 years vs 35 years, respectively). There was no gender predilection or specific clinical symptoms associated with early duodenal adenocarcinoma. The size of the cancer in the sporadic group was larger as compared to that in the FAP group (mean 13.2 mm vs 7.6 mm, respectively), which might have been related to regular endoscopic surveillance provided to patients in the FAP group. There were adenomatous components in 50% of sporadic adenocarcinomas and 57% of FAP associated lesions. Prior to this study, there was evidence that the sequence of progression from adenoma to carcinoma in the duodenum was similar to that in the colon for FAP-associated carcinoma[24]. These data also support a progression from sporadic duodenal adenomas to carcinomas.
Adenomas involving the major (or minor) papillae can occur either sporadically or in the context of a genetic syndrome and have the potential to undergo malignant transformation to adenocarcinoma. The rate of reported malignant transformation of ampullary (major papilla) adenomas is higher than for other sporadic duodenal polyps, ranging from 26% to 65%[25]. Ampullary adenomas can cause symptoms because of their location, such as obstructive jaundice, pancreatitis, and bleeding. On endoscopic visualization alone, it may be difficult to differentiate an ampullary adenoma from an ampullary carcinoid tumor or gangliocytic paraganglioma, and biopsies should typically be obtained for diagnosis prior to endoscopic resection[25].
ENDOSCOPIC ASSESSMENT
“Every endoscopic procedure has a cognitive and a technical component”[26]. This statement underscores the need for planning and careful evaluation of any lesion in the luminal GI tract prior to attempted endoscopic intervention. For duodenal polyps or lesions, obvious but critical factors include size, location (particularly in relationship to major and minor papillae), and whether it is mucosally-based or subepithelial. A duodenoscope will often be required to definitively determine a lesion’s exact relationship to the major and minor papillae, which can dramatically change the subsequent approach to endoscopic resection. As with any invasive procedure, each individual patient’s comorbidities (e.g., neuro-cardiovascular conditions, bleeding diatheses, etc.) and the specific clinical context must be considered prior to pursing endoscopic resection, irrespective of a lesion’s degree of dysplasia.
For mucosally-based lesions, the visual endoscopic appearance is very important. Macroscopic morphological features can be described by using the Paris classification for superficial neoplasia, which can provide insight into the potential for endoscopic resectability based on lesion type[27].
High-definition narrow-band imaging (NBI) and other commercially available enhanced optical imaging modalities now offer endoscopists the ability to better diagnose and differentiate neoplastic lesions from lesions with no malignant potential. While the data for NBI is not as robust for duodenal neoplasia as it is for other areas of the GI tract, the utility of NBI in the evaluation of duodenal adenomas in the setting of FAP[28] and for ampullary adenomas has been described[29].
Chromoendoscopy by using topical dyes, such as indigo carmine, and magnification endoscopy can be used to evaluate the mucosal pit pattern of GI lesions, which can indicate dysplasia and even invasive cancers. While applicable to the duodenum, the “Kudo” pit pattern has been better described in other parts of the luminal GI tract[30]. However, true magnification endoscopy with optical zoom is not available on most commercially available endoscopes in North and South America[31].
In general, either on high-definition white-light or NBI endoscopy, depressed areas (Paris type 0-IIc) or areas with a markedly disordered vascular pattern or avascularity are indicative of invasive cancers; such lesions are not appropriate for endoscopic resection. Other mucosal features such as bridging folds or tenting can also be indicative of submucosal fibrosis or invasion.
For visualization of non-ampullary, mucosally-based, duodenal lesions, we typically prefer a high-definition, forward-viewing, gastroscope with a low-profile cap. The use of a soft distal attachment cap can be invaluable for visualization, stabilization, and manipulation of mucosal folds in the duodenum. However, some lesions (particularly those along the anterior and medial duodenal walls) can only be properly visualized by using a duodenoscope, and familiarity or skill with endoscopic retrograde cholangiopancreatography (ERCP) can be very helpful when assessing and resecting these duodenal lesions.
In the following sections we will describe in detail advanced endoscopic techniques (beyond conventional cold biopsy or hot-snare polypectomy) that are primarily used for the resection of large mucosally-based neoplasms located in the duodenum or at the ampulla (Table 2). The indications and types of resection recommended for subepithelial tumors have been described above. When embarking on advanced endoscopic resection of GI neoplasia, one central axiom is that if you are not confident that you can completely remove a lesion endoscopically, it would be best to refer the patient to a high-volume interventional endoscopist. Failed resection attempts lead to submucosal fibrosis, which can increase the risk of a future endoscopic resection and in some instances can even make subsequent endoscopic resection impossible. Furthermore, when placing submucosal tattoos to help identify lesions that might be referred for advanced endoscopic resection, it is important not to tattoo close by or underneath a lesion, as the tattoo ink can also cause significant submucosal fibrosis.
Table 2.
Endoscopic procedures for the potential resection of elevated or sessile mucosally-based duodenal lesions (typically for duodenal adenomas)
| Procedure | Appropriate lesion size | Used for non-ampullary lesions | Used for papillectomy | Piecemeal resection possible | Can be done using a duodenoscope | Degree of difficulty |
| Snare polypectomy (en bloc) | ≤ 10 mm | Y | Y | N | Y | + |
| Cap-assisted EMR | ≤ 18 mm | Y | N | Y | N | +++ |
| (requires submucosal lifting) | ||||||
| Cap-band-assisted EMR | ≤ 11 mm | Y | N | Y | N | ++ |
| Conventional EMR (with submucosal injection) | Any size | Y | Y | Y | Y | +++ |
| Underwater EMR | Any size | Y | Y | Y | Y | +++ |
| (forward-viewing scope with a cap is preferred) | ||||||
| ESD | Any size | Y | N | N/A | N | ++++ |
| (goal of ESD is en bloc resection) |
For degree of difficulty, + denotes the easiest and ++++ denotes the hardest procedures to perform. EMR: Endoscopic mucosal resection; ESD: Endoscopic submucosal dissection.
ENDOSCOPIC MUCOSAL RESECTION
Endoscopic mucosal resection (also called mucosectomy) is a well-established method for removing lesions throughout the luminal GI tract, including in the duodenum[32,33]. Conventionally, sterile normal saline is first injected into the submucosa around and underneath a mucosally-based duodenal polyp, typically an adenoma. This submucosal-saline cushion then enables the safe resection of raised, flat, and sessile mucosal lesions, which otherwise might be prone to perforation of the thin duodenal wall. After adequate lifting is achieved, hot-snare polypectomy is then used to perform EMR. En bloc resection is preferable whenever possible, in order to minimize polyp recurrence. In general, polyps greater than 20 mm in size will need to be removed in a piecemeal fashion by repeated, overlapping snare resections (piecemeal EMR)[34].
The blue dyes indigo carmine and methylene blue can be used to tint the saline injectate used for saline-assisted EMR. Use of indigo carmine is preferred, as it is a plant-based dye that does not intercalate DNA, as does methylene blue. Furthermore, indigo carmine will stain the submucosa but not the deep muscle layer, which helps to visualize the MP following resection to ensure that it remains intact. Some endoscopists also add epinephrine to the injectate when performing EMR in vascular areas such as the duodenum at concentrations of 1:100000 or more dilute, to help reduce the risk of intraprocedural bleeding.
Success rates for eventual complete removal of duodenal adenomas range from about 70% to 100%[12,20,21,34-39] Kedia et al[20] illustrated the success rate of EMR as it pertains to percentage of luminal circumference. A complete resection rate of 95% was achieved for polyps involving < 25% luminal circumference, as compared to 46% for polyps involving 25% to 50% of the luminal circumference, and 0% for polyps encompassing > 50% of the luminal circumference[20]. In a review by Basford and Bhandari[12] of several studies that recorded number of sessions to achieve complete resection, 80% of the cases were completed within one session, 17% within two sessions, and 3% in three sessions.
With respect to adverse events, in considering the largest recently published series of conventional EMR of non-ampullary duodenal adenomas, the rate of perforation was reported from 0% to 1.9% and acute or delayed bleeding occurred in 0% to 14% of cases[20,21,34,38]. Overall, the rate of residual or recurrent adenoma is probably around 25%[21,34], particularly when very large adenomas are removed in piecemeal fashion.
In general (and as in other sites of the luminal GI tract), the larger the duodenal adenoma, the lower the chance for complete resection (traditionally defined as circumferential and deep margins clear of dysplasia, also termed “R0” resection) and the higher the risk of immediate or delayed complications (bleeding or perforation) and residual or recurrent dysplasia at time of follow-up endoscopy. However, one issue with piecemeal resection of large adenomas is that the pathologists cannot re-orient several resected specimens to definitely call histologically negative margins. Thus, we are limited to the endoscopic appearance for determination of complete resection following piecemeal EMR, and we must wait until follow-up endoscopy is performed 3-6 mo later to see if the resection was actually curative.
When considering very large duodenal adenomas, Fanning et al[40] compared outcomes between EMR of giant hemicircumferential or larger adenomas - also called laterally spreading tumors (LSTs) - and smaller conventional duodenal adenomas or other lesions. This study included 19 LSTs (≥ 30 mm) with a mean size of 40.5 mm and 31 smaller lesions (< 30 mm) with a mean size of 14.5 mm. There were statistically more significant major complications (26.3% vs 3.2%, P = 0.014) in the LST group, which were mainly cases of bleeding. Intraprocedural bleeding occurred in 57.8% of LST cases vs in only 19.3% where smaller lesions were resected (P = 0.005). Notably, complete resection was achieved in 84% of LSTs, and 76% of these patients had no evidence of recurrence (thereby achieving curative resection) on surveillance endoscopy.
CAP-ASSISTED AND CAP-BAND-ASSISTED EMR
Cap-assisted EMR (EMR-C) is an alternative to free-hand EMR for the resection of duodenal polyps. EMR caps come in various shapes and sizes. Commercially available kits exist that include an EMR cap and a special snare that can be loaded inside the cap. A soft oblique EMR cap is available that has an outer diameter of 18 mm, with which a lesion up to about 2 cm in size could be potentially resected. Submucosal injection is mandatory when performing EMR-C. After a submucosal bleb is created, the cap and loaded snare are placed over the mucosal lesion of interest and endoscope suction is applied to bring the lesion into the cap. Next, the snare is closed around mucosa brought into the cap for subsequent hot-snare resection.
Initially, there were concerns that use of EMR-C in the duodenum would be associated with higher rates of perforation due to the risk of inadvertently grabbing the MP of the thin duodenal wall. However, Conio et al[41] reported the success of EMR-C of 26 duodenal polyps with a median size of 15 mm. Complete resection was achieved in 96% of patients, and the rate of recurrence was less than 12%. There were no cases of perforation, three cases of intraprocedural bleeding, and no cases of delayed bleeding. Two important points were that a large amount of submucosal fluid was injected in these cases and controlled suction was employed so as to avoid entrapment of the MP.
Anecdotally, a technique has been described whereby after the snare has been tightened around the tissue sucked in to the cap, the snare is then slightly loosened and then quickly retightened. The theory is that this allows any inadvertently captured MP to be released from the ensnared specimen, thereby allowing safer subsequent hot-snare resection.
In contrast, cap-band-assisted mucosectomy/EMR is a modification of variceal band ligator technology, whereby the mucosa is suctioned into a cap and then a rubber band is released over the suctioned mucosa creating a pseudopolyp, which can then be resected by using a hot snare. This technique does not require submucosal injection of fluid for polyp lifting, and the pseudopolyp can be resected either above or below the level of the constricting band (Figure 10).
Figure 10.
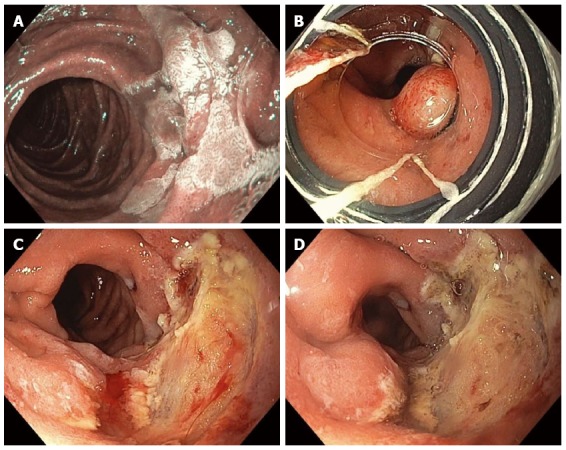
Piecemeal cap-band-assisted mucosectomy (endoscopic mucosal resection). A large, 2.5-cm, nearly flat, duodenal adenoma was found in the second portion of the duodenum along the lateral wall and was examined with narrow-band-imaging, which did not reveal any invasive cancer (A); Cap-band-assisted EMR was performed. Two bands were sequentially deployed (B) and the resultant pseudopolyps were resected leaving behind no macroscopic evidence of residual adenoma (C); Argon plasma coagulation was used to ablate the borders of the polyp and exposed submucosal vessels in order to reduce the risk of adenoma recurrence and delayed bleeding, respectively (D).
Again, commercially available EMR-C and cap-band-assisted EMR systems have United States Food and Drug Administration 510(k) premarketing clearance for “gastrointestinal mucosal resection” (Olympus America, Center Valley, PA, 510(k) number: K984358) and “endoscopic mucosal resection in the upper gastrointestinal tract” (Cook Medical, Winston Salem, NC, 510(k) number: K050578). A similar device from Boston Scientific that is also indicated for “endoscopic mucosal resection in the upper GI tract” (Natick, MA, 510(k) number: K140726) has just been released for purchase in the United States.
UNDERWATER EMR
Dr. Kenneth Binmoeller first described the novel technique of “underwater” EMR (UEMR) in the colorectum in 2012[42] and in the duodenum in 2013[43]. Our group has also described UEMR for the removal of large duodenal adenomas in 2015[44,45]. The inspiration for water-immersion EMR was stimulated by the observation that the MP layer of the GI lumen remains circular and “distant” from the EUS transducer in both an insufflated GI lumen and also in a lumen that is decompressed, in which only enough water is infused to allow for adequate visualization. Furthermore, while the mucosa is stretched thin in an insufflated viscus, in the decompressed GI lumen the mucosa is involuted and folded upon itself, and portions of the mucosa are then “farther away” from the deep muscle layer. In this fashion, UEMR allows for the safe resection of larger mucosal lesions en bloc. When piecemeal UEMR is performed, very large lesions can be removed in relatively fewer pieces, which theoretically will result in fewer mucosal bridges or islands left behind that can be the nucleus for a recurrent lesion (Figure 11). Other advantages of UEMR are that submucosal lifting with saline or other solutions is not necessary when this technique is used for mucosectomy. Furthermore, if intraprocedural bleeding occurs the infused water prevents clot formation and thus a bleeding vessel can be more easily visualized and treated[46].
Figure 11.

Piecemeal underwater endoscopic mucosal resection. A large, 3-cm, non-ampullary, laterally spreading adenoma was found in the second portion of the duodenum (A); Narrow-band imaging (NBI) demonstrated adenoma without evidence of invasive cancer (B); Carbon dioxide gas that was used for insufflation was removed and water infused (C); A 15-mm crescent snare (D) was used for piecemeal underwater endoscopic mucosal resection (UEMR). The electrosurgical settings used for snare resection were DRYCUT 60 W, Effect 4, using a VIO 300D generator (ERBE USA, Marietta, GA). NBI was used to identify a residual island of adenoma (E, seen just inside the “9 o’clock” margin of the resection). Final images showed complete endoscopic resection (F).
In order to ensure procedural success during UEMR, a low-profile, transparent, distal attachment cap is mandatory for underwater visualization. The cap also enables manipulation of the mucosa, which can be essential in complex underwater piecemeal resections. A high-definition endoscope with a water-jet feature and an irrigation pump are also necessary to perform this procedure efficiently. We have found that crescent snares are quite useful to approximate the often non-uniform borders of polyps, particularly underwater. When performing UEMR we have generally adopted settings similar to those first described by Binmoeller et al[43,46], which we have previously described[47]. Specifically, we use DRYCUT 60 W, EFFECT 4 or 5, using VIO 300D generator (ERBE United States, Marietta, GA) for underwater hot-snare resection.
As mentioned earlier, Binmoeller et al[43] were the first to describe UEMR for the resection of large, laterally spreading, non-ampullary duodenal tumors. In this prospective study of twelve patients referred for endoscopic resection of duodenal polyps (median size of 35 mm), 83% of the patients achieved technical success (endoscopic evidence of complete removal of all adenomatous tissue) during their first session, and 92% of patients achieved technical success after two procedures. All patients who had technically successful resections met the secondary endpoint of durable complete resection as evidenced by lack of endoscopic or histologic evidence of residual or recurrent adenoma at follow-up endoscopy. Adverse events included delayed bleeding, which was found in 25% (3/12) of patients - two of whom required blood transfusions. One patient developed a significant duodenal stricture that responded to balloon dilation. One patient developed altered mental status secondary to hyponatremia from water intoxication following 5 L of intraprocedural water infusion during resection of a circumferential adenoma that took 151 min. This patient’s mentation and electrolyte levels normalized following hospitalization and administration of intravenous hypertonic saline[43].
From this experience, it is evident that when performing UEMR in the upper GI tract that tracking the amount of water infused and removed is critical. Binmoeller et al[43] recommend checking serum electrolytes when more than a net of 2 L of water is infused during UEMR in the upper GI tract. We have found that by using only as much water as is needed to visualize a lesion, and by removing all remaining water from the GI lumen by the end of the procedure, that typically the procedural “ins and outs” are matched and water intoxication has not occurred even for UEMR of large duodenal adenomas[44,45].
It is our experience that UEMR can be easily learned by an endoscopist who is already skilled at performing EMR and/or ESD after observation of a few cases[47] or potentially by hands-on training in ex vivo animal models under the guidance of an UEMR expert.
ENDOSCOPIC SUBMUCOSAL DISSECTION
ESD involves a submucosal injection to lift a lesion confined to the mucosa or superficial submucosa in a manner similar to conventional EMR. Specialized endoscopic electrosurgical knives are then used to make a circumferential incision around the dysplastic lesion, after which these endoscopic knives are used to dissect through the submucosal plane with the goal of removing the lesion en bloc with negative lateral and deep margins[26,48].
The advantage of ESD over EMR is increased rates of en bloc curative resection at the cost of increased perforation risk and procedure time. The complete resection rate of sporadic non-ampullary duodenal adenomas by ESD has been reported to range from 86% to 100%[39]. Honda et al[3] described some of the earliest experiences with ESD in the duodenum. While complete en bloc resection was successful in all 9 duodenal ESD patients, the rate of perforation was high at 22% (2 of 9 patients). Generally speaking, the higher rate of perforation during ESD in the duodenum, as opposed to the other luminal organs, is related to the thinner MP layer. Two more recent retrospective studies comparing EMR and ESD, performed by Japanese experts at ESD centers, showed greater en bloc and complete resection rates following duodenal ESD, which was offset by longer procedure times and higher perforation rates[49,50]. In these two studies, the perforation rate for ESD ranged from 20% to 25% while EMR had a perforation rate of 0%. Given the increased frequency of severe complications during or following duodenal ESD, this procedure should be reserved for those endoscopists with extensive experience in ESD involving the stomach, rectum, esophagus, and colon.
One other advantage of ESD, as opposed to EMR, is that ESD potentially enables the endoscopic resection of duodenal subepithelial tumors (SETs). Matsumoto and Yoshida[49] included in their series (mentioned above) nine submucosal duodenal carcinoids; eight of which were removed en bloc by ESD.
AMPULLECTOMY
Traditionally, surgery was the preferred method for the removal of ampullary tumors. Surgical options for ampullary tumors include pancreaticoduodenectomy and local operative excision (surgical ampullectomy). With the advent of therapeutic ERCP, endoscopic ampullectomy is widely accepted as a less invasive treatment with less morbidity as compared to surgery[51-53]. While the term “ampullectomy” is commonly used when the major papilla is removed by endoscopic means, this procedure is better described as “papillectomy” of the major papilla, as the entire area of the ampulla can only be removed by surgery[25]. In patients without intraductal growth or invasion of adenoma, endoscopic papillectomy can offer rates of curative resection upwards of 81% to 93%[54,55]. When considering large series, the recurrence rates following endoscopic papillectomy range from 8% to 20%[51,52].
While these data are encouraging, enthusiasm should be tempered by the not so insignificant rate of adverse events associated with endoscopic papillectomy, which ranges from 8% to 31%[51,52,54], with rates of pancreatitis around 10%[54] and rates of perforation as high as 3.6%[54]. Procedure-related bleeding has been reported in 2% to 13% of cases, and rates of post-ampullectomy papillary stenosis can range from 0% to 8%[56]. Reassuringly, mortality following endoscopic papillectomy by an experienced pancreaticobiliary endoscopist should be 0.4% or lower[52,56]. Given the risks associated with this procedure, endoscopic papillectomy should only be performed in high-volume ERCP centers (with the requisite radiological and surgical backup) and by expert pancreaticobiliary endoscopists, who are accustomed to caring for post-ERCP complications.
It should be underscored that the indications for endoscopic papillectomy are not standardized[25]. An adenoma limited to the ampullary region, without extension into the biliary or pancreatic ducts, with no evidence of malignancy, no invasion into the muscle layer, and with size less than 4 cm[57-59] would be considered reasonable for endoscopic papillectomy. While intraductal growth of adenoma is not an absolute contraindication to endoscopic papillectomy, it is associated with low rates of complete resection and high recurrence rates[60], particularly when there is 1 cm or more of ingrowth. While not absolute, adenomas smaller than 1 cm in size in the proper clinical context (e.g., in an elderly patient or one with life-limiting comorbidities) may not require removal, and might be better served by surveillance alone or by doing nothing, unless the lesion causes a subsequent problem (such as biliary obstruction or pancreatitis)[25].
The general procedure for endoscopic papillectomy includes hot-snare resection of the ampulla followed by pancreatic duct (PD) stenting of the the ventral PD, except in cases of pancreas divisum, which greatly reduces the risk of post-ERCP pancreatitis following major papillectomy (Figure 12). Various techniques to facilitate post-papillectomy PD stenting have been devised, including first accessing the pancreas duct to perform a pancreatic sphincterotomy (which can sometimes make en bloc resection more difficult), or by injection of contrast tinted with indigo carmine or methylene blue into the PD so as to better identify the PD orifice after ampullectomy. Papillectomy over a guidewire left in the PD has also been described, which can enable easier post-papillectomy PD stenting[61]. Submucosal lifting of the area of the major papilla is not mandatory, and this technique can be helpful in some situations but not in others. Completion biliary and pancreatic sphincterotomies are often performed following major papillectomy, as the diathermic effect of hot-snare resection can adversely affect the function of any remaining biliary or pancreatic sphincters. In addition, biliary stenting may be required if bile drainage appears to be suboptimal to reduce the risk of cholangitis. Lastly, unless there is an absolute contraindication, 100 mg of per rectal indomethacin should be given in all instances to adult patients following papillectomy of the major papilla to reduce the risk of post-ERCP pancreatitis.
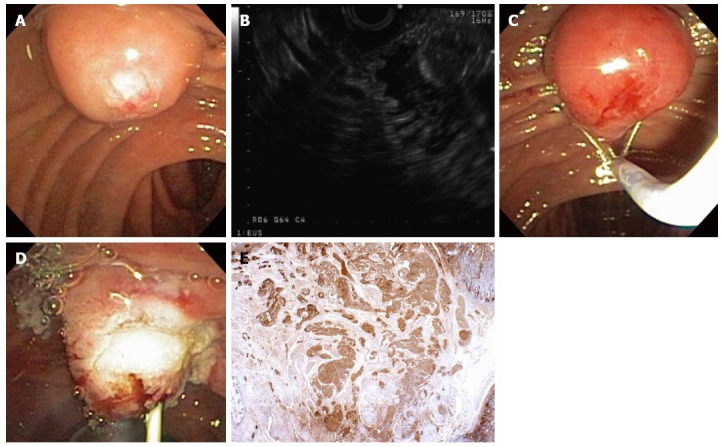
Figure 12.

Ampullary evaluation followed by endoscopic papillectomy and endoscopic retrograde cholangiopancreatography. A 2-cm adenoma of the Ampulla of Vater (major papilla) was referred for endoscopic management (A); Endoscopic ultrasonography showed no invasion of the ampullary lesion into the duodenal muscularis propria or pancreas (B); Sterile normal saline was injected to lift the ampullary adenoma (C); after which hot-snare resection was accomplished (D); The adenoma was resected en bloc and the cut distal bile duct was evident following papillectomy (E); A biliary sphincterotomy was performed (F) and a 5-Fr, 4-cm-long, polyethylene, single-pigtail stent was placed into the pancreatic duct of Wirsung (G). Rectal indomethacin was also administered to reduce the risk of post-ERCP pancreatitis. Endoclips were used to provide mucosal closure of the inferior margin of the resection.
In rare instances, an adenoma of the minor papilla may be found that requires resection. Just as of the major papilla, papillectomy of the minor papilla can be carried out by hot-snare resection with or without submucosal saline injection. In cases of normal pancreatic ductal anatomy (no pancreas divisum), prophylactic stenting of the main PD (via the ventral duct of Wirsung) is not necessary following minor papilla resection. If a minor papillotomy (sphincterotomy of the putative sphincter of Helly[62]) is done before or after minor papillectomy, a small-caliber prophylactic stent could be placed into the dorsal PD of Santorini, as long as it is in continuity with the main PD. However, in patients who require minor papillectomy who have pancreas divisum, prophylactic stenting of the dorsal duct is mandatory (Figure 13). Unless a patient has an absolute contraindication, rectal indomethacin should also be given following minor papillectomy.
Figure 13.

A 63-year-old woman presented with chronic abdominal pain and recurrent acute pancreatitis in the setting of pancreas divisum. Endoscopic retrograde cholangiopancreatography (ERCP) for minor papillotomy and pancreatic duct (PD) stenting was intended. During ERCP, the minor papilla was found to be enlarged to about 1-cm in size and was somewhat hard on palpation with the sphincterotome (A). A minor papillotomy was performed (B), the minor papilla was biopsied, and a pancreatic duct (PD) stent was placed. Pathology showed adenoma, and the patient returned for minor papillectomy several weeks later. The prior papillotomy had healed (C), and hot-snare resection was used to resect the minor papilla without a saline lift (D); No residual adenomatous tissue was seen after minor papillectomy (E); and biopsies of the resection margins provided pathologic confirmation of complete resection. A PD stent was left at the conclusion of the procedure (F) and rectal indomethacin was administered.
As with the resection of non-ampullary adenomas, en bloc resection is preferred during papillectomy. Larger ampullary lesions might require piecemeal resection, and large laterally spreading adenomas that involve the ampulla can be removed by a multimodality approach, such as with adjunctive use of underwater EMR[44].
ROLE OF ENDOSCOPIC ULTRASOUND IN DUODENAL LESION RESECTION
Endoscopic ultrasonography is a widely available and effective imaging modality that enables real-time assessment of a lesion’s depth of invasion (T-stage) and nodal status (N-stage). When considering duodenal lesions, EUS has a clear role in the evaluation and differentiation of subepithelial lesions. By using EUS-FNA, cytopathology of a subepithelial lesion can be obtained to diagnose carcinoids, leiomyomas, GISTs, pancreatic heterotopia, lymphomas, etc. Also, EUS-fine needle biopsy is now available and can provide a core sample for histopathological analysis.
However, the role of EUS in the evaluation of mucosally-based lesions in the GI tract, including in the duodenum, is more limited. High-frequency miniprobes (12-20 MHz) are better at delineating the walls of the GI tract than conventional echoendoscopes, but even high-frequency miniprobes can have difficulty in differentiating T1a (tumor invades the lamina propria) from T1b (tumor invades the submucosa) lesions[26,63]. As such, the surface characteristics seen by using high-definition white-light, with or without NBI or magnification chromoendoscopy, are often more helpful than EUS at evaluating a lesion for invasive cancer and for determining if endoscopic resection is feasible or if surgical resection will be required.
EUS does have an important role in assessing for intraductal extension of ampullary tumors, as well as for tumor invasion into or beyond the MP[25]. This allows the proper triage of patients directly to surgical intervention who might otherwise fail endoscopic papillectomy[64]; thereby sparing patients the risk of papillectomy and ERCP. The accuracy of EUS in this setting has been shown to be very high, and it has even been proposed that EUS be used in all cases prior to endoscopic papillectomy[65].
POST-RESECTION ENDOSCOPIC SURVEILLANCE
There is no standardization as to when patients should return for surveillance following endoscopic resection of various lesions in the duodenum. After resection of a large duodenal or ampullary adenoma, we and others favor a short-term, follow-up, surveillance endoscopy in 3 to 6 mo, which typically includes biopsies of the post-polypectomy scar even if there is no obvious nodularity. As mentioned earlier, data regarding the use of NBI and the “Kudo” pit-pattern in evaluation of mucosally-based duodenal neoplasia are limited. However, in our experience, if patients are brought back too soon (typically < 3 mo after resection), it can be difficult to differentiate the normal inflammatory response associated with mucosal healing from the vascular pattern seen with recurrent adenoma. Short-term surveillance endoscopy is particularly important following piecemeal resection, as any remaining microscopic neoplasia can lead to an endoscopically visible residual or recurrent lesion after a few months. Also, any recurrent or residual adenoma is best resected or ablated while it is still small, so as to maximize the chance for a curative resection. If the 3- to 6-mo follow-up endoscopy shows no residual or recurrent neoplasia, then we suggest another surveillance endoscopy at 1 year.
Additionally, patients with duodenal adenomas are probably at increased risk for colorectal neoplasia[66,67]. As such, the American Society for Gastrointestinal Endoscopy Guideline on “The role of endoscopy in ampullary and duodenal adenomas”[25] suggests “offer[ing] screening colonoscopy to all patients who have duodenal or ampullary adenomas.”
WHEN TO REFER TO SURGERY
The decision as to which duodenal lesions should be referred to surgery can be complex. In general, GISTs or large or multifocal duodenal carcinoids should be referred for surgical resection following endoscopic diagnosis (which can be done by bite-on-bite biopsies, EUS-FNA, endoscopic unroofing of a lesion for histopathological confirmation, etc.). For dysplasia arising from the mucosa, the central issue is one of lymph node metastasis. Lesions that do not invade into the submucosa, even if high-grade dysplasia is present, should not be associated with any risk of lymph node metastasis. Such lesions can be amenable to endoscopic resection, and the decision to refer certain lesions to surgery then centers around whether or not the lesion is endoscopically resectable from a technical perspective.
While some early adenocarcinomas that invade only the superficial duodenal submucosa could potentially be removed by endoscopic resection, it is generally recommended to refer these lesions for surgical resection[68]. Furthermore, as the duodenum is the most dangerous location in which to perform ESD, and as piecemeal duodenal EMR has been associated with recurrence rates of upwards of 25%, early duodenal adenocarcinomas are probably best treated by surgery, except in situations where a patient’s decision-making or comorbidities make surgery impossible.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest, financial or otherwise, to report that are relevant to this manuscript. Dr. Wang discloses research funding from Cook Medical on the topic of metal biliary stents. Dr. Gaspar and Dr. Stelow have no disclosures to make with regard to this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 12, 2015
First decision: September 11, 2015
Article in press: November 9, 2015
P- Reviewer: Chen MJ, De Palma GD S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Ghazi A, Ferstenberg H, Shinya H. Endoscopic gastroduodenal polypectomy. Ann Surg. 1984;200:175–180. doi: 10.1097/00000658-198408000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jepsen JM, Persson M, Jakobsen NO, Christiansen T, Skoubo-Kristensen E, Funch-Jensen P, Kruse A, Thommesen P. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483–487. doi: 10.3109/00365529409092458. [DOI] [PubMed] [Google Scholar]
- 3.Honda T, Yamamoto H, Osawa H, Yoshizawa M, Nakano H, Sunada K, Hanatsuka K, Sugano K. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270–274. doi: 10.1111/j.1443-1661.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- 4.Oka S, Tanaka S, Nagata S, Hiyama T, Ito M, Kitadai Y, Yoshihara M, Haruma K, Chayama K. Clinicopathologic features and endoscopic resection of early primary nonampullary duodenal carcinoma. J Clin Gastroenterol. 2003;37:381–386. doi: 10.1097/00004836-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Endo M, Abiko Y, Oana S, Kudara N, Chiba T, Suzuki K, Koizuka H, Uesugi N, Sugai T. Usefulness of endoscopic treatment for duodenal adenoma. Dig Endosc. 2010;22:360–365. doi: 10.1111/j.1443-1661.2010.01014.x. [DOI] [PubMed] [Google Scholar]
- 6.Bal A, Joshi K, Vaiphei K, Wig JD. Primary duodenal neoplasms: a retrospective clinico-pathological analysis. World J Gastroenterol. 2007;13:1108–1111. doi: 10.3748/wjg.v13.i7.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tung CF, Chow WK, Peng YC, Chen GH, Yang DY, Kwan PC. Bleeding duodenal lipoma successfully treated with endoscopic polypectomy. Gastrointest Endosc. 2001;54:116–117. doi: 10.1067/mge.2001.113916. [DOI] [PubMed] [Google Scholar]
- 8.He Z, Sun C, Zheng Z, Yu Q, Wang T, Chen X, Cao H, Liu W, Wang B. Endoscopic submucosal dissection of large gastrointestinal stromal tumors in the esophagus and stomach. J Gastroenterol Hepatol. 2013;28:262–267. doi: 10.1111/jgh.12056. [DOI] [PubMed] [Google Scholar]
- 9.Miki Y, Kurokawa Y, Hirao M, Fujitani K, Iwasa Y, Mano M, Nakamori S, Tsujinaka T. Survival analysis of patients with duodenal gastrointestinal stromal tumors. J Clin Gastroenterol. 2010;44:97–101. doi: 10.1097/MCG.0b013e3181b8e754. [DOI] [PubMed] [Google Scholar]
- 10.Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28:524–530. doi: 10.1007/s00464-013-3197-8. [DOI] [PubMed] [Google Scholar]
- 11.Binmoeller KF, Shah JN, Bhat YM, Kane SD. Suck-ligate-unroof-biopsy by using a detachable 20-mm loop for the diagnosis and therapy of small subepithelial tumors (with video) Gastrointest Endosc. 2014;79:750–755. doi: 10.1016/j.gie.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Basford PJ, Bhandari P. Endoscopic management of nonampullary duodenal polyps. Therap Adv Gastroenterol. 2012;5:127–138. doi: 10.1177/1756283X11429590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Palma GD, Masone S, Siciliano S, Maione F, Falleti J, Mansueto G, De Rosa G, Persico G. Endocrine carcinoma of the major papilla: report of two cases and review of the literature. Surg Oncol. 2010;19:235–242. doi: 10.1016/j.suronc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Kim GH, Kim JI, Jeon SW, Moon JS, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Lee YC. Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J Gastroenterol Hepatol. 2014;29:318–324. doi: 10.1111/jgh.12390. [DOI] [PubMed] [Google Scholar]
- 15.Culver EL, McIntyre AS. Sporadic duodenal polyps: classification, investigation, and management. Endoscopy. 2011;43:144–155. doi: 10.1055/s-0030-1255925. [DOI] [PubMed] [Google Scholar]
- 16.Gao YP, Zhu JS, Zheng WJ. Brunner’s gland adenoma of duodenum: a case report and literature review. World J Gastroenterol. 2004;10:2616–2617. doi: 10.3748/wjg.v10.i17.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akino K, Kondo Y, Ueno A, Yamazaki K, Hosokawa M, Shimoji H, Adachi T, Honda S, Ichiyanagi S, Akahonai Y, et al. Carcinoma of duodenum arising from Brunner’s gland. J Gastroenterol. 2002;37:293–296. doi: 10.1007/s005350200038. [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Jung IS, Cheon GJ, Cho JY, Lee JS, Jin SY, Shim CS. Endoscopic removal of giant Brunneroma presenting as a large pedunculated polyp. Endoscopy. 2007;39 Suppl 1:E72. doi: 10.1055/s-2006-945153. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Hirasaki S, Ikeda F, Yumoto E, Yamane H, Matsubara M. Three cases of Solitary Peutz-Jeghers-type hamartomatous polyp in the duodenum. World J Gastroenterol. 2008;14:944–947. doi: 10.3748/wjg.14.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kedia P, Brensinger C, Ginsberg G. Endoscopic predictors of successful endoluminal eradication in sporadic duodenal adenomas and its acute complications. Gastrointest Endosc. 2010;72:1297–1301. doi: 10.1016/j.gie.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Navaneethan U, Lourdusamy D, Mehta D, Lourdusamy V, Venkatesh PG, Sanaka MR. Endoscopic resection of large sporadic non-ampullary duodenal polyps: efficacy and long-term recurrence. Surg Endosc. 2014;28:2616–2622. doi: 10.1007/s00464-014-3512-z. [DOI] [PubMed] [Google Scholar]
- 22.Bülow S, Björk J, Christensen IJ, Fausa O, Järvinen H, Moesgaard F, Vasen HF. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381–386. doi: 10.1136/gut.2003.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783–785. doi: 10.1016/s0140-6736(89)90840-4. [DOI] [PubMed] [Google Scholar]
- 24.Spigelman AD, Talbot IC, Penna C, Nugent KP, Phillips RK, Costello C, DeCosse JJ. Evidence for adenoma-carcinoma sequence in the duodenum of patients with familial adenomatous polyposis. The Leeds Castle Polyposis Group (Upper Gastrointestinal Committee) J Clin Pathol. 1994;47:709–710. doi: 10.1136/jcp.47.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, Rajan E, Shen B, Zuckerman MJ, Fanelli RD, Van Guilder T, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849–854. doi: 10.1016/j.gie.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 26.Wang AY. The international emergence of endoscopic submucosal dissection for early gastric cancer. Gastrointest Endosc. 2011;73:928–931. doi: 10.1016/j.gie.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 27.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Ceron M, van den Broek FJ, Mathus-Vliegen EM, Boparai KS, van Eeden S, Fockens P, Dekker E. The role of high-resolution endoscopy and narrow-band imaging in the evaluation of upper GI neoplasia in familial adenomatous polyposis. Gastrointest Endosc. 2013;77:542–550. doi: 10.1016/j.gie.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Uchiyama Y, Imazu H, Kakutani H, Hino S, Sumiyama K, Kuramochi A, Tsukinaga S, Matsunaga K, Nakayoshi T, Goda K, et al. New approach to diagnosing ampullary tumors by magnifying endoscopy combined with a narrow-band imaging system. J Gastroenterol. 2006;41:483–490. doi: 10.1007/s00535-006-1800-7. [DOI] [PubMed] [Google Scholar]
- 30.Kudo SE, Kashida H. Flat and depressed lesions of the colorectum. Clin Gastroenterol Hepatol. 2005;3:S33–S36. doi: 10.1016/s1542-3565(05)00283-1. [DOI] [PubMed] [Google Scholar]
- 31.ASGE Technology Committee. High-definition and high-magnification endoscopes. Gastrointest Endosc. 2014;80:919–927. doi: 10.1016/j.gie.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 33.Wang AY, Ahmad NA, Zaidman JS, Brensinger CM, Lewis JD, Long WB, Kochman ML, Ginsberg GG. Endoluminal resection for sessile neoplasia in the GI tract is associated with a low recurrence rate and a high 5-year survival rate. Gastrointest Endosc. 2008;68:160–169. doi: 10.1016/j.gie.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos) Gastrointest Endosc. 2009;69:66–73. doi: 10.1016/j.gie.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 35.Hirasawa R, Iishi H, Tatsuta M, Ishiguro S. Clinicopathologic features and endoscopic resection of duodenal adenocarcinomas and adenomas with the submucosal saline injection technique. Gastrointest Endosc. 1997;46:507–513. doi: 10.1016/s0016-5107(97)70005-1. [DOI] [PubMed] [Google Scholar]
- 36.Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806–810. doi: 10.1055/s-2008-1077619. [DOI] [PubMed] [Google Scholar]
- 37.Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc. 2010;71:754–759. doi: 10.1016/j.gie.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 38.Kim HK, Chung WC, Lee BI, Cho YS. Efficacy and long-term outcome of endoscopic treatment of sporadic nonampullary duodenal adenoma. Gut Liver. 2010;4:373–377. doi: 10.5009/gnl.2010.4.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marques J, Baldaque-Silva F, Pereira P, Arnelo U, Yahagi N, Macedo G. Endoscopic mucosal resection and endoscopic submucosal dissection in the treatment of sporadic nonampullary duodenal adenomatous polyps. World J Gastrointest Endosc. 2015;7:720–727. doi: 10.4253/wjge.v7.i7.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fanning SB, Bourke MJ, Williams SJ, Chung A, Kariyawasam VC. Giant laterally spreading tumors of the duodenum: endoscopic resection outcomes, limitations, and caveats. Gastrointest Endosc. 2012;75:805–812. doi: 10.1016/j.gie.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 41.Conio M, De Ceglie A, Filiberti R, Fisher DA, Siersema PD. Cap-assisted EMR of large, sporadic, nonampullary duodenal polyps. Gastrointest Endosc. 2012;76:1160–1169. doi: 10.1016/j.gie.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video) Gastrointest Endosc. 2012;75:1086–1091. doi: 10.1016/j.gie.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Binmoeller KF, Shah JN, Bhat YM, Kane SD. “Underwater” EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video) Gastrointest Endosc. 2013;78:496–502. doi: 10.1016/j.gie.2013.03.1330. [DOI] [PubMed] [Google Scholar]
- 44.Flynn MM, Cox DG, Strand DS, Mann JA, Sauer BG, Shami VM, Wang AY. Wide-field endoscopic resection of a large laterally spreading adenoma that encompassed the major papilla by combined ampullectomy, EMR, and underwater EMR. Gastrointest Endosc. 2015;81:1270–1271. doi: 10.1016/j.gie.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 45.Flynn MM, Wang AY. Underwater endoscopic mucosal resection of large duodenal adenomas. Video J Encyclop GI Endosc. 2014;3-4:84–86. [Google Scholar]
- 46.Binmoeller KF. Underwater endoscopic mucosal resection. J Interv Gastroenterol. 2014;4:113–116. [Google Scholar]
- 47.Wang AY, Flynn MM, Patrie JT, Cox DG, Bleibel W, Mann JA, Sauer BG, Shami VM. Underwater endoscopic mucosal resection of colorectal neoplasia is easily learned, efficacious, and safe. Surg Endosc. 2014;28:1348–1354. doi: 10.1007/s00464-013-3297-5. [DOI] [PubMed] [Google Scholar]
- 48.Wang AY, Emura F, Oda I, Cox DG, Kim HS, Yeaton P. Endoscopic submucosal dissection with electrosurgical knives in a patient on aspirin therapy (with video) Gastrointest Endosc. 2010;72:1066–1071. doi: 10.1016/j.gie.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto S, Yoshida Y. Selection of appropriate endoscopic therapies for duodenal tumors: an open-label study, single-center experience. World J Gastroenterol. 2014;20:8624–8630. doi: 10.3748/wjg.v20.i26.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nonaka S, Oda I, Tada K, Mori G, Sato Y, Abe S, Suzuki H, Yoshinaga S, Nakajima T, Matsuda T, et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47:129–135. doi: 10.1055/s-0034-1390774. [DOI] [PubMed] [Google Scholar]
- 51.Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, Gluck M, Jiranek G, Patterson D, Schembre D, et al. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923–932. doi: 10.1016/j.gie.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 52.Ceppa EP, Burbridge RA, Rialon KL, Omotosho PA, Emick D, Jowell PS, Branch MS, Pappas TN. Endoscopic versus surgical ampullectomy: an algorithm to treat disease of the ampulla of Vater. Ann Surg. 2013;257:315–322. doi: 10.1097/SLA.0b013e318269d010. [DOI] [PubMed] [Google Scholar]
- 53.De Palma GD. Endoscopic papillectomy: indications, techniques, and results. World J Gastroenterol. 2014;20:1537–1543. doi: 10.3748/wjg.v20.i6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Napoleon B, Gincul R, Ponchon T, Berthiller J, Escourrou J, Canard JM, Boyer J, Barthet M, Ponsot P, Laugier R, et al. Endoscopic papillectomy for early ampullary tumors: long-term results from a large multicenter prospective study. Endoscopy. 2014;46:127–134. doi: 10.1055/s-0034-1364875. [DOI] [PubMed] [Google Scholar]
- 55.De Palma GD, Luglio G, Maione F, Esposito D, Siciliano S, Gennarelli N, Cassese G, Persico M, Forestieri P. Endoscopic snare papillectomy: a single institutional experience of a standardized technique. A retrospective cohort study. Int J Surg. 2015;13:180–183. doi: 10.1016/j.ijsu.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 56.Moon JH, Choi HJ, Lee YN. Current status of endoscopic papillectomy for ampullary tumors. Gut Liver. 2014;8:598–604. doi: 10.5009/gnl14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Binmoeller KF, Boaventura S, Ramsperger K, Soehendra N. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc. 1993;39:127–131. doi: 10.1016/s0016-5107(93)70051-6. [DOI] [PubMed] [Google Scholar]
- 58.Cheng CL, Sherman S, Fogel EL, McHenry L, Watkins JL, Fukushima T, Howard TJ, Lazzell-Pannell L, Lehman GA. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757–764. doi: 10.1016/s0016-5107(04)02029-2. [DOI] [PubMed] [Google Scholar]
- 59.Desilets DJ, Dy RM, Ku PM, Hanson BL, Elton E, Mattia A, Howell DA. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202–208. doi: 10.1067/mge.2001.116564. [DOI] [PubMed] [Google Scholar]
- 60.Kahaleh M, Shami VM, Brock A, Conaway MR, Yoshida C, Moskaluk CA, Adams RB, Tokar J, Yeaton P. Factors predictive of malignancy and endoscopic resectability in ampullary neoplasia. Am J Gastroenterol. 2004;99:2335–2339. doi: 10.1111/j.1572-0241.2004.40391.x. [DOI] [PubMed] [Google Scholar]
- 61.Moon JH, Cha SW, Cho YD, Ryu CB, Cheon YK, Kwon KW, Kim YS, Kim YS, Lee JS, Lee MS, et al. Wire-guided endoscopic snare papillectomy for tumors of the major duodenal papilla. Gastrointest Endosc. 2005;61:461–466. doi: 10.1016/s0016-5107(04)02649-5. [DOI] [PubMed] [Google Scholar]
- 62.Singh I. The terminal part of the accessory pancreatic duct and its musculature. J Anat. 1962;96:505–508. [PMC free article] [PubMed] [Google Scholar]
- 63.Edge SB. American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 64.Gaspar J, Shami VM. The role of EUS in ampullary lesions: is the answer black and white? Gastrointest Endosc. 2015;81:389–390. doi: 10.1016/j.gie.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 65.Ridtitid W, Schmidt SE, Al-Haddad MA, LeBlanc J, DeWitt JM, McHenry L, Fogel EL, Watkins JL, Lehman GA, Sherman S, et al. Performance characteristics of EUS for locoregional evaluation of ampullary lesions. Gastrointest Endosc. 2015;81:380–388. doi: 10.1016/j.gie.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Apel D, Jakobs R, Weickert U, Riemann JF. High frequency of colorectal adenoma in patients with duodenal adenoma but without familial adenomatous polyposis. Gastrointest Endosc. 2004;60:397–399. doi: 10.1016/s0016-5107(04)01712-2. [DOI] [PubMed] [Google Scholar]
- 67.Murray MA, Zimmerman MJ, Ee HC. Sporadic duodenal adenoma is associated with colorectal neoplasia. Gut. 2004;53:261–265. doi: 10.1136/gut.2003.025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsumoto S, Miyatani H, Yoshida Y. Future directions of duodenal endoscopic submucosal dissection. World J Gastrointest Endosc. 2015;7:389–395. doi: 10.4253/wjge.v7.i4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]


