Abstract
Laparoscopic gastrectomy has been widely accepted as a standard alternative for the treatment of early-stage gastric adenocarcinoma because of its favorable short-term outcomes. Although controversies exist, such as establishing clear indications, proper preoperative staging, and oncologic safety, experienced surgeons and institutions have applied this approach, along with various types of function-preserving surgery, for the treatment of advanced gastric cancer. With technical advancement and the advent of state-of-the-art instruments, indications for laparoscopic gastrectomy are expected to expand as far as locally advanced gastric cancer. Laparoscopic gastrectomy appears to be promising; however, scientific evidence necessary to generalize this approach to a standard treatment for all relevant patients and care providers remains to be gathered. Several multicenter, prospective randomized trials in high-incidence countries are ongoing, and results from these trials will highlight the short- and long-term outcomes of the approach. In this review, we describe up-to-date findings and critical issues regarding laparoscopic gastrectomy for gastric cancer.
Keywords: Gastrectomy, Laparoscopic resection, Early gastric cancer, Stomach neoplasms, Advanced gastric cancer, Minimally invasive surgery
Core tip: Laparoscopic gastrectomy has been widely accepted as a standard alternative to open gastrectomy for the treatment of early gastric cancer. Its clinical indications are expanding to include more extensive surgeries along with more sophisticated conserving or function-preserving surgeries. Although some controversies limit extensive application of the procedure, including a lack of evidence of oncologic safety and discrepancy between high- and low-incidence countries, laparoscopic gastrectomy for gastric adenocarcinoma could become widespread as new technologies, improved surgical techniques, and evidence from ongoing multicenter trials emerge.
INTRODUCTION
Surgical resection is the main curative modality to treat gastric adenocarcinoma. Although this multidisciplinary approach can also provide effective and individualized care for the gastric cancer patient, it has shown only incremental progress to improve survival. Meanwhile, over the last two decades, the surgical modality for gastric cancer has been dramatically altered and improved in various ways that limit surgical insult to the patient, subsequently facilitating recovery[1,2]. Using laparoscopic gastrectomy to treat early gastric cancer in Eastern countries, where detection is increasing, could expedite the development and propagation of this approach for the treatment of gastric cancer[3-5]. In this review, we describe the current evidence promoting laparoscopic gastrectomy for gastric adenocarcinoma and concerns regarding its use.
INDICATIONS OF LAPAROSCOPIC SURGERY
Early gastric cancer has a favorable long-term survival when treated with curative surgical resection and lymph node dissection. When the tumor is confined to the mucosal layer, long-term survival is almost 99%; survival is 96% when the tumor is limited to the submucosa[6,7]. Ideally, endoscopic mucosal resection or endoscopic submucosal dissection is the best way to preserve patient quality of life. However, this approach can only be applied to limited number of patients that meet strict criteria and can only prudently be performed at specialized centers in high-incidence countries[8]. The initial indication for laparoscopic surgery for gastric adenocarcinoma is a tumor limited to the submucosal layer of the mid-to-lower part of the stomach with no evidence of regional lymph node metastasis, which does not necessarily require extended lymph node dissection, except in those cases suitable for endoscopic treatment[9,10]. Laparoscopic total gastrectomy is indicated for a proximal lesion such as T1N0[10]. In Eastern Asian countries, the current indication for laparoscopic gastrectomy has been expanded to serosa-negative advanced cancers with or without limited involvement of perigastric lymph nodes[11]. However, experienced surgeons have explored the use of laparoscopic surgery for advanced gastric cancer beyond these indications[12-14], and the use of the laparoscopic approach for selected advanced cancers has no detrimental effects compared to open conventional surgery. Despite this early promise, data from well-designed studies are still too scarce to recommend the use of this approach in cases requiring extensive lymph node dissection for the treatment of advanced gastric cancer. Randomized clinical trials examining outcomes of laparoscopic gastrectomy vs open gastrectomy for gastric cancer are listed in Table 1[15-22].
Table 1.
Recent randomized clinical trials of laparoscopic vs open gastrectomy for the treatment of gastric cancer
| Study | Year | Eligibility | Procedure | LND extent | No. of patient (LAG/OG) | Operative time | Blood loss | No. of retrieved LN | Hospital stay | Morbidity | Mortality |
| Kitano et al[1] | 2002 | cT1 | LADG/ODG | Not mentioned | 14/14 | ODG | LADG | Equivalent | Equivalent | Equivalent | Equivalent |
| Fujii et al[15] | 2003 | cT1 | LADG/ODG | Not mentioned | 10/10 | ODG | Equivalent | NE | NE | Equivalent | Equivalent |
| Hayashi et al[16] | 2005 | cT1 | LADG/ODG | Not mentioned | 14/14 | ODG | Equivalent | Equivalent | LADG | NE | Equivalent |
| Huscher et al[17] | 2005 | cT1-4N0-2 | TLDG/ODG | D1, D2 | 30/29 | Equivalent | TLDG | Equivalent | TLDG | Equivalent | Equivalent |
| Lee et al[18] | 2005 | cT1 | LADG/ODG | D2 | 24/23 | ODG | Equivalent | Equivalent | Equivalent | LADG | NE |
| Kim et al[19] | 2008 | cT1N0-1 | LADG/ODG | D1, D2 | 82/82 | ODG | LADG | ODG | LADG | NE | NE |
| Kim et al[11] | 2010 | cT1-2N0-1 | LADG/ODG | D1 + beta, D2 | 179/163 | ODG | LADG | NE | NE | Equivalent | Equivalent |
| Cai et al[14] | 2011 | cT2-3 | LAG/OG (DG, TG, PG) | D2 | 49/47 | OG | Equivalent | Equivalent | Equivalent | Equivalent | NE |
| Sakuramoto et al[20] | 2013 | cT1 | LADG/ODG | D1+beta | 31/32 | ODG | LADG | Equivalent | Equivalent | Equivalent | NE |
| Takiguchi et al[21] | 2013 | cTNMI | LADG/ODG | D1, D2 | 20/20 | ODG | LADG | Equivalent | LADG | NE | NE |
| Aoyama et al[22] | 2014 | cTNMI | LADG/ODG | D1 + beta, D2 | 13/13 | ODG | LADG | Equivalent | NE | Equivalent | Equivalent |
LND: Lymph node dissection; LADG: Laparoscopy-assisted distal gastrectomy; TLDG: Totally laparoscopic distal gastrectomy; ODG: Open distal gastrectomy; LAG; Laparoscopy-assisted gastrectomy; OG: Open gastrectomy; DG: Distal gastrectomy; TG: Total gastrectomy; PG: Proximal gastrectomy; LN: Lymph node; NE: Not evaluated. Favored group in each value were shown.
EVIDENCE FOR THE USE OF LAPAROSCOPIC SURGERY IN EARLY GASTRIC CANCER
Since the first published report regarding laparoscopic gastrectomy for early gastric cancer[2], many retrospective studies and small randomized clinical trials have shown the short-term benefits of laparoscopic gastrectomy over open conventional surgery and long-term outcomes that are comparable between the two[1,11,16,17,23,24]. Several meta-analyses also confirm that laparoscopic gastrectomy is an equivalent method to conventional open surgery[25-28]. However, these reports were conducted mainly with randomized clinical trials and with distal subtotal gastrectomy. Generally, laparoscopic distal gastrectomy causes less blood loss, shorter or similar hospital stays, lower complication rates, and comparable mortality rates compared to open conventional surgery[11,25]. However, a reduced number of retrieved lymph nodes, as well as longer operation time, is a weaknesses of the approach[25]. An interim analysis of 179 laparoscopic and 161 open gastrectomy patients from the largest randomized clinical trial for preoperatively diagnosed early gastric cancer (finished enrollment: 1416 patients; 705 laparoscopic vs 711 open distal gastrectomy) revealed similar complication and mortality rates between the groups[11].
Among all gastric cancer cases, the penetration rate of laparoscopic total gastrectomy is quite low because of its technical difficulties - even for experienced surgeons - compared to laparoscopic distal gastrectomy[27,29-33]; this low rate of use may be attributed especially to the difficulties of lymph node dissection and anastomosis[31,33-37]. Well-designed trials regarding this procedure are, therefore, scarce, and only some experienced surgeons report the safety and feasibility of laparoscopic lymph node dissection and their anastomosis methods in their retrospective analyses[34,38,39]. A multicenter, single-arm, phase II clinical trial in Korea is expected to highlight long-term outcomes, as well as the feasibility and safety of laparoscopic total gastrectomy, for stage I cancer [Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) 03 trial, NCT01584336].
EVIDENCE FOR THE USE OF LAPAROSCOPIC SURGERY FOR ADVANCED GASTRIC CANCER
For the treatment of advanced gastric cancer, lymph nodes around the common hepatic artery (LN 8), celiac axis (LN 9), and splenic artery (LN 11p) should be completely removed, as should the lymph nodes around the proper hepatic artery (LN 12a) in cases of distal subtotal gastrectomy, and those around the distal part of the splenic artery (LN #11d) and splenic hilum (LN #10) in cases of total gastrectomy, according to Japanese guideline[40,41]. Figure 1 shows an intraoperative view during and after laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy for advanced gastric cancer. Regardless of the discrepancy in what constitutes adequate lymph node dissection for advanced cancer between Eastern and Western countries, lymph node dissection using laparoscopy is irrefutably technically demanding[8,40,42]. The difficulties in laparoscopic lymph node dissection might explain recent meta-analyses’ reports of fewer lymph nodes retrieved in laparoscopy than open surgery[25,27,28,43]. However, this finding could be clinically unimportant because there was no significant difference in the proportion within each group with 15 or more lymph nodes retrieved for proper staging[25]. Furthermore, studies evaluating laparoscopic gastrectomy for advanced cancer reported acceptable short-term outcomes when the procedure was performed by experienced surgeons[12,17,44-47]. Although evidence is still preliminary, laparoscopic gastrectomy with D2 lymph node dissection showed less blood loss and postoperative pain, shorter hospital stays, and similar complication and overall survival rates[48]. Ongoing, large-scale phase II or III multicenter trials are evaluating the feasibility and safety of laparoscopic surgery for advanced cancer in the Eastern countries: the KLASS 02 (NCT 01456598), the Japanese Laparoscopic Gastric Surgery Study group (JLSSG0901; UMIN-CTR 000003420), and the Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS01; NCT01609309). The T-stage inclusion criteria are similar among studies (T2-T4a); however, regarding N-stage, more advanced disease is included in the JLSSG and CLASS trials. The primary outcome in the KLASS and CLASS trials is 3-year disease free survival, whereas the incidence of anastomosis leakage and pancreatic fistula (phase II) and relapse-free survival (phase III) is the primary outcome of the JLSSG trial. These well-designed clinical trials could give answers regarding the short- and long-term safety of laparoscopic distal gastrectomy for advanced gastric cancer. In terms of laparoscopic total gastrectomy for advanced gastric cancer, several experienced surgeons report similar short-term results to open conventional surgery in their retrospective analyses[49-53]. However, their data include selected cases in terms of depth of tumor invasion, extent of nodal involvement, tumor size, and status of adjacent organ invasion; thus, the results are biased. The feasibility and safety of more difficult laparoscopic total gastrectomy procedures, such as those requiring removal of bulky, hard, or fixed tumors or lymph nodes, multi-organ resection, and operations for remnant stomach should be elucidated for even the most experienced surgeons[34,54-57]. So far, there is no large-scale, multicenter randomized controlled trial for assessing the role of laparoscopic total gastrectomy for advanced proximal gastric cancer.
Figure 1.
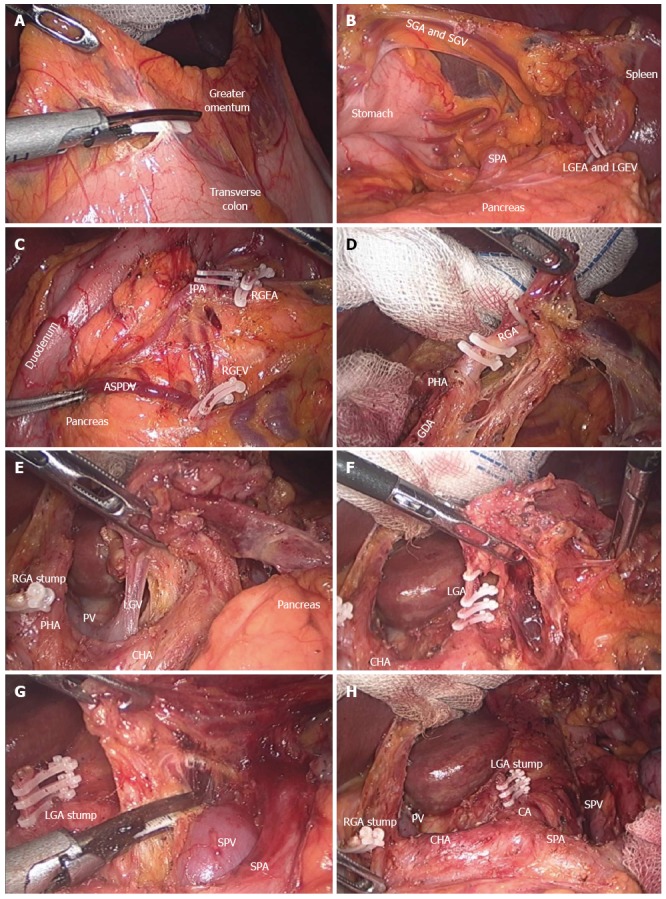
Intraoperative view during distal subtotal gastrectomy with D2 lymph node dissection. A: Division of the greater omentum; B: Isolation of the LGEA and LGEV; C: Exposure of the RGEA, RGEV, and ASPDV; D: Isolation of the RGA; E: Dissection of the hepatoduodenal ligament, exposure of the PV and isolation of the LGV; F: Isolation and ligation of the LGA; G: Dissection along the SPA and SPV; H: Suprapancreatic view after D2 lymph node dissection. RGEA: Right gastroepiploic artery; LGEA: Left gastroepiploic artery; LGEV: Left gastroepiploic vein; RGEV: Right gastroepiploic vein; ASPDV: Anterior superior pancreaticoduodenal vein; RGA: Right gastric artery; LGA: Left gastric artery; SPA: Splenic artery; SPV: Splenic vein; CHA: Common hepatic artery; GDA: Gastroduodenal artery; IPA: Infrapyloric artery; LGV: Left gastric vein; PHA: Proper hepatic artery; PV: Portal vein; SGA: Short gastric artery; SGV: Short gastric vein.
TECHNICAL CONSIDERATIONS
Laparoscopy-assisted gastrectomy requires mini-laparotomy for specimen removal and extracorporeal anastomoses. It is still widely performed and applied in most clinical trials. However, this method is troublesome, especially in obese patients[37,58,59]. Intracorporeal anastomosis, which made this procedure the so-called “totally laparoscopic surgery,” enables more sophisticated reconstruction methods. It could reduce operation time, decrease length of the mini-laparotomy, and shift the incision for specimen retrieval below the umbilicus, which results in reduced incision-related pain[60,61]. After intracorporeal Billroth I anastomosis was safely performed with delta-shaped anastomosis, totally laparoscopic distal subtotal gastrectomy gained popularity[62,63]. Many efforts have also been made to restore esophagojejunal anastomosis intracorporeally during total gastrectomy[36,37,64-68]. Recently, intracorporeal side-to-side esophagojejunostomy using linear staples was introduced, demonstrating its safety and feasibility[37,69]. Hand-sewn anastomosis is no longer an attractive choice for the procedure; however, reconstruction methods vary according to each surgeon’s preference. The laparoscopic hand-sewn technique is still commonly performed to close the common entry hole of endolinear staplers during intracorporeal anastomosis.
Most surgeons use at least five trocars for laparoscopic gastrectomy. However, with advanced techniques and improved technology, reduced port or even single-port (incision) laparoscopic gastrectomy has been safely performed[70-73]. However, because these procedures were reported from experienced surgeons or exceptionally high-volume institutions, technical feasibility, safety, and standardization of the procedure have not yet been fully elucidated[72,73]. So far, it is difficult to assess whether reduced port or single incision gastrectomy is less invasive than conventional laparoscopic surgery in terms of postoperative clinical outcomes[74]. However, a recent comparison of 50 cases of single-port laparoscopic distal gastrectomy with 50 multi-port surgery demonstrated better short-term results for single-port procedures, including reduced blood loss, lower maximum pain score on the operative day and postoperative day 1, less use of parenteral analgesics, and lower C-reactive protein levels on postoperative day 5, although the reduction of operative insult in single-port surgery did not extend to reducing length of hospital stays[73].
LIMITED EXTENT SURGERY USING LAPAROSCOPY
Limiting the extent of surgery is a goal of minimally invasive gastrectomy because of its ability to preserve patient function. Thus, laparoscopic techniques have potential to ease patients’ recovery and have been investigated for feasibility and efficacy in sentinel lymph node navigation surgery, pylorus-preserving gastrectomy, and proximal gastrectomy.
Partial resection of the stomach with sentinel basin lymph node dissection for small, T1N0M0 cancer is currently indicated for sentinel lymph node navigation surgery for gastric cancer[75,76]. A randomized clinical trial (JCOG 0302) using an intraoperative dye injection method showed a higher false-negative rate (46%), and accordingly stopped patient accrual[77]. Several meta-analyses have also pointed out the unsatisfactorily higher false-negative rate of sentinel lymph node navigation surgery[78-80]. However, a recent clinical trial by Kitagawa et al[75] highlighted acceptable results, with 93% accuracy of detecting lymph node metastasis and only a 7% false-negative rate. The learning curve effect is likely to reduce false-negative rates. Standardization of the procedure and relevant resources are required to widely adopt sentinel lymph node navigation surgery for gastric cancer. A multicenter phase III trial [Sentinel Node Oriented Tailored Approach (SENORITA) trial, NCT01804998] of laparoscopic sentinel node biopsy and stomach-preserving surgery has been launched to examine the benefits of sentinel lymph node navigation surgery compared to conventional laparoscopic gastrectomy. Indications for this trial include preoperatively-diagnosed T1N0 tumors less than 3 cm and receipt of laparoscopic segmental or wedge resection with sentinel basin lymph node dissection. The primary endpoint is 3-year disease free survival.
Another method capable of preserving function is pylorus-preserving gastrectomy. This procedure has been cautiously developed based on our understanding of patterns of lymph node metastasis around the pylorus. The likelihood of lymph node metastasis in the suprapyloric (LN 5) and the infrapyloric (LN 6) area is quite insignificant in the selected patients on whom the procedure has been performed[81-85]. Pylorus-preserving gastrectomy is indicated for middle third, stage cT1N0 cancer located more than 4 cm from the pylorus[81-83]. Advantages compared to conventional distal subtotal gastrectomy include decreased incidences of dumping syndrome, bile reflux, and gallstone formation[81,82,86]. Long-term oncologic results showed acceptable survival rates[87,88] if the procedure was performed by experienced surgeons on carefully selected patients; thus, the advantages for nutritional profiles and long-term consequences for quality of life of the patients should be elucidated by well-designed multicenter trials.
Proximal gastrectomy is a suitable option for proximal early gastric cancer that preserves gastric function after operation; higher rates of postoperative complication and long-term nutritional deterioration can occur in patients after total gastrectomy[89-91]. However, laparoscopic proximal gastrectomy - especially its reconstruction methods - is yet to be standardized and has not shown significant benefits over conventional total gastrectomy[92]. Its major drawbacks include complications involving esophagojejunostomy (i.e., stenosis), reflux esophagitis, and trivial improvement of nutritional profiles. A recent multicenter retrospective comparison study of nutritional profiles and some aspects associated with dumping syndrome revealed that proximal gastrectomy was superior to those of total gastrectomy[93]. However, there were no significant changes in body weight during the study period. The aforementioned function-preserving surgery should, therefore, be first standardized technically and conceptually and evaluated by multicenter clinical trials.
FUTURE PERSPECTIVES ON LAPAROSCOPIC GASTRECTOMY FOR GASTRIC CANCER
As surgical techniques and state-of-the-art technology improve, numerous innovative procedures will be developed and refined to increase quality of life for patients receiving gastrectomy for gastric cancer. Experienced surgeons in high-volume centers already apply laparoscopic approaches to complicated cases that were once contraindicated because of safety issues and a lack of robust evidence. Several important multicenter randomized clinical trials in Eastern Asian countries are expected to give answers regarding the issues related to laparoscopic gastrectomy. It will be very interesting to see how far this approach will grow and expand. On the other hand, there are few high-level evidences advocating laparoscopic gastrectomy for gastric cancer. Until now, laparoscopic distal subtotal gastrectomy for early gastric cancer was the sole standard alternative to conventional open surgery. There are abundant issues to be solved in terms of technical feasibility, oncologic safety, proper selection of indicated patients, cost, education, and credentialing. Therefore, the above-mentioned, newly developed operative techniques should be practiced in carefully selected patients, ensuring that patients understand the procedures.
CONCLUSION
Laparoscopic gastrectomy for gastric adenocarcinoma has evolved due to the advent of new technologies and improved surgical techniques. Clinical indications are expanding to more extensive surgeries in conjunction with more sophisticated, conserving surgeries. Although discrepancies in what constitutes a proper environment for laparoscopic treatment of gastric cancer between the high- and low-incidence countries hinders the implementation of non-biased, large-scale clinical trials and massive application of the procedure, strict validation should be followed before adopting the use of laparoscopy for the treatment of gastric cancer.
Footnotes
Supported by ETRI R&D Program (14ZC1400, The Development of a Realistic Surgery Rehearsal System based on Patient Specific Surgical Planning), funded by the Government of South Korea.
Conflict-of-interest statement: Taeil Son has no conflicts of interest or financial obligations to disclose. Woo Jin Hyung has received fees for serving as a consultant for Ethicon, LLC.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 31, 2015
First decision: July 14, 2015
Article in press: November 19, 2015
P- Reviewer: Rios-Cruz D S- Editor: Yu J L- Editor: A E- Editor: Liu XM
References
- 1.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 2.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 3.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, Arai K, Kodera Y, Nashimoto A. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66. doi: 10.1007/s10120-006-0370-y. [DOI] [PubMed] [Google Scholar]
- 5.Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419–424. doi: 10.1136/pgmj.2004.029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 7.Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br J Surg. 1992;79:241–244. doi: 10.1002/bjs.1800790319. [DOI] [PubMed] [Google Scholar]
- 8.Ajani JA, Buyse M, Lichinitser M, Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha R, Carrato A, et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: Results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer. 2013;49:3616–3624. doi: 10.1016/j.ejca.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Brar S, Law C, McLeod R, Helyer L, Swallow C, Paszat L, Seevaratnam R, Cardoso R, Dixon M, Mahar A, et al. Defining surgical quality in gastric cancer: a RAND/UCLA appropriateness study. J Am Coll Surg. 2013;217:347–357.e1. doi: 10.1016/j.jamcollsurg.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 10.Lutz MP, Zalcberg JR, Ducreux M, Ajani JA, Allum W, Aust D, Bang YJ, Cascinu S, Hölscher A, Jankowski J, et al. Highlights of the EORTC St. Gallen International Expert Consensus on the primary therapy of gastric, gastroesophageal and oesophageal cancer - differential treatment strategies for subtypes of early gastroesophageal cancer. Eur J Cancer. 2012;48:2941–2953. doi: 10.1016/j.ejca.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 12.Son T, Hyung WJ, Lee JH, Kim YM, Noh SH. Minimally invasive surgery for serosa-positive gastric cancer (pT4a) in patients with preoperative diagnosis of cancer without serosal invasion. Surg Endosc. 2014;28:866–874. doi: 10.1007/s00464-013-3236-5. [DOI] [PubMed] [Google Scholar]
- 13.Huscher CG, Mingoli A, Sgarzini G, Brachini G, Binda B, Di Paola M, Ponzano C. Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg. 2007;194:839–844; discussion 844. doi: 10.1016/j.amjsurg.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28:331–337. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 15.Fujii K, Sonoda K, Izumi K, Shiraishi N, Adachi Y, Kitano S. T lymphocyte subsets and Th1/Th2 balance after laparoscopy-assisted distal gastrectomy. Surg Endosc. 2003;17:1440–1444. doi: 10.1007/s00464-002-9149-3. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 17.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 19.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 20.Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Watanabe M, Okutomi T, Wang G, Bax L. Laparoscopy versus open distal gastrectomy by expert surgeons for early gastric cancer in Japanese patients: short-term clinical outcomes of a randomized clinical trial. Surg Endosc. 2013;27:1695–1705. doi: 10.1007/s00464-012-2658-9. [DOI] [PubMed] [Google Scholar]
- 21.Takiguchi S, Fujiwara Y, Yamasaki M, Miyata H, Nakajima K, Sekimoto M, Mori M, Doki Y. Laparoscopy-assisted distal gastrectomy versus open distal gastrectomy. A prospective randomized single-blind study. World J Surg. 2013;37:2379–2386. doi: 10.1007/s00268-013-2121-7. [DOI] [PubMed] [Google Scholar]
- 22.Aoyama T, Yoshikawa T, Hayashi T, Hasegawa S, Tsuchida K, Yamada T, Cho H, Ogata T, Fujikawa H, Yukawa N, et al. Randomized comparison of surgical stress and the nutritional status between laparoscopy-assisted and open distal gastrectomy for gastric cancer. Ann Surg Oncol. 2014;21:1983–1990. doi: 10.1245/s10434-014-3509-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee SI, Choi YS, Park DJ, Kim HH, Yang HK, Kim MC. Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg. 2006;202:874–880. doi: 10.1016/j.jamcollsurg.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc. 2009;23:1759–1763. doi: 10.1007/s00464-008-0198-0. [DOI] [PubMed] [Google Scholar]
- 25.Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446–456. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 26.Memon MA, Khan S, Yunus RM, Barr R, Memon B. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc. 2008;22:1781–1789. doi: 10.1007/s00464-008-9925-9. [DOI] [PubMed] [Google Scholar]
- 27.Kodera Y, Fujiwara M, Ohashi N, Nakayama G, Koike M, Morita S, Nakao A. Laparoscopic surgery for gastric cancer: a collective review with meta-analysis of randomized trials. J Am Coll Surg. 2010;211:677–686. doi: 10.1016/j.jamcollsurg.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Zorcolo L, Rosman AS, Pisano M, Marcon F, Restivo A, Nigri GR, Fancellu A, Melis M. A meta-analysis of prospective randomized trials comparing minimally invasive and open distal gastrectomy for cancer. J Surg Oncol. 2011;104:544–551. doi: 10.1002/jso.21980. [DOI] [PubMed] [Google Scholar]
- 29.Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69–77. doi: 10.5230/jgc.2011.11.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mochiki E, Toyomasu Y, Ogata K, Andoh H, Ohno T, Aihara R, Asao T, Kuwano H. Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc. 2008;22:1997–2002. doi: 10.1007/s00464-008-0015-9. [DOI] [PubMed] [Google Scholar]
- 31.Tanimura S, Higashino M, Fukunaga Y, Takemura M, Tanaka Y, Fujiwara Y, Osugi H. Laparoscopic gastrectomy for gastric cancer: experience with more than 600 cases. Surg Endosc. 2008;22:1161–1164. doi: 10.1007/s00464-008-9786-2. [DOI] [PubMed] [Google Scholar]
- 32.Topal B, Leys E, Ectors N, Aerts R, Penninckx F. Determinants of complications and adequacy of surgical resection in laparoscopic versus open total gastrectomy for adenocarcinoma. Surg Endosc. 2008;22:980–984. doi: 10.1007/s00464-007-9549-5. [DOI] [PubMed] [Google Scholar]
- 33.Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, Cho SJ, Lee JY, Kim CG, Choi IJ, et al. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol. 2009;100:392–395. doi: 10.1002/jso.21345. [DOI] [PubMed] [Google Scholar]
- 34.Hyung WJ, Lim JS, Song J, Choi SH, Noh SH. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008;207:e6–e11. doi: 10.1016/j.jamcollsurg.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Okabe H, Obama K, Kan T, Tanaka E, Itami A, Sakai Y. Medial approach for laparoscopic total gastrectomy with splenic lymph node dissection. J Am Coll Surg. 2010;211:e1–e6. doi: 10.1016/j.jamcollsurg.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Okabe H, Obama K, Tanaka E, Nomura A, Kawamura J, Nagayama S, Itami A, Watanabe G, Kanaya S, Sakai Y. Intracorporeal esophagojejunal anastomosis after laparoscopic total gastrectomy for patients with gastric cancer. Surg Endosc. 2009;23:2167–2171. doi: 10.1007/s00464-008-9987-8. [DOI] [PubMed] [Google Scholar]
- 37.Okabe H, Tsunoda S, Tanaka E, Hisamori S, Kawada H, Sakai Y. Is laparoscopic total gastrectomy a safe operation? A review of various anastomotic techniques and their outcomes. Surg Today. 2015;45:549–558. doi: 10.1007/s00595-014-0901-9. [DOI] [PubMed] [Google Scholar]
- 38.Hara T, Fujiwara Y, Sugimura K, Kishi K, Motoori M, Miyoshi N, Akita H, Gotoh K, Takahashi H, Marubashi S, et al. [Comparison of early clinical outcomes between laparoscopic total gastrectomy and open total gastrectomy for early-stage gastric cancer] Gan To Kagaku Ryoho. 2014;41:1476–1478. [PubMed] [Google Scholar]
- 39.Kim HS, Kim MG, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and laparoscopic-assisted total gastrectomy methods for the surgical treatment of early gastric cancer near the gastroesophageal junction. J Laparoendosc Adv Surg Tech A. 2013;23:204–210. doi: 10.1089/lap.2012.0393. [DOI] [PubMed] [Google Scholar]
- 40.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 41.Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100. doi: 10.1007/s10120-011-0040-6. [DOI] [PubMed] [Google Scholar]
- 42.Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v50–v54. doi: 10.1093/annonc/mdq164. [DOI] [PubMed] [Google Scholar]
- 43.Ohtani H, Tamamori Y, Noguchi K, Azuma T, Fujimoto S, Oba H, Aoki T, Minami M, Hirakawa K. Meta-analysis of laparoscopy-assisted and open distal gastrectomy for gastric cancer. J Surg Res. 2011;171:479–485. doi: 10.1016/j.jss.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Uyama I, Suda K, Satoh S. Laparoscopic surgery for advanced gastric cancer: current status and future perspectives. J Gastric Cancer. 2013;13:19–25. doi: 10.5230/jgc.2013.13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SS, Kim CS, Mok YJ, Kim SJ, Kim HI. Gastric cancer confined to the muscularis propria: a possible candidate for laparoscopic surgery or adjuvant therapy. Scand J Gastroenterol. 2005;40:450–454. doi: 10.1080/00365520410009302. [DOI] [PubMed] [Google Scholar]
- 46.Hur H, Jeon HM, Kim W. Laparoscopy-assisted distal gastrectomy with D2 lymphadenectomy for T2b advanced gastric cancers: three years’ experience. J Surg Oncol. 2008;98:515–519. doi: 10.1002/jso.21155. [DOI] [PubMed] [Google Scholar]
- 47.Strong VE, Devaud N, Allen PJ, Gonen M, Brennan MF, Coit D. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case-control study. Ann Surg Oncol. 2009;16:1507–1513. doi: 10.1245/s10434-009-0386-8. [DOI] [PubMed] [Google Scholar]
- 48.Wei HB, Wei B, Qi CL, Chen TF, Huang Y, Zheng ZH, Huang JL, Fang JF. Laparoscopic versus open gastrectomy with D2 lymph node dissection for gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2011;21:383–390. doi: 10.1097/SLE.0b013e31822d02dc. [DOI] [PubMed] [Google Scholar]
- 49.Li P, Huang CM, Zheng CH, Xie JW, Wang JB, Lin JX, Lu J, Wang Y, Chen QY. Laparoscopic spleen-preserving splenic hilar lymphadenectomy in 108 consecutive patients with upper gastric cancer. World J Gastroenterol. 2014;20:11376–11383. doi: 10.3748/wjg.v20.i32.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang CM, Chen QY, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J. Laparoscopic spleen-preserving splenic hilar lymphadenectomy performed by following the perigastric fascias and the intrafascial space for advanced upper-third gastric cancer. PLoS One. 2014;9:e90345. doi: 10.1371/journal.pone.0090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bo T, Peiwu Y, Feng Q, Yongliang Z, Yan S, Yingxue H, Huaxing L. Laparoscopy-assisted vs. open total gastrectomy for advanced gastric cancer: long-term outcomes and technical aspects of a case-control study. J Gastrointest Surg. 2013;17:1202–1208. doi: 10.1007/s11605-013-2218-1. [DOI] [PubMed] [Google Scholar]
- 52.Lee JH, Ahn SH, Park do J, Kim HH, Lee HJ, Yang HK. Laparoscopic total gastrectomy with D2 lymphadenectomy for advanced gastric cancer. World J Surg. 2012;36:2394–2399. doi: 10.1007/s00268-012-1669-y. [DOI] [PubMed] [Google Scholar]
- 53.Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230–234. doi: 10.1007/s101200050069. [DOI] [PubMed] [Google Scholar]
- 54.Shinohara T, Uyama I, Kanaya S, Inaba K, Isogaki J, Horiguchi A, Miyakawa S. Totally laparoscopic pancreaticoduodenectomy for locally advanced gastric cancer. Langenbecks Arch Surg. 2009;394:733–737. doi: 10.1007/s00423-009-0492-x. [DOI] [PubMed] [Google Scholar]
- 55.Song J, Kim JY, Kim S, Choi WH, Cheong JH, Hyung WJ, Choi SH, Noh SH. Laparoscopic completion total gastrectomy in remnant gastric cancer: technical detail and experience of two cases. Hepatogastroenterology. 2009;56:1249–1252. [PubMed] [Google Scholar]
- 56.Uyama I, Sugioka A, Matsui H, Fujita J, Komori Y, Hasumi A. Laparoscopic pancreas-preserving total gastrectomy for proximal gastric cancer. A case and technical report. Surg Endosc. 2001;15:217–218. doi: 10.1007/s004640040037. [DOI] [PubMed] [Google Scholar]
- 57.Kwon IG, Cho I, Guner A, Choi YY, Shin HB, Kim HI, An JY, Cheong JH, Noh SH, Hyung WJ. Minimally invasive surgery for remnant gastric cancer: a comparison with open surgery. Surg Endosc. 2014;28:2452–2458. doi: 10.1007/s00464-014-3496-8. [DOI] [PubMed] [Google Scholar]
- 58.Kim MG, Kim KC, Kim BS, Kim TH, Kim HS, Yook JH, Kim BS. A totally laparoscopic distal gastrectomy can be an effective way of performing laparoscopic gastrectomy in obese patients (body mass index≥30) World J Surg. 2011;35:1327–1332. doi: 10.1007/s00268-011-1034-6. [DOI] [PubMed] [Google Scholar]
- 59.Kim MG, Kawada H, Kim BS, Kim TH, Kim KC, Yook JH, Kim BS. A totally laparoscopic distal gastrectomy with gastroduodenostomy (TLDG) for improvement of the early surgical outcomes in high BMI patients. Surg Endosc. 2011;25:1076–1082. doi: 10.1007/s00464-010-1319-0. [DOI] [PubMed] [Google Scholar]
- 60.Zhang YX, Wu YJ, Lu GW, Xia MM. Systematic review and meta-analysis of totally laparoscopic versus laparoscopic assisted distal gastrectomy for gastric cancer. World J Surg Oncol. 2015;13:116. doi: 10.1186/s12957-015-0532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han G, Park JY, Kim YJ. Comparison of short-term postoperative outcomes in totally laparoscopic distal gastrectomy versus laparoscopy-assisted distal gastrectomy. J Gastric Cancer. 2014;14:105–110. doi: 10.5230/jgc.2014.14.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284–287. doi: 10.1016/s1072-7515(02)01239-5. [DOI] [PubMed] [Google Scholar]
- 63.Hosogi H, Kanaya S. Intracorporeal anastomosis in laparoscopic gastric cancer surgery. J Gastric Cancer. 2012;12:133–139. doi: 10.5230/jgc.2012.12.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Usui S, Nagai K, Hiranuma S, Takiguchi N, Matsumoto A, Sanada K. Laparoscopy-assisted esophagoenteral anastomosis using endoscopic purse-string suture instrument “Endo-PSI (II)” and circular stapler. Gastric Cancer. 2008;11:233–237. doi: 10.1007/s10120-008-0481-8. [DOI] [PubMed] [Google Scholar]
- 65.Kim HI, Cho I, Jang DS, Hyung WJ. Intracorporeal esophagojejunostomy using a circular stapler with a new purse-string suture technique during laparoscopic total gastrectomy. J Am Coll Surg. 2013;216:e11–e16. doi: 10.1016/j.jamcollsurg.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Sakuramoto S, Kikuchi S, Futawatari N, Moriya H, Katada N, Yamashita K, Watanabe M. Technique of esophagojejunostomy using transoral placement of the pretilted anvil head after laparoscopic gastrectomy for gastric cancer. Surgery. 2010;147:742–747. doi: 10.1016/j.surg.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. 2010;211:e25–e29. doi: 10.1016/j.jamcollsurg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 68.Nagai E, Ohuchida K, Nakata K, Miyasaka Y, Maeyama R, Toma H, Shimizu S, Tanaka M. Feasibility and safety of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy: inverted T-shaped anastomosis using linear staplers. Surgery. 2013;153:732–738. doi: 10.1016/j.surg.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 69.Kim HS, Kim MG, Kim BS, Yook JH, Kim BS. Totally laparoscopic total gastrectomy using endoscopic linear stapler: early experiences at one institute. J Laparoendosc Adv Surg Tech A. 2012;22:889–897. doi: 10.1089/lap.2012.0238. [DOI] [PubMed] [Google Scholar]
- 70.Park do J, Lee JH, Ahn SH, Eng AK, Kim HH. Single-port laparoscopic distal gastrectomy with D1+β lymph node dissection for gastric cancers: report of 2 cases. Surg Laparosc Endosc Percutan Tech. 2012;22:e214–e216. doi: 10.1097/SLE.0b013e318253df9b. [DOI] [PubMed] [Google Scholar]
- 71.Kunisaki C, Ono HA, Oshima T, Makino H, Akiyama H, Endo I. Relevance of reduced-port laparoscopic distal gastrectomy for gastric cancer: a pilot study. Dig Surg. 2012;29:261–268. doi: 10.1159/000341677. [DOI] [PubMed] [Google Scholar]
- 72.Kim SM, Ha MH, Seo JE, Kim JE, Choi MG, Sohn TS, Bae JM, Kim S, Lee JH. Comparison of Reduced Port Totally Laparoscopic Distal Gastrectomy (Duet TLDG) and Conventional Laparoscopic-Assisted Distal Gastrectomy. Ann Surg Oncol. 2015;22:2567–2572. doi: 10.1245/s10434-014-4333-y. [DOI] [PubMed] [Google Scholar]
- 73.Ahn SH, Son SY, Jung do H, Park do J, Kim HH. Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg. 2014;219:933–943. doi: 10.1016/j.jamcollsurg.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 74.Kawamura H, Tanioka T, Shibuya K, Tahara M, Takahashi M. Comparison of the invasiveness between reduced-port laparoscopy-assisted distal gastrectomy and conventional laparoscopy-assisted distal gastrectomy. Int Surg. 2013;98:247–253. doi: 10.9738/INTSURG-D-12-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–3710. doi: 10.1200/JCO.2013.50.3789. [DOI] [PubMed] [Google Scholar]
- 76.Park JY, Ryu KW, Eom BW, Yoon HM, Kim SJ, Cho SJ, Lee JY, Kim CG, Kook MC, Choi IJ, et al. Proposal of the surgical options for primary tumor control during sentinel node navigation surgery based on the discrepancy between preoperative and postoperative early gastric cancer diagnoses. Ann Surg Oncol. 2014;21:1123–1129. doi: 10.1245/s10434-013-3427-2. [DOI] [PubMed] [Google Scholar]
- 77.Miyashiro I, Hiratsuka M, Sasako M, Sano T, Mizusawa J, Nakamura K, Nashimoto A, Tsuburaya A, Fukushima N. High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer. 2014;17:316–323. doi: 10.1007/s10120-013-0285-3. [DOI] [PubMed] [Google Scholar]
- 78.Cardoso R, Bocicariu A, Dixon M, Yohanathan L, Seevaratnam R, Helyer L, Law C, Coburn NG. What is the accuracy of sentinel lymph node biopsy for gastric cancer? A systematic review. Gastric Cancer. 2012;15 Suppl 1:S48–S59. doi: 10.1007/s10120-011-0103-8. [DOI] [PubMed] [Google Scholar]
- 79.Can MF, Yagci G, Cetiner S. Systematic review of studies investigating sentinel node navigation surgery and lymphatic mapping for gastric cancer. J Laparoendosc Adv Surg Tech A. 2013;23:651–662. doi: 10.1089/lap.2012.0311. [DOI] [PubMed] [Google Scholar]
- 80.Wang Z, Dong ZY, Chen JQ, Liu JL. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol. 2012;19:1541–1550. doi: 10.1245/s10434-011-2124-2. [DOI] [PubMed] [Google Scholar]
- 81.Suh YS, Han DS, Kong SH, Kwon S, Shin CI, Kim WH, Kim HH, Lee HJ, Yang HK. Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg. 2014;259:485–493. doi: 10.1097/SLA.0b013e318294d142. [DOI] [PubMed] [Google Scholar]
- 82.Hiki N, Kaminishi M. Pylorus-preserving gastrectomy in gastric cancer surgery--open and laparoscopic approaches. Langenbecks Arch Surg. 2005;390:442–447. doi: 10.1007/s00423-005-0573-4. [DOI] [PubMed] [Google Scholar]
- 83.Takeuchi H, Kitagawa Y. Is pylorus-preserving gastrectomy universally applicable to early gastric cancer of the mid stomach? Ann Surg Oncol. 2014;21:356–357. doi: 10.1245/s10434-013-3256-3. [DOI] [PubMed] [Google Scholar]
- 84.Kodera Y, Yamamura Y, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Morimoto T, Kato T. Lymph node metastasis in cancer of the middle-third stomach: criteria for treatment with a pylorus-preserving gastrectomy. Surg Today. 2001;31:196–203. doi: 10.1007/s005950170168. [DOI] [PubMed] [Google Scholar]
- 85.Morii Y, Arita T, Shimoda K, Hagino Y, Yoshida T, Kitano S. Indications for pylorus-preserving gastrectomy for gastric cancer based on lymph node metastasis. Hepatogastroenterology. 2002;49:1477–1480. [PubMed] [Google Scholar]
- 86.Sano T, Hollowood A. Early gastric cancer: diagnosis and less invasive treatments. Scand J Surg. 2006;95:249–255. doi: 10.1177/145749690609500407. [DOI] [PubMed] [Google Scholar]
- 87.Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 2008;95:1131–1135. doi: 10.1002/bjs.6295. [DOI] [PubMed] [Google Scholar]
- 88.Hiki N, Sano T, Fukunaga T, Ohyama S, Tokunaga M, Yamaguchi T. Survival benefit of pylorus-preserving gastrectomy in early gastric cancer. J Am Coll Surg. 2009;209:297–301. doi: 10.1016/j.jamcollsurg.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 89.Jiang X, Hiki N, Nunobe S, Fukunaga T, Kumagai K, Nohara K, Katayama H, Ohyama S, Sano T, Yamaguchi T. Long-term outcome and survival with laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Surg Endosc. 2011;25:1182–1186. doi: 10.1007/s00464-010-1336-z. [DOI] [PubMed] [Google Scholar]
- 90.Ogoshi K, Okamoto Y, Nabeshima K, Morita M, Nakamura K, Iwata K, Soeda J, Kondoh Y, Makuuchi H. Focus on the conditions of resection and reconstruction in gastric cancer. What extent of resection and what kind of reconstruction provide the best outcomes for gastric cancer patients? Digestion. 2005;71:213–224. doi: 10.1159/000087046. [DOI] [PubMed] [Google Scholar]
- 91.Son MW, Kim YJ, Jeong GA, Cho GS, Lee MS. Long-Term Outcomes of Proximal Gastrectomy versus Total Gastrectomy for Upper-Third Gastric Cancer. J Gastric Cancer. 2014;14:246–251. doi: 10.5230/jgc.2014.14.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bo T, Zhihong P, Peiwu Y, Feng Q, Ziqiang W, Yan S, Yongliang Z, Huaxin L. General complications following laparoscopic-assisted gastrectomy and analysis of techniques to manage them. Surg Endosc. 2009;23:1860–1865. doi: 10.1007/s00464-008-0312-3. [DOI] [PubMed] [Google Scholar]
- 93.Masuzawa T, Takiguchi S, Hirao M, Imamura H, Kimura Y, Fujita J, Miyashiro I, Tamura S, Hiratsuka M, Kobayashi K, et al. Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: a multi-institutional retrospective study. World J Surg. 2014;38:1100–1106. doi: 10.1007/s00268-013-2370-5. [DOI] [PubMed] [Google Scholar]


