Abstract
Currently, pancreatic adenocarcinoma mainly occurs after 60 years of age, and its prognosis remains poor despite modest improvements in recent decades. The aging of the population will result in a rise in the incidence of pancreatic adenocarcinoma within the next years. Thus, the management of pancreatic cancer in the elderly population is gaining increasing relevance. Older cancer patients represent a heterogeneous group with different biological, functional and psychosocial characteristics that can modify the usual management of this disease, including pharmacokinetic and pharmacodynamic changes, polypharmacy, performance status, comorbidities and organ dysfunction. However, the biological age, not the chronological age, of the patient should be the limiting factor in determining the most appropriate treatment for these patients. Unfortunately, despite the increased incidence of this pathology in older patients, there is an underrepresentation of these patients in clinical trials, and the management of older patients is thus determined by extrapolation from the results of studies performed in younger patients. In this review, the special characteristics of the elderly, the multidisciplinary management of localized and advanced ductal adenocarcinoma of the pancreas and the most recent advances in the management of this condition will be discussed, focusing on surgery, chemotherapy, radiation and palliative care.
Keywords: Pancreatic ductal adenocarcinoma, Elderly, Management, Treatment, Pancreatic cancer
Core tip: Pancreatic cancer is a disease that mainly affects the elderly. The older patients have different biological, functional and psychosocial characteristics compared with the young population. The infrequent participation of these patients in clinical trials have raised challenges in the management of this disease. In this review, the special features of the elderly as well as the current multidisciplinary management of pancreatic cancer will be discussed.
INTRODUCTION
In the United States, approximately 48960 patients are diagnosed with pancreatic cancer annually, and this disease represents the fourth leading cause of cancer-related death in the United States among both men and women. Advancing age is a high risk factor for cancer, and more than 60% of new cancer cases and over 70% of cancer mortalities occur in elderly people. The incidence of pancreatic cancer increases with age; in the United States, only 13% of all patients with pancreatic cancer are diagnosed before 60 years of age.
Elderly patients represent a special subgroup because of the presence of related pharmacodynamic and pharmacokinetic changes. Hence, a standard clinical evaluation of these patients may not be sufficient to determine individual treatment strategies. New assessment methods have been proposed, and several studies have demonstrated the value of these techniques in routine clinical practice.
At present, surgical resection is the only potentially curative treatment for pancreatic cancer, but only 15%-20% of patients are candidates for pancreatectomy because the majority of them are diagnosed with disseminated or locally advance disease. Although it is the best option, many older patients are not recommended for surgery. In addition, they are also less likely to receive chemotherapy compared with younger patients.
Due to the aging population, it is estimated that the number of elderly patients with pancreatic cancer will continue to rise. Unfortunately, very little data are available regarding the management of these patients; therefore, therapeutic approaches to this subgroup are a daily challenge.
The aim of this review is summarize current knowledge regarding the management and therapeutic approach in elderly patients with pancreatic cancer.
CHARACTERISTICS OF THE ELDERLY AND THEIR EFFECTS ON THERAPEUTIC DECISIONS
The population of western countries is aging, and because the incidence of cancer increases with age, the population of patients with cancer is growing. More than 50% of all newly diagnosed patients with cancer are older than 60 years, and more than one third are over the age of 70[1]. By the year 2030, 70% of all malignancies and 85% of all cancer-related deaths are expected to occur in individuals aged 65 years or older, and therefore, older people will likely represent the prototypical cancer patient in the future[2]. Age is an important risk factor for the development of pancreatic cancer. Whereas the overall incidence rate of pancreatic cancer for all ages is 11.7%, the incidence rate among individuals older than 65 years and older than 80 years is 66.4% and up to as many as 91.1%, respectively[3].
The association between aging and cancer is well established: carcinogenesis is a time-consuming process with a final product (cancer) that is more likely to occur late in life; older tissues are more vulnerable to environmental carcinogens; and changes in the environment of the body (chronic inflammation, immunosenescence) may favor the development of cancer[4,5]. Additionally, the immune system plays an important role in the progression of pancreatic cancer[6-8]. Very little data are available regarding pancreatic cancer in the elderly. Kamisawa et al[9] compared the pathologic features of pancreatic cancer in elderly vs younger patients and found no differences in the grade, location or incidence of the local spread, although elderly patients developed fewer hematogenous metastases. Other reports have indicated that older patients present more diploid tumors or that p53 mutations, which are associated with a worse prognosis[10].
Several studies have shown that older cancer patients are often undertreated and have poorer outcomes compared with younger individuals[11,12]. This outcome may be due to the less aggressive treatment of elderly patients. Focusing on pancreatic cancer, some studies have shown that nearly half of all elderly patients did not receive any treatment for locoregional pancreatic cancer. Moreover, only 11% received a multimodal therapy (surgery +/- chemoradiotherapy)[13].
Despite the rapidly growing oncogeriatric population, older cancer patients are underrepresented in clinical trials[14]. Talarico et al[15] analyzed the age-related enrollment of cancer patients in clinical trials and found that the proportions of the overall patient population aged ≥ 65, ≥ 70, and ≥ 75 years were 36%, 20%, and 9% in clinical trials compared with 60%, 46%, and 31% in the United States cancer population, respectively. This under-representation generates challenges because the results from clinical trials in younger patients cannot be extrapolated to the treatment of the elderly. The diverse effects of aging on organ function and the variety of potential comorbid disease results in a heterogeneous elderly population. For example, pharmacokinetic and pharmacodynamic differences between young and elderly patients, and indeed among elderly patients themselves, could result in considerable variability in the efficacy and safety of cancer treatments. The most important pharmacokinetic changes are described in Table 1. Pharmacodynamic changes may cause resistance to cytotoxic drugs in older individuals due to resistance to apoptosis and poorer oxygenation in these neoplasms[16,17]. Specifically, in patients with advanced pancreatic adenocarcinoma treated with gemcitabine who were aged 75 or older, the median survival time was approximately six to eight weeks shorter than that in trial patients[18]. Another important question concerns the effects of toxicity on older people. While grade 2 adverse events are not important in young people and, in fact, are often not reported, the same level of toxicity may result in a considerable deterioration of functionality in elderly patients.
Table 1.
Pharmacokinetic changes in the elderly
| Physiological process | Situation in the elderly | Changes | Effect |
| Absorption | Decreased | Atrophy of the intestinal mucosa Decreases in gastrointestinal motility | Reduced absorption of protein, vitamins and drugs |
| Decreased splanchnic blood flow | |||
| Decreased secretion of digestive enzymes | |||
| Metabolism | Decreased | Reduced liver size | Reduced protein synthesis |
| Reduced hepatic blood flow | Reduced activation/deactivation of drugs and carcinogens | ||
| Reduced activity of cytochrome p450-dependant reactions | |||
| Drug distribution | Decreased | Reduced total body water | Reduced Vd of water-soluble drugs |
| Reduced concentration of plasma albumin | Increased Vd of liposoluble drugs | ||
| Reduced red blood cell concentration | |||
| Excretion | Decreased | Reduced glomerular filtration rate | Reduced elimination of drugs and of their active metabolites |
| Reduced tubular function |
Vd: Volume of distribution.
A widespread occurrence in the management of older individuals is the intake of multiple medications[19]. Polypharmacy is at least as common as it is in age-matched individuals without cancer. Prithviraj et al[20] showed that 80% of newly diagnosed cancer patients aged 65 or older were taking five or more medications. Polypharmacy increases the risk of side effects, drug-drug interactions, and treatment costs and decreases medical adherence. Moreover, it may represent a risk factor for additional complications of cytotoxic chemotherapy and affect patient outcomes[21]. Hence, periodic reviews of prescribed medications is necessary to abolish these challenges associated with polypharmacy.
Because aging is a highly individualized process, chronological age is not adequate to estimate the individual life expectancy and functional reserve. In other words, “biological age” is more important than “chronological age” to define who is an old patient. In medical oncology, treatment decisions are mostly based on clinical judgment and performance scales such as the Karnofsky performance score (KPS). However, in older cancer patients, these scales are not as sensitive as in the adult population because relevant information is not taken into account, such as comorbidities, the functionality of the patients and support from family. To improve the assessment and to determine individual treatment strategies for an optimal outcome, one concept of geriatric medicine is being incorporated in geriatric oncology: the comprehensive geriatric assessment (CGA). CGA is defined as a multidimensional, interdisciplinary diagnostic process that focuses on the determination of medical, psychosocial, and functional capabilities in older people to develop an integrated treatment plan. CGA has been shown to improve overall survival, quality of life and physical functioning in the non-oncologic geriatric population. Several recent reports have strongly suggested that different components of comprehensive geriatric assessment can be useful in oncology to predict early death, functional decline, toxicity and overall survival[22,23]. Important domains in a geriatric assessment are functional status, comorbidities, cognition, mental health status and support, fatigue, and the assessment of polypharmacy and presence of geriatric syndromes[24]. Many tools are available to assess these domains (Table 2). Despite the recommendations for a CGA by The International Society of Geriatric Oncology (SIOG), it is not widely implemented in the practice of oncology likely because it is a time- and resource-consuming endeavor[25]. In addition, true CGA is conducted by an experienced geriatrician; nonetheless, they are rarely available in most cancer structures. A suitable tool is one that is performed quickly by a trained nurse or physician and that has a high sensitivity and specificity to discriminate patients who require a more detailed assessment and possible geriatric interventions. The most widely used screening tool for older cancer patients is the VES-13 (vulnerable elders survey-13), which has a sensitivity range from 68%-87%[26].
Table 2.
Elements of a comprehensive geriatric assessment
| Parameter assessed | Elements and tools of the assessment |
| Demographic and social status | Questions on living situation, marital status, educational level, safety of the environment, financial resources, caregiver burden |
| Functional status | Performance status index |
| ADLs | |
| IADLs | |
| Barthel index | |
| Pepper assessment tool for disability | |
| Visual and/or hearing impairment, regardless of use of glasses or hearing aid | |
| Mobility problem (requiring help or use of walking aid) | |
| Timed Get Up and Go | |
| One-leg standing balance test | |
| Walking problems, gait assessment, and gait speed | |
| Karnofsky health care professional-rated performance rating scale | |
| Comorbidity | Charlson comorbidity index |
| CIRS | |
| No. of comorbid conditions | |
| Summary of comorbidities | |
| NYHA | |
| Cognition | Mini Mental State Examination Informant Questionnaire on Cognitive Decline in the Elderly Modified Mini Mental State Examination |
| Clock-drawing test | |
| Blessed Orientation-Memory-Concentration | |
| Emotional conditions (Depression) | Geriatric Depression Scale |
| Hospital Anxiety and Depression Scale | |
| Mental health index | |
| Presence of depression (as a geriatric syndrome) | |
| Nutrition | Weight loss (unintentional loss in 3 or 6 mo) |
| Mini Nutritional Assessment Short Nutritional | |
| DETERMINE Nutritional Index | |
| Polypharmacy | Number of medications |
| Appropriateness of medications | |
| Risk of drug interactions | |
| Geriatric syndromes | Dementia |
| Delirium | |
| Depression | |
| Falls | |
| Neglect and abuse | |
| Spontaneous bone fractures and osteoporosis | |
| Incontinence (fecal and/or urinary) | |
| Constipation | |
| Sarcopenia |
Data adapted from Wildiers et al[25]. ADL: Activity of daily living; CIRS: Cumulative Illness Rating Scale; CIRS-G: Cumulative Illness Rating Scale-Geriatrics; DETERMINE: Disease, Eating poorly, Tooth loss/mouth pain, Economic hardship, Reduced social contact, Multiple medicines, Involuntary weight loss/gain, Needs assistance in self-care, Elder years > 80; ECOG: Eastern Cooperative Oncology Group; IADL: Instrumental activity of daily living; PS: Performance status; NYHA: New York Heart Association.
Because elderly patients are a heterogeneous group, routine individual assessments of frailty and fitness are required. Such assessments may guide treatment decisions through evaluations of the balance of benefits and harms associated with performing or omitting specific oncologic interventions.
SURGERY FOR PANCREATIC CANCER IN THE ELDERLY
Surgical resection is the only potentially curative treatment for pancreatic cancer. Unfortunately, only 15% to 20% patients are candidates for pancreatectomy due to the late presentation of symptoms and/or detection of the disease[27-29]. Furthermore, the rate of resectability diminishes with age. Likewise, some authors reported that 40% of patients between the ages of 66-70 years are candidates for a pancreatectomy, but by the age of 85 years, only 7% are eligible candidates[30,31].
Resection of the pancreas, either by pancreaticoduodenectomy (PD) (the Whipple procedure), total or by partial pancreatectomy, is a complex surgical procedure with a high rate of morbidity and mortality. Mortality rates after pancreatic surgery have dropped to less than 2%-5% at experienced centers, but complication rates are high, reaching at least 30% in many centers[29]. Mortality also increases proportionally with age: 6.7% of patients aged 65-69 years, 9.3% of patients aged 70-79 years, and 15.5% of patients aged 80 years or older. However, hospitals with a low pancreatic surgery volume (< 11 resections per year) have higher mortality rates than high volume hospitals (7.3% vs 3.2%, P < 0.0001)[32,33]. These differences were accentuated with each increasing age group. The Hopkins study showed that 33% of patients older than 80 years presented delayed gastric emptying compared with 18.6% of the patients younger than 80 years (P = 0.03)[34]. Other studies reported a similar trend in the occurrence of delayed gastric emptying but without statistical significance[35]. Ito et al[36] showed a higher incidence of pancreatic fistulas in the elderly, but the results were not significant (45.1% vs 29.9%, respectively, P = 0.14). In the study by Hodul et al[35] the rate of neurologic complications was 9.4% in the older group and 0% in the younger group. The length of hospital stay was also proportionally increased according to age[29].
The number of patients requiring ongoing inpatient nursing care at the time of discharge increased significantly with age. The proportion of patients who could not be discharged home was 10.6%, 19.2% and 36.7% for ages 65-69, 70-79 and 80 years or older, respectively (P < 0.0001)[34].
The Memorial Sloan Kettering Group showed a significant difference in 5-year survival between patients aged 70 years or older (21%, median = 18 mo) and < 70 years (29%, median = 24 mo, P = 0.03)[37]. Finlayson et al[34] evaluated the 5-year survival of patients following surgery for pancreatic cancer and demonstrated a decrease from 16.4% in patients aged 65-69 years to 15.6% in patients aged 70-79 years and 11.3% in patients aged 80 years or older, but this difference did not achieve statistical significance. Patients with more than two comorbidities undergoing pancreatectomy for pancreatic cancer had a 5-year-survival rate of 10% compared with 14% in patients with fewer than two comorbidities (P = NS).
To reduce the rates of morbidity and mortality in elderly patients undergoing pancreatic surgery, an accurate pre-anesthesia and cardiovascular risk assessment is needed. The perioperative management should also be standardized. Patients should be routinely admitted to the intensive care unit (ICU) or to recovery for the first 48-72 h post-surgery. All patients must receive broad-spectrum antibiotics for two to three days and an H2 blocker during the entire postoperative hospital stay[38].
Minimally invasive surgery is associated with a lower rate of cardio-respiratory complications, diminished post-operative pain, shorter hospital stays, and a faster reincorporation into daily activities. Therefore, it is a very good option for elderly patients.
In summary, surgery is the only curative treatment in patients with pancreatic cancer. The benefits of surgery do not diminish with age, and therefore, elderly patients should not be denied the surgery a priori based on their age[39]. Although the elderly have a higher surgical mortality rate, this depends, among other factors, on the presence of comorbidities and the experience of the surgeon. Consequently, a proper preoperative assessment must be performed, as well as strict post-operative monitoring. Furthermore, this surgery must be conducted in a center with a high volume of pancreatic surgery patients. Under these conditions, surgical resection can be performed safely in older individuals.
ADJUVANT THERAPIES FOR PANCREATIC CANCER IN THE ELDERLY
Approximately one of each five patients with pancreatic adenocarcinoma is diagnosed at a resectable stage, which is the only option for cure. However, most of these patients will present local or metastatic relapse during the subsequent two years after resection.
Despite the benefits of adjuvant therapies in clinical trials, multimodal therapy clearly seems to be underutilized in the elderly. Parmar et al[13] identified only 1166 (11.1%) of 10505 patients older than 65 years (median age of 77 years) with locoregional pancreatic adenocarcinoma who received surgery and chemotherapy. Moreover, less than a half of the patients undergoing operative resection received chemotherapy. Nonetheless, other authors have reported an equivalent observation of only approximately 30% of elderly patients undergoing treatment with adjuvant therapies[40-42].
These findings could be explained by the perception of a limited life expectancy in patients with pancreatic cancer, the increased risk of an independent cancer cause of death and the longer post-surgical recovery of elderly patients. However, the role of adjuvant therapy has been demonstrated extensively, increasing the 5-year overall survival up to 25%. In addition, as described below, the prognosis and adjuvant therapy benefits in patients with pancreatic cancer are independent of age.
The adjuvant approach includes systemic chemotherapy to reduce the risk of distant metastases and chemoradiotherapy to reduce the risk of locoregional failure[43].
As discussed previously, the role of adjuvant chemotherapy has been well described in different prospective studies. In the CONKO-001 phase III trial, 368 patients were randomized to assess the effect of adjuvant chemotherapy with gemcitabine on the prognosis of resected pancreatic cancer. The median disease-free survival time was 13.4 mo vs 6.7 mo (HR = 0.55, P < 0.001) for the gemcitabine and observation groups, respectively. Treatment with the adjuvant gemcitabine also prolonged overall survival (HR = 0.76, P = 0.01). The median age of the patients was 62 years, and 219 patients (62%) were older than 65 years. No differences in progression-free survival were found in the multivariable analysis for age (P = 0.06)[44].
One thousand and eighty-eight patients were included in the ESPAC-3 trial, in which the superiority of fluorouracil vs gemcitabine as an adjuvant treatment after pancreatic cancer resection was assessed. No differences in the median overall survival were found (23 mo vs 23.6 mo for fluorouracil and gemcitabine, respectively; P = 0.039). As in other studies, age was not significantly associated with the prognosis[45].
As a consequence of the limited representation of elderly patients in clinical trials, several retrospective studies have been published to assess the use and efficacy of adjuvant chemotherapy in this population. In a multicenter series from 1990 to 2011, Nagrial et al[42] identified patients with resected pancreatic ductal adenocarcinoma, including 178 patients aged 70 years or older (median age of 75 years). Only 30% of the older patients received adjuvant chemotherapy. Not receiving adjuvant chemotherapy was a poor independent prognostic factor, with a median overall survival of 13.1 mo vs 21.8 mo for treated patients (HR = 1.89, P = 0.002)[42].
Davila et al[46] described the utilization and survival effects of adjuvant therapies among 1383 patients who were older than 65 years (41% older than 75 years). Forty-nine percent of the patients received adjuvant therapy. Patients younger than 75 years of age were significantly more likely to receive adjuvant chemotherapy or chemoradiotherapy. In contrast to other studies, the administration of adjuvant chemotherapy did not decrease the risk of mortality compared with surgery alone[46].
While the benefits of adjuvant chemotherapy are evident, the value of adjuvant chemoradiation therapy is controversial due to contradictory results between the European and United States clinical trials. The ESPAC-1 trial evaluated the role of chemotherapy and chemoradiotherapy using a two-by-two factorial design in 289 patients. This study showed a five-year survival rate benefit for chemotherapy compared with no chemotherapy (21% vs 8%; P = 0.009). However, chemoradiotherapy had a deleterious effect on survival compared with no chemoradiotherapy, with an estimated five-year survival of 10% vs 20%, respectively (P = 0.05). No significant differences in survival were found with respect to an age of 60 years or older compared with that younger than 60 years[47]. In contrast, the GITSG 9173 trial evaluated the benefit of adjuvant chemoradiation in 43 pancreatic cancer patients. The median survival was significantly longer for the treatment group (20 mo vs 11 mo)[48]. A similar study was performed by Klinkenbijl et al[49] in Europe. In that study, which included 218 patients up to 80 years old who were diagnosed with pancreatic head or periampullary adenocarcinoma, no significant differences in median survival were observed. The median duration of survival was 19.0 mo for the observation group and 24.5 mo for the treatment group (P = 0.208)[49].
There are doubts concerning the benefits of adjuvant chemoradiotherapy in the elderly. As observed for the adjuvant chemotherapy trials, the previously described studies did not focus on this subgroup of the patient population.
Horowitz et al[41] prospectively collected the clinical and pathological data for patients undergoing treatment for pancreatic cancer. Among the 166 patients aged 75 years or older, the 2-year survival improved for the patients who received adjuvant chemoradiation compared with surgery alone (49.0% vs 31.6%, respectively, P = 0.013)[41]. Another study of 1383 patients who were older than 65 years showed a benefit of adjuvant chemoradiation with a 23% lower risk of mortality compared with surgery alone (HR = 0.77)[46]. Miyamoto et al[50] retrospectively reviewed patients aged 75 years or more who were treated with chemoradiation for pancreatic cancer (eighteen patients as adjuvant therapy after surgery). The median overall survival for the adjuvant group was 20.6 mo, although the patients suffered from significant toxicity[50].
In conclusion, despite the lack of specific adjuvant data for the elderly, age does not seem to be a determinant factor in decisions regarding the administration of adjuvant chemotherapy. In our opinion, similarly to younger patients, older patients with a good performance status and no significant comorbidities can be treated with adjuvant chemotherapy preferably based on gemcitabine.
LOCALLY ADVANCED PANCREATIC CANCER IN THE ELDERLY
Locally advanced pancreatic cancer is defined as a tumor that encases a vascular structure, such as the superior mesenteric artery, celiac axis or superior or mesenteric vein-portal confluence[51]. It represents approximately 20%-25% of all newly diagnosed pancreatic cancers. In the strictest sense, in locally advanced pancreatic carcinoma, resection is not an option. In cases of locally non-resectable disease, the results of previous randomized trials indicated that concurrent external beam radiation therapy (EBRT) and 5-fluorouracil (5-FU) therapy resulted in significantly improved survival compared with EBRT alone or chemotherapy alone[52]. Thus, for locally advanced pancreatic cancer, chemoradiation is the standard of care[53,54].
In elderly patients with locally advanced pancreatic adenocarcinoma, oncologists are hesitant to indicate chemoradiotherapy because of the associated poor prognosis and high risk of severe toxicities. In fact, in a retrospective cohort study conducted by Kryzanowska, only 21% to 27 % of the elderly patients with locally advanced pancreatic adenocarcinoma received chemoradiation therapy in the United States[55].
In the elderly, the tolerability, efficacy and long-term outcomes associated with chemoradiotherapy remain unclear. Morizane et al[56] reported the efficacy and tolerability of chemoradiotherapy (fluorouracil infusion 200 mg/m2 per day plus concurrent radiotherapy (50.4 Gy in 28 fractions over 5.5 wk) in elderly (> 70 years) vs younger patients (< 70 years) with locally advanced pancreatic cancer. The median survival time was longer in the elderly patients (11.3 mo vs 9.5 mo), and there were no significant differences in the frequency of severe toxicity[56].
Similarly, Miyamoto et al[50] analyzed a subset of elderly patient (median age of 78 years) receiving definitive chemoradiation therapy. The median overall survival was 8.6 mo, which is comparable to the survival of younger historic controls. The authors also concluded that chemoradiation therapy in selected elderly patients with locally advanced pancreatic cancer can be considered an appropriate treatment and that further research is needed to reduce the high toxicity associated with this treatment approach[50].
Stereotactic body radiation therapy (SBRT) has been an important recent advance in RT for pancreatic cancer. Pioneered in the locally advance setting, the majority of the literature has shown that SBRT is well tolerated and effective, providing excellent local control and minimal toxicity[57]. Therefore, SBRT is a promising alternative modality as a definitive treatment for elderly patients with unresectable tumors. Several small studies have evaluated the efficacy and tolerability of SBRT in locally advanced setting. Chang et al[58] evaluated 77 patients with unresectable pancreatic adenocarcinoma who received 25 Gy in 1 fraction. In their study, the local free progression rates at 6 and 12 mo were 91% and 84%, respectively, and the rate of grade > 2 toxicity was 9%[58]. Another interesting recently reported study investigated twenty-six patients aged 80 years or greater who were treated with SBRT (24 Gy in one fraction) alone or with chemotherapy[59]. The median OS from SBRT was 7.6 mo. More interestingly, there were no acute or late ≥ grade 3 toxicities, and the treatment was very effective for achieving symptom relief, particularly abdominal and back pain.
Chemotherapy alone without radiotherapy remains an option in the elderly subgroup of patients. Recent phase III studies comparing chemoradiotherapy with chemotherapy alone in patients with locally advanced non-resectable pancreatic cancer have shown that chemotherapy alone is more beneficial in terms of survival outcomes and is more tolerable than combined chemoradiation therapy[60,61].
With the development of more sophisticated imaging tools, a greater number of patients have been included in a new subgroup: borderline resectable pancreatic cancer[62]. Several criteria have been proposed to define this group. MDACC criteria defines borderline resectable pancreatic cancer following possible tumor-vessel relationships as follows: a tumor abutment of the superior mesenteric artery or celiac axis measuring ≤ 180 degrees; or encasement of the hepatic artery amenable to resection and reconstruction; or short - segment reconstructable occlusion of the superior mesenteric vein, portal vein, or superior mesenteric-portal vein confluence[63].
In this scenario, neoadjuvant therapies have been proposed as an alternative option with the aim of downstaging tumor in order to improve microscopic resection rates[64]. This approach could be interesting for elderly patients with borderline or resectable pancreatic cancer in which the initiation of adjuvant chemotherapy is frequently delayed due to surgical complications or comorbidities, or is even discarded. Preoperative therapies also provide a time window in which patients who progress or develop distant metastases during treatment, or those with a significant functional impairment, can be identified to avoid unnecessary surgery[65]. Few studies have explored the role of neoadjuvant therapy in elderly patients. At the 2014 ASCO Gastrointestinal Cancer Symposium, Miura et al[66] reported the outcomes associated with neoadjuvant therapy (chemotherapy or chemoradiotherapy) in older patients with resectable or borderline resectable pancreatic cancer. They showed that the elderly group (aged 75 years or older) compared with the young group (aged younger than 75 years) had more hospitalizations during neoadjuvant therapy (50% vs 28%, respectively) and were also less likely to complete the therapy (72.4% vs 89.5%, respectively). Among the patients who completed the therapy, there were no significant differences in complication rates or median overall survival between the two groups. In conclusion, the neoadjuvant approach is an effective treatment option in elderly patients with borderline resectable pancreatic cancer and could be included in the treatment selection process for older patients who are not candidates for surgery.
METASTATIC PANCREATIC CANCER IN THE ELDERLY
In the United States, as many as 53% of pancreatic cancer patients are diagnosed during the metastatic stage of the disease. The prognosis of patients with metastatic stage pancreatic cancer is poor, with a 5-year survival rate of 2%[67]. It is not clear that age is a prognostic factor for survival in patients with metastatic pancreatic cancer. Some studies suggest that age is a major contributing factor to a poor prognosis, while other studies reported no survival differences according to age[68,69]. Some of these studies are retrospective and small, and thus, their findings may be questionable. As described below, in the prospective PRODIGE clinical trial, age was an adverse prognostic factor[70]. However, the data obtained in retrospective studies and some large clinical trials show that the use of systemic therapy could provide a survival benefit in selected elderly and very elderly patients with metastatic pancreatic cancer[69-73]. Unfortunately, as mentioned above, the underrepresentation of elderly patients in clinical trials has resulted in treatment decisions in this patient population that are extrapolated from studies performed in younger patients. However, a change is currently underway with the development of clinical trials in elderly metastatic patients to explore the safety and efficacy of new combinations of chemotherapy.
From the end of the 1990s to 2011, gemcitabine has been considered the standard of care for patients with metastatic pancreatic cancer according to pivotal phase III clinical trial results. Burris et al[74] showed a significant survival advantage with gemcitabine compared with 5FU as well as an improvement in clinical benefits. The inclusion of older patients was permitted, but the median age was only 62 years old, and the data were not separately analyzed for the elderly subgroup[74]. Maréchal et al[69] retrospectively analyzed the efficacy and tolerability of gemcitabine-based first-line chemotherapy in patients aged 70 years or older who were included in phase 2 and phase 3 trials. The results revealed a similar efficacy and toxicity compared with patients who were younger than 70 years old[69].
In the last 5 years, great advances have been achieved in the treatment of metastatic disease. The ACCORD-11 phase III trial compared the efficacy and safety of FOLFIRINOX or gemcitabine as first-line therapy for metastatic pancreatic cancer[70]. In that study, the primary endpoint of overall survival was achieved (11.1 mo vs 6.8 mo for FOLFIRINOX and gemcitabine, respectively; HR = 0.57, P < 0.001). These robust efficacy results were achieved at the expense of a greater number of adverse events in the FOLFIRINOX group. It is relevant to consider that a performance status score > 1 and an age older than 75 years were exclusion criteria in this study. Only 98 of 342 patients (29%) were older than 65 years. Although an age of 65 years or older was identified as an independent prognostic factor for poor overall survival, this subgroup of patients also demonstrated a benefit in overall survival with FOLFIRINOX (HR = 0.48). The limited safety data for this regimen in older patients has prompted some authors to conduct small retrospective studies of advanced pancreatic cancer using modified FOLFIRINOX, and the results suggest an improved toxicity profile with an apparently similar efficacy[75].
Moreover, new clinical trials are being developed to examine the efficacy and tolerability of dose-adjusted FOLFIRINOX in patients with metastatic pancreatic cancer who are aged 70 years or older[76].
In the MPACT phase III clinical trial, the combination of gemcitabine and nab-paclitaxel also demonstrated superiority in terms of efficacy for metastatic pancreatic cancer compared with gemcitabine monotherapy[71]. In contrast to the PRODIGE trial, in this study, an age older than 75 years and a Karnofsky performance status of 70 (corresponding to an ECOG performance status of 2) were not exclusion criteria. The median age of the patients was higher than that in PRODIGE; in fact, 365 patients (42%) were at least 65 years of age, and 10% of the patients were 75 years or older (Table 3). The apparently better tolerance of this schedule despite the inclusion of patients who were older and had a worse ECOG performance status support nab-paclitaxel-gemcitabine as an interesting option for this group of patients.
Table 3.
Baseline characteristics of the Patients in the ACCORD-11 and MPACT trials
| Variable | ACCORD-11(FOLFIRINOX) | MPACT (Gemcitabine-Nab-paclitaxel) |
| Age | ||
| Median year (min, max) | 61 (25, 76) | 63 (27, 88) |
| ≥ 65-yr-old | 29.0% | 42% |
| ≥ 75-yr-old | - | 10% |
| Performance status (ECOG) | ||
| 0 | 37.4% | 16% |
| 1 | 61.9% | 44% |
| 2 | 32% | |
| 3 | 7% |
Many other randomized trials based on other combinations of gemcitabine such as gemcitabine plus oxaliplatin (GEMOX) or capecitabine-gemcitabine have been conducted without positive results[77-80]. Moreover, to our knowledge, the data collected in these studies have not been independently analyzed for the older subpopulation, and therefore, it is not yet possible to draw conclusions.
Moore et al[81] conducted a clinical trial evaluating the addition of erlotinib to gemcitabine. In their study, the primary end-point was met, with an overall survival that was significantly longer in the erlotinib group (5.91 mo vs 6.24 mo, HR = 0.82, P = 0.038). However, it remains questionable whether this difference is clinically relevant. Moreover, there were no differences in overall survival in the 268 patients (47%) who were older than 65 years (HR = 0.96).
The special characteristics of older patients and the observation that chemotherapy is not curative in this setting indicate that the risks and benefits of palliative chemotherapy should be carefully evaluated in terms of overall survival, tolerance and quality of life according to physiological rather than chronological age.
Considering the results and inclusion criteria obtained for large clinical trials of patients with metastatic pancreatic cancer, it is our opinion that FOLFIRINOX should be reserved for patients up to 75 years of age with an ECOG performance status of 0-1. This subgroup of patients could also be treated with the gemcitabine-nab-paclitaxel schedule. Patients with an ECOG performance status of 2 or who are older than 75 years with an ECOG performance status of 0-1 could be treated with nab-paclitaxel-gemcitabine. The most fragile patients would benefit from treatment with gemcitabine monotherapy (Figure 1).
Figure 1.
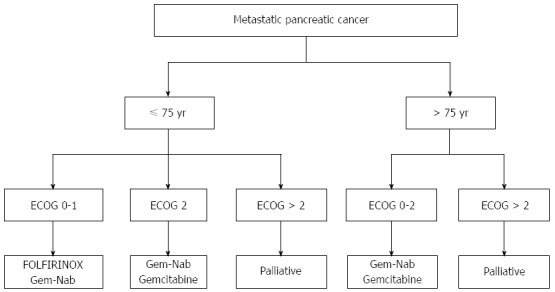
Choices of chemotherapy regimens for metastatic pancreatic cancer according to age and performance status. ECOG: Eastern cooperative oncology group; Gem-Nab: Gemcitabine-nab-paclitaxel.
PALLIATIVE CARE FOR PANCREATIC CANCER IN THE ELDERLY
Pancreatic cancer is characterized by a high symptom burden at the time of diagnosis and a short survival expectancy because most patients present with incurable locally advanced or metastatic disease. Hence, palliative care plays a key role in the management of these patients.
The most common complications associated with pancreatic cancer are due to tumor growth and infiltration of adjacent structures (biliary obstruction, duodenal obstruction, pancreatic insufficiency, pain) and systemic phenomena (cachexia, thromboembolic events).
More than 75% of patients with pancreatic cancer experience pain due to pancreatic and celiac plexus infiltration[82]. In general, elderly patients with cancer pain are undertreated, and many of them underreport their pain[83]. Adequate treatment for pain is essential to avoid a decrease in quality of life, depression and deterioration of performance status[84]. Pain should be treated according to the WHO analgesic recommendations. Major opioids are effective in the elderly, but some precautions must be considered because these patients often receive multiple medications[85]. Another treatment option that is available for control pain is a celiac plexus block[86]. This invasive technique achieves adequate pain control in 70%-90% of cases and significantly reduces the consumption of narcotics[87]. However, the duration of the analgesic effect does not exceed 2-3 mo.
Biliary obstruction is observed in up to 70% of pancreatic cancer patients. Most of these patients are treated successfully with an endoscopically placed stent, with resolution of the obstruction in 90% of cases[88]. Older age was found to be an unfavorable prognostic factor for stent patency when a plastic stent was used[89]. Surgical biliary bypass is usually the last option for patients in whom stent placement is ineffective. Duodenal obstruction occurs in approximately 20% of patients, and metal stents and palliative surgery are feasible therapeutic approaches[90]. Therefore, elderly patients with a reasonable life expectancy should be offered these palliative procedures which may improve patient’s quality of life, essential in this group of patients.
Cachexia is closely related to pancreatic cancer. It is a multifactorial syndrome that is defined by an ongoing loss of skeletal muscle mass (with or without a loss of fat mass) that cannot be fully reversed via conventional nutritional support and leads to progressive functional impairments. Its physiopathology is characterized by a negative protein and energy balance that is driven by a variable combination of reduced food intake and abnormal metabolism[91]. There is currently no single or combined treatment strategy that has been shown to be successful in all patients[92]. Glucocorticoids and megestrol acetate are effective for improvement of cachexia in 30%-50% of cases. Thus, the improved management of cancer cachexia may require a multimodal approach by a multi-disciplinary team[93].
In summary, the increasing incidence of pancreatic cancer in the elderly, the special features of this patient population, and the poor information available from clinical trials regarding the management of older patients has resulted in challenges in the treatment of these patients. However, age should not be the determining factor in decisions regarding the best approach. An integral evaluation of the patient in accordance with appropriate tools should be conducted. Some clinical trials targeting the elderly population are currently underway to gain a better understanding of this disease in the elderly.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 12, 2015
First decision: June 2, 2015
Article in press: November 9, 2015
P- Reviewer: Amedei A, Ghiorzo P, Ogura T S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Yancik R, Ries LA. Cancer in older persons: an international issue in an aging world. Semin Oncol. 2004;31:128–136. doi: 10.1053/j.seminoncol.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, et al. SEER Cancer Statistics Review, 1975-2007, National Cancer Institute 2010. Available from: http://seer.cancer.gov/csr/1975_2007/
- 4.Lasry A, Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36:217–228. doi: 10.1016/j.it.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Grimes A, Chandra SB. Significance of cellular senescence in aging and cancer. Cancer Res Treat. 2009;41:187–195. doi: 10.4143/crt.2009.41.4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amedei A, Niccolai E, Benagiano M, Della Bella C, Cianchi F, Bechi P, Taddei A, Bencini L, Farsi M, Cappello P, et al. Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol Immunother. 2013;62:1249–1260. doi: 10.1007/s00262-013-1429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amedei A, Niccolai E, Prisco D. Pancreatic cancer: role of the immune system in cancer progression and vaccine-based immunotherapy. Hum Vaccin Immunother. 2014;10:3354–3368. doi: 10.4161/hv.34392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazhin AV, Shevchenko I, Umansky V, Werner J, Karakhanova S. Two immune faces of pancreatic adenocarcinoma: possible implication for immunotherapy. Cancer Immunol Immunother. 2014;63:59–65. doi: 10.1007/s00262-013-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamisawa T, Yuyang T, Egawa N, Ishiwata J, Tsuruta K, Okamoto A, Koike M. Characteristics of pancreatic carcinoma in the elderly. Int J Pancreatol. 1998;24:31–34. doi: 10.1007/BF02787528. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Nio Y, Song MM, Sumi S, Hirahara N, Minari Y, Tamura K. p53 protein expression as prognostic factor in human pancreatic cancer. Anticancer Res. 1997;17:2779–2788. [PubMed] [Google Scholar]
- 11.Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–1869. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 12.Quaglia A, Tavilla A, Shack L, Brenner H, Janssen-Heijnen M, Allemani C, Colonna M, Grande E, Grosclaude P, Vercelli M. The cancer survival gap between elderly and middle-aged patients in Europe is widening. Eur J Cancer. 2009;45:1006–1016. doi: 10.1016/j.ejca.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Parmar AD, Vargas GM, Tamirisa NP, Sheffield KM, Riall TS. Trajectory of care and use of multimodality therapy in older patients with pancreatic adenocarcinoma. Surgery. 2014;156:280–289. doi: 10.1016/j.surg.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 15.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 16.Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5:224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 17.Balducci L, Extermann M. Cancer and aging. An evolving panorama. Hematol Oncol Clin North Am. 2000;14:1–16. doi: 10.1016/s0889-8588(05)70274-4. [DOI] [PubMed] [Google Scholar]
- 18.Lamont EB, Schilsky RL, He Y, Muss H, Cohen HJ, Hurria A, Meilleur A, Kindler HL, Venook A, Lilenbaum R, et al. Generalizability of trial results to elderly Medicare patients with advanced solid tumors (Alliance 70802) J Natl Cancer Inst. 2015;107:336. doi: 10.1093/jnci/dju336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balducci L, Corcoran MB. Antineoplastic chemotherapy of the older cancer patient. Hematol Oncol Clin North Am. 2000;14:193–212, x-xi. doi: 10.1016/s0889-8588(05)70284-7. [DOI] [PubMed] [Google Scholar]
- 20.Prithviraj GK, Koroukian S, Margevicius S, Berger NA, Bagai R, Owusu C. Patient Characteristics Associated with Polypharmacy and Inappropriate Prescribing of Medications among Older Adults with Cancer. J Geriatr Oncol. 2012;3:228–237. doi: 10.1016/j.jgo.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lees J, Chan A. Polypharmacy in elderly patients with cancer: clinical implications and management. Lancet Oncol. 2011;12:1249–1257. doi: 10.1016/S1470-2045(11)70040-7. [DOI] [PubMed] [Google Scholar]
- 22.Puts MT, Santos B, Hardt J, Monette J, Girre V, Atenafu EG, Springall E, Alibhai SM. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol. 2014;25:307–315. doi: 10.1093/annonc/mdt386. [DOI] [PubMed] [Google Scholar]
- 23.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamaker ME, Vos AG, Smorenburg CH, de Rooij SE, van Munster BC. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist. 2012;17:1439–1449. doi: 10.1634/theoncologist.2012-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, Falandry C, Artz A, Brain E, Colloca G, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, Roth C, MacLean CH, Shekelle PG, Sloss EM, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 27.Riall TS. What is the effect of age on pancreatic resection? Adv Surg. 2009;43:233–249. doi: 10.1016/j.yasu.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riall TS, Reddy DM, Nealon WH, Goodwin JS. The effect of age on short-term outcomes after pancreatic resection: a population-based study. Ann Surg. 2008;248:459–467. doi: 10.1097/SLA.0b013e318185e1b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riall TS, Sheffield KM, Kuo YF, Townsend CM, Goodwin JS. Resection benefits older adults with locoregional pancreatic cancer despite greater short-term morbidity and mortality. J Am Geriatr Soc. 2011;59:647–654. doi: 10.1111/j.1532-5415.2011.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohn TA, Yeo CJ, Cameron JL, Lillemoe KD, Talamini MA, Hruban RH, Sauter PK, Coleman J, Ord SE, Grochow LB, et al. Should pancreaticoduodenectomy be performed in octogenarians? J Gastrointest Surg. 1998;2:207–216. doi: 10.1016/s1091-255x(98)80014-0. [DOI] [PubMed] [Google Scholar]
- 31.Meguid RA, Ahuja N, Chang DC. What constitutes a “high-volume” hospital for pancreatic resection? J Am Coll Surg. 2008;206:622.e1–622.e9. doi: 10.1016/j.jamcollsurg.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Makary MA, Winter JM, Cameron JL, Campbell KA, Chang D, Cunningham SC, Riall TS, Yeo CJ. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg. 2006;10:347–356. doi: 10.1016/j.gassur.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222:638–645. doi: 10.1097/00000658-199511000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg. 2007;205:729–734. doi: 10.1016/j.jamcollsurg.2007.06.307. [DOI] [PubMed] [Google Scholar]
- 35.Hodul P, Tansey J, Golts E, Oh D, Pickleman J, Aranha GV. Age is not a contraindication to pancreaticoduodenectomy. Am Surg. 2001;67:270–275; discussion 275-276. doi: 10.1016/s0016-5085(00)81967-8. [DOI] [PubMed] [Google Scholar]
- 36.Ito Y, Kenmochi T, Irino T, Egawa T, Hayashi S, Nagashima A, Kitagawa Y. The impact of surgical outcome after pancreaticoduodenectomy in elderly patients. World J Surg Oncol. 2011;9:102. doi: 10.1186/1477-7819-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bathe OF, Levi D, Caldera H, Franceschi D, Raez L, Patel A, Raub WA, Benedetto P, Reddy R, Hutson D, Sleeman D, Livingstone AS, Levi JU. Radical resection of periampullary tumors in the elderly: evaluation of long-term results. World J Surg. 2000;24:353–358. doi: 10.1007/s002689910056. [DOI] [PubMed] [Google Scholar]
- 38.Aloia TA, Lee JE, Vauthey JN, Abdalla EK, Wolff RA, Varadhachary GR, Abbruzzese JL, Crane CH, Evans DB, Pisters PW. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347–355. doi: 10.1016/j.jamcollsurg.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Targarona J, Callacondo D, Pino C, Rodriguez C, Coayla G, Garatea R, Barreda C, Barreda L. [Impact of duodenopancreatectomy in elder patients] Rev Gastroenterol Peru. 2013;33:217–222. [PubMed] [Google Scholar]
- 40.Frakes JM, Strom T, Springett GM, Hoffe SE, Balducci L, Hodul P, Malafa MP, Shridhar R. Resected pancreatic cancer outcomes in the elderly. J Geriatr Oncol. 2015;6:127–132. doi: 10.1016/j.jgo.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Horowitz DP, Hsu CC, Wang J, Makary MA, Winter JM, Robinson R, Schulick RD, Cameron JL, Pawlik TM, Herman JM. Adjuvant chemoradiation therapy after pancreaticoduodenectomy in elderly patients with pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2011;80:1391–1397. doi: 10.1016/j.ijrobp.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagrial AM, Chang DK, Nguyen NQ, Johns AL, Chantrill LA, Humphris JL, Chin VT, Samra JS, Gill AJ, Pajic M, et al. Adjuvant chemotherapy in elderly patients with pancreatic cancer. Br J Cancer. 2014;110:313–319. doi: 10.1038/bjc.2013.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 44.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 45.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 46.Davila JA, Chiao EY, Hasche JC, Petersen NJ, McGlynn KA, Shaib YH. Utilization and determinants of adjuvant therapy among older patients who receive curative surgery for pancreatic cancer. Pancreas. 2009;38:e18–e25. doi: 10.1097/MPA.0b013e318187eb3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 48.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 49.Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–82; discussion 782-4. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyamoto DT, Mamon HJ, Ryan DP, Willett CG, Ancukiewicz M, Kobayashi WK, Blaszkowsky L, Fernandez-del Castillo C, Hong TS. Outcomes and tolerability of chemoradiation therapy for pancreatic cancer patients aged 75 years or older. Int J Radiat Oncol Biol Phys. 2010;77:1171–1177. doi: 10.1016/j.ijrobp.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 51.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 52.Shinchi H, Takao S, Noma H, Matsuo Y, Mataki Y, Mori S, Aikou T. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2002;53:146–150. doi: 10.1016/s0360-3016(01)02806-1. [DOI] [PubMed] [Google Scholar]
- 53.Sultana A, Tudur Smith C, Cunningham D, Starling N, Tait D, Neoptolemos JP, Ghaneh P. Systematic review, including meta-analyses, on the management of locally advanced pancreatic cancer using radiation/combined modality therapy. Br J Cancer. 2007;96:1183–1190. doi: 10.1038/sj.bjc.6603719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moertel CG, Frytak S, Hahn RG, O’Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 55.Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol. 2003;21:3409–3414. doi: 10.1200/JCO.2003.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Morizane C, Okusaka T, Ito Y, Ueno H, Ikeda M, Takezako Y, Kagami Y, Ikeda H. Chemoradiotherapy for locally advanced pancreatic carcinoma in elderly patients. Oncology. 2005;68:432–437. doi: 10.1159/000086985. [DOI] [PubMed] [Google Scholar]
- 57.Rwigema JC, Parikh SD, Heron DE, Howell M, Zeh H, Moser AJ, Bahary N, Quinn A, Burton SA. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34:63–69. doi: 10.1097/COC.0b013e3181d270b4. [DOI] [PubMed] [Google Scholar]
- 58.Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, Ford JM, Desser T, Quon A, Koong AC. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 59.Kim CH, Ling DC, Wegner RE, Flickinger JC, Heron DE, Zeh H, Moser AJ, Burton SA. Stereotactic body radiotherapy in the treatment of pancreatic adenocarcinoma in elderly patients. Radiat Oncol. 2013;8:240. doi: 10.1186/1748-717X-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L, Azzedine A, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 61.Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–46; discussion 846-8. doi: 10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim KH, Chung E, Khan A, Cao D, Linehan D, Ben-Josef E, Wang-Gillam A. Neoadjuvant therapy of pancreatic cancer: the emerging paradigm? Oncologist. 2012;17:192–200. doi: 10.1634/theoncologist.2011-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao WC, Chien KL, Lin YL, Wu MS, Lin JT, Wang HP, Tu YK. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:1095–1103. doi: 10.1016/S1470-2045(13)70388-7. [DOI] [PubMed] [Google Scholar]
- 66.Miura JT, Krepline AN, Duelge KD, George B, Ritch PS, Erickson B, Thomas JP, Mahmoud A, Quebbeman EJ, Turaga K, et al. Neoadjuvant therapy for pancreatic cancer in patients older than age 75. J Clin Oncol. 2014;32 suppl 3:abstr 287. [Google Scholar]
- 67.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 68.Tas F, Sen F, Keskin S, Kilic L, Yildiz I. Prognostic factors in metastatic pancreatic cancer: Older patients are associated with reduced overall survival. Mol Clin Oncol. 2013;1:788–792. doi: 10.3892/mco.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maréchal R, Demols A, Gay F, de Maertelaer V, Arvanitaki M, Hendlisz A, Van Laethem JL. Tolerance and efficacy of gemcitabine and gemcitabine-based regimens in elderly patients with advanced pancreatic cancer. Pancreas. 2008;36:e16–e21. doi: 10.1097/MPA.0b013e31815f3920. [DOI] [PubMed] [Google Scholar]
- 70.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 71.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li D, Capanu M, Yu KH, Lowery MA, Kelsen DP, O’Reilly EM. Treatment, outcomes, and clinical trial participation in very elderly patients (pts) with metastatic pancreas cancer (mPC) ASCO Meeting Abstracts. 2014;32:4119. doi: 10.1016/j.clcc.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aldoss IT, Tashi T, Gonsalves W. Role of chemotherapy in the very elderly patients with metastatic pancreatic cancer - a Veterans Affairs Cancer Registry analysis. J Geriatr Oncol. 2011;2:209–214. [Google Scholar]
- 74.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 75.Alessandretti MB, Moreira RB, Brandao EP, Gomes JR, Fernandes Amarante MP, Lino AD. Safety and efficacy of modified dose-attenuated FOLFIRINOX chemotherapy in patients over 65 years with advanced pancreatic adenocarcinoma. J Clin Oncol. 2015:33. [Google Scholar]
- 76.Clinicaltrials. Available from: https://clinicaltrials.gov/ct2/show/NCT02143219.
- 77.Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 78.Poplin E, Feng Y, Berlin J, Rothenberg ML, Hochster H, Mitchell E, Alberts S, O’Dwyer P, Haller D, Catalano P, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–2217. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 80.Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 81.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 82.Wyse JM, Chen YI, Sahai AV. Celiac plexus neurolysis in the management of unresectable pancreatic cancer: when and how? World J Gastroenterol. 2014;20:2186–2192. doi: 10.3748/wjg.v20.i9.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc. 1990;38:409–414. doi: 10.1111/j.1532-5415.1990.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 84.Torgerson S, Wiebe LA. Supportive care of the patient with advanced pancreatic cancer. Oncology (Williston Park) 2013;27:183–190. [PubMed] [Google Scholar]
- 85.Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13:e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 86.Seicean A. Celiac plexus neurolysis in pancreatic cancer: the endoscopic ultrasound approach. World J Gastroenterol. 2014;20:110–117. doi: 10.3748/wjg.v20.i1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McGreevy K, Hurley RW, Erdek MA, Aner MM, Li S, Cohen SP. The effectiveness of repeat celiac plexus neurolysis for pancreatic cancer: a pilot study. Pain Pract. 2013;13:89–95. doi: 10.1111/j.1533-2500.2012.00557.x. [DOI] [PubMed] [Google Scholar]
- 88.Maire F, Hammel P, Ponsot P, Aubert A, O’Toole D, Hentic O, Levy P, Ruszniewski P. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol. 2006;101:735–742. doi: 10.1111/j.1572-0241.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 89.Matsuda Y, Shimakura K, Akamatsu T. Factors affecting the patency of stents in malignant biliary obstructive disease: univariate and multivariate analysis. Am J Gastroenterol. 1991;86:843–849. [PubMed] [Google Scholar]
- 90.Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7:18. doi: 10.1186/1471-230X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–1350. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 92.Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44:1124–1132. doi: 10.1016/j.ejca.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 93.Tuca A, Jimenez-Fonseca P, Gascón P. Clinical evaluation and optimal management of cancer cachexia. Crit Rev Oncol Hematol. 2013;88:625–636. doi: 10.1016/j.critrevonc.2013.07.015. [DOI] [PubMed] [Google Scholar]


