Abstract
Pancreatic carcinoma is a common cancer of the digestive system with a poor prognosis. It is characterized by insidious onset, rapid progression, a high degree of malignancy and early metastasis. At present, radical surgery is considered the only curative option for treatment, however, the majority of patients with pancreatic cancer are diagnosed too late to undergo surgery. The sensitivity of pancreatic cancer to chemotherapy or radiotherapy is also poor. As a result, there is no standard treatment for patients with advanced pancreatic cancer. Cryoablation is generally considered to be an effective palliative treatment for pancreatic cancer. It has the advantages of minimal invasion and improved targeting, and is potentially safe with less pain to the patients. It is especially suitable in patients with unresectable pancreatic cancer. However, our initial findings suggest that cryotherapy combined with 125-iodine seed implantation, immunotherapy or various other treatments for advanced pancreatic cancer can improve survival in patients with unresectable or metastatic pancreatic cancer. Although these findings require further in-depth study, the initial results are encouraging. This paper reviews the safety and efficacy of cryoablation, including combined approaches, in the treatment of pancreatic cancer.
Keywords: Pancreatic cancer, Cryoablation, Combination therapy, Cryoimmunotherapy
Core tip: Pancreatic carcinoma is a devastating cancer of the digestive system. The majority of patients present too late to undergo radical surgery and there is no standard treatment for patients with advanced pancreatic cancer. Cryoablation is considered a palliative therapy in the treatment of pancreatic cancer; however, our results suggest that cryoablation may be an effective and safe treatment that can improve survival and quality of life in patients with unresectable or metastatic pancreatic cancer, particularly in combination with other modalities such as 125-iodine seed implantation and immunotherapy. As such, cryoablation may be a potential standard treatment for pancreatic cancer.
INTRODUCTION
Pancreatic cancer is one of the most common and lethal forms of gastrointestinal cancer. It is characterized by insidious onset, rapid progression, a high degree of malignancy, early metastasis and poor prognosis[1]. In 2011, mortality rates of pancreatic cancer relative to all cancers worldwide ranked eighth and ninth for males and females, respectively[2]. Furthermore, there has been almost no improvement in survival rates in the past four years and the incidence of pancreatic cancer has risen in the United States over the last three years[1,3,4]. Currently, radical surgical resection is the only curative option but is only possible in 5%-25% of cases[5]. The majority of patients present with unresectable pancreatic cancer and 40%-45% have metastatic disease, primarily hepatic. Consequently, the median survival rate is only 3-6 mo. Even the most favorable patients who undergo resection with adjuvant therapy show 5-year survival rates < 29%; for patients with metastatic advanced pancreatic cancer, surgery offers no advantage over palliative treatment and the prognosis is poor[6]. Although the spread of pancreatic cancer cells can be controlled in part by chemotherapy, it is often administered too late to be of therapeutic benefit and the toxic side effects are severe, causing some patients to give up treatment. The therapeutic effect of chemotherapy is even poorer in advanced pancreatic cancer with metastatic disease. As such, pancreatic cancer remains one of the most lethal types of cancer due to its lack of early symptoms and rapid progression.
A large phase III trial carried out in 2012 studied combination chemotherapy with Folfirinox in 61 cases of pancreatic cancer. The majority of patients (86.9%) had an ECOG (Eastern Cooperative Oncology Group) performance status of 0 or 1; 62.3% had metastatic disease; and 37.7% had been treated for locally advanced or borderline resectable disease. The response rate was around 25%, with median progression-free survival and overall survival of 7.5 mo and 13.5 mo, respectively. A total of 21 (34.4%) patients were hospitalized as a result of therapy, however, there were no therapy-related deaths. Therapy was discontinued in 23 (37.7%) patients due to adverse events[7]. Although the Folfirinox regimen was found to be effective, its application has been limited due to adverse side effects and it should be administered with caution.
Radiochemotherapy has been shown to extend median survival time in patients with pancreatic cancer by 9-13 mo, however, the disease progressed rapidly in many cases after therapy was completed[8], and its application has been limited due to a high level of complications.
Ablative therapies have the advantages of minimal invasion with improved targeting and less pain to the patients. However, the complex biological characteristics of the pancreas mean that radiofrequency ablation (RFA) imposes a high risk to the surrounding tissues and its impact on patient survival needs further clinical research. In contrast, microwave ablation is less affected by current conduction, dry tissue or carbonization and perfusion compared to RFA. It is also faster and can cover a greater area than RFA. Multiple applications of microwaves can also be administered without impacting on each other[9]. High-intensity focused ultrasound ablation has been used for a relatively long time in the treatment of cancers compared to RFA and microwave ablation, especially in larger tumors. However, its efficacy is affected by various factors in the treatment of pancreatic cancer, including the effects of gastrointestinal tract and respiratory movement. Research into the application of laser ablation in pancreatic cancer is scarce and further data are required to support its use. In summary, the complex anatomy of the pancreas and peripheral vascular tissues, its close association with the gastrointestinal tract and the solidity of the pancreatic mass mean that ablation therapies are usually administered in combination with surgery in the treatment of pancreatic cancer. Although they are often the treatment of choice as a palliative therapy, their clinical application in the treatment of unresectable advanced pancreatic cancer has been limited.
Cryosurgery is a novel therapeutic approach in the treatment of benign and malignant tumors, especially unresectable tumors[10]. A number of clinical trials have reported encouraging results in its treatment of lung cancer, liver cancer, prostate cancer, kidney tumors and breast cancer[11-15]. We have recently shown that cryotherapy can be effective in improving survival time and quality of life in patients with pancreatic cancer[16,17].
SAFETY
To date, cryoablation has not been widely used in the treatment of pancreatic tumors primarily due to the small volume of the pancreatic gland, fragile pancreatic parenchyma and its proximity to structures such as the stomach, duodenum, colon, common bile duct and vessels. In comparison, the liver has a large volume of normal hepatic parenchyma surrounding the tumor-bearing tissue. Based on our clinical experience, we believe cryosurgery has several advantages over other techniques in the treatment of unresectable pancreatic cancer: (1) Conventional management of unresectable pancreatic cancer involves a bypass operation without removal of the tumor and is primarily used as a palliative therapy; cryosurgery can overcome this shortcoming by enabling this procedure to be converted to radical surgery; (2) Cryosurgery is less invasive and has a lower level of complications compared to conventional resection procedures; (3) Unresectable tumors can be treated with percutaneous cryosurgery under ultrasound (US) or computed tomography (CT) guidance, with efficacies similar to those achieved with intraoperative cryosurgery; (4) Percutaneous cryosurgery can be performed simultaneously with other modalities, such as 125-iodine seed implantation, for the treatment of metastatic tumors; (5) Immune enhancement or activation can occur following cryosurgery due to quantitative and qualitative changes in antigens on the cell-surface components of the tumor cells, termed cryoimmunity[18,19]; and (6) The main purpose of most pancreatic cancer treatments is to debulk the tumor rather than provide “radical treatment”. Cryoablation can achieve this goal. It is reported that cryoablated cancerous tissue has an increased sensitivity to chemo/radiotherapy[20].
Intraoperative cryosurgery
Cryotherapy of pancreatic cancer can be divided into intraoperative cryosurgery and percutaneous cryosurgery. Complications associated with intraoperative cryosurgery are similar to those found in other abdominal and thoracic surgical operations; however, a study into intraoperative cryosurgery carried out between 1995 and 1999 in 10 patients with unresectable pancreatic cancer reported a reduction in cancer-related pain in all cases following cryosurgery with no postoperative complications, such as pancreatitis, pancreatic fistulas and sepsis, or death directly related to surgery[21]. A later study carried out in 2008 in 46 patients with pancreatic cancer who underwent intraoperative cryosurgery reported that 31 (67%) patients presented with gastroparesis, there were no surgery-related deaths, 29 patients presented with the tumor located in the pancreatic head and uncinate process, and in 2 cases the tumor was located in the body and tail of the pancreas[22]. This gastroparesis may be related to the surgical procedure. In general, patients with pancreatic cancer respond well to cryosurgery and the method is less invasive than conventional pancreatic resection with fewer surgical-related complications or occurrences of postoperative death. Korpan considered that cryosurgery should be the modality of choice in most cases of pancreatic cancer[23].
Percutaneous cryotherapy
Freezing has long been used in the treatment of cancers; however, the technique is only possible in deep-seated tumors by using argon-helium knife technology. By exploiting recent advances in imaging technology, three novel techniques in percutaneous cryosurgery were pioneered: As the liver and stomach heal rapidly following surgery, we used transabdominal approaches for tumors located in the pancreatic head; whereas transdorsal approaches were adopted for tumors located in the pancreatic body or tail. The percutaneous probe can be administered in four ways depending on the position of the needle in the tumor: through the stomach (Figure 1); through the left lobe of the liver (Figure 2); between the stomach and transverse colon (Figure 3); or by using transdorsal approaches (Figure 4).
Figure 1.
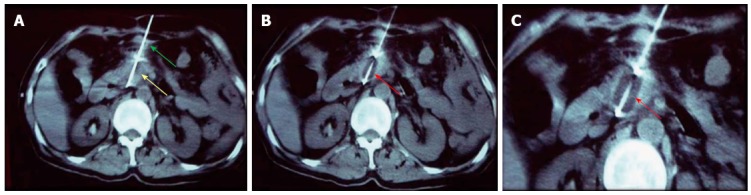
Percutaneous cryoablation of pancreatic cancer under computed tomography guidance. The images were taken via the stomach. A: The cryoprobe (green arrow) was percutaneously inserted into the tumor at the head of the pancreas via the stomach (yellow arrow); B: The ice ball beginning to form (red arrow) following the application of argon gas; C: The ice ball (red arrow) surrounding the probe shows a gradual increase in size.
Figure 2.
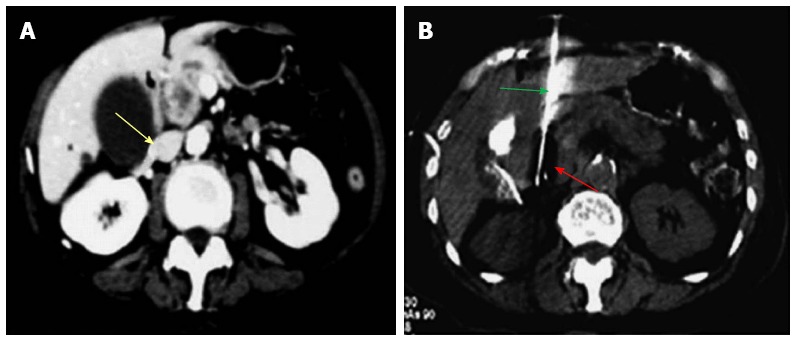
Percutaneous cryoablation of pancreatic cancer under enhanced computed tomography guidance. The images were taken via the left lobe of the liver. A: The tumor at the head of the pancreas (yellow arrow) is visible with high intensity; B: The probe (green arrow) was percutaneously inserted into the tumor via the left lobe of the liver; the ice ball appears as a dark area (red arrow).
Figure 3.
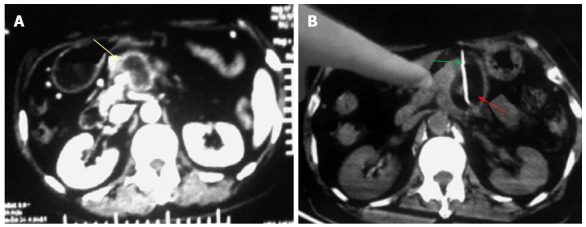
Percutaneous cryoablation of pancreatic cancer under enhanced computed tomography guidance. The images were taken between the stomach and transverse colon. A: The tumor at the head of the pancreas (yellow arrow) is visible with high intensity; B: The probe (green arrow) was percutaneously inserted into the tumor between the stomach and transverse colon; the ice ball is shown as a dark area (red arrow).
Figure 4.
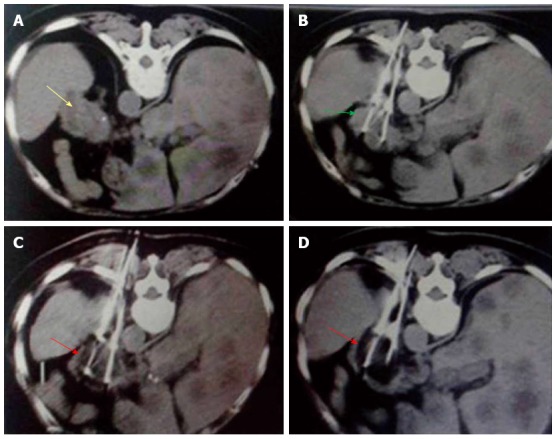
Percutaneous cryoablation of pancreatic cancer under enhanced computed tomography guidance. The images represent transdorsal approaches. A: The tumor at the head of the pancreas can be seen with high intensity (yellow arrow); B: The probe (green arrow) was inserted percutaneously into the tumor via a transdorsal approach; C: Ice ball formation appears as a dark area (red arrow); D: The size of the ice ball covers the entire area of tumor.
We have carried out multiple retrospective analyses in our institution (Guangzhou Fuda Cancer Hospital) into the application of percutaneous cryoablation in the treatment of pancreatic cancer. In 2008 Xu et al[24] reported that the treatment of locally advanced pancreatic cancer by percutaneous cryoablation gave satisfactory results. Argon-helium knife percutaneous cryosurgery was used in the treatment of 49 cases of locally advanced pancreatic cancer between 2001 and 2008. The technique involved two freeze-thaw cycles, each of which involved freezing for 5 min followed by rewarming for 10 min. Adverse effects included upper abdominal pain with an increase in serum amylase; six cases of acute pancreatitis, one of whom developed severe pancreatitis; and intra-abdominal bleeding in three patients which stopped within four days[24,25]. This freeze-thaw treatment was also administered to 59 patients with locally advanced pancreatic cancer between 2008 and 2009. The adverse effects included 45 cases of postoperative abdominal pain, 29 cases of fever and 34 cases with an increase in serum amylase. A further 5 cases developed more serious complications including intra-abdominal hemorrhage, pancreatic leakage, obstruction and cryoprobe needle tract metastasis[26]. US and CT guided percutaneous cryoablation was used to treat 32 patients between February 2009 and February 2010 with unresectable pancreatic cancer or resectable pancreatic cancer staged from II-IV which was not amenable to surgery. They included 15 tumors smaller than 5 cm (46.9%) that were successfully ablated in one session of cryoablation. There were no serious complications and 27 patients experienced a ≥ 50% reduction in pain, 22 experienced a 50% decrease in analgesic consumption and 16 experienced an increase in Karnofsky Performance Status (KPS) score ≥ 20. In addition, partial response and stable disease were reported in 9 and 21 patients, respectively, and their lesions could be controlled by CT or enhanced CT[16]. US and CT guided percutaneous cryoablation has three key advantages in the treatment of pancreatic cancer: (1) It provides real-time and accurate monitoring of ice ball formation, vascular visualization and optimal positioning and control of the cryoprobe throughout the procedure; (2) Improved visualization as a result of US and CT imaging protects adjacent vascular and organs during cryoprobe positioning and freezing; and (3) The procedure is simpler and has a shorter duration (approximately 1 h) than conventional approaches, thereby improving recovery times and reducing the length of hospitalization. All of the analyses showed that complications resulting from percutaneous cryoablation could be minimized by symptomatic treatment, and there were no treatment-related deaths.
In theory, intraoperative cryosurgery should offer an advantage over percutaneous cryoablation by protecting visceral tissues through cooling while the ablation procedure is performed under direct vision; however, we found that US or CT imaging during percutaneous cryoablation can reveal adjacent structures more clearly than laparotomy[16]. Furthermore, the complications associated with intraoperative cryosurgery were similar to those observed in open abdominal or thoracic surgery, whereas percutaneous cryoablation in the treatment of pancreatic cancer appeared safer with fewer therapy-related complications. The advantages of US and CT guided percutaneous cryoablation, including improved accuracy and prolonged patient survival, suggested that this approach may provide an alternative independent or comprehensive therapy in the treatment of pancreatic cancer. Most of complications can be prevented or mitigated with the improvement of cryoablation techniques and in-depth knowledge on the anatomy of the pancreas. In summary, cryotherapy in the treatment of pancreatic cancer can be safe and reliable.
EFFICACY
To date there have been relatively few studies on the efficacy of cryotherapy in the treatment pancreatic cancer and most have been confined to intraoperative cryosurgery or cryosurgery under endoscopic ultrasound. Those relating to percutaneous cryotherapy are rare, with the majority being carried out in Fuda Hospital Guangzhou. A study involving 49 patients with locally advanced pancreatic cancer who received intraoperative cryosurgery or percutaneous cryosurgery at our hospital between March 2001 and November 2007 showed that median survival was 16.2 mo and overall survival rates at 6, 12, 24 and 36 mo were 94.9%, 63.1%, 22.8% and 9.5%, respectively. At the time of this report, the patient with the longest survival time (40 mo) is still alive with no evidence of tumor recurrence[24]. A retrospective analysis of follow-up data from 59 patients treated between 2008 and 2009 found that their median overall survival was 8.4 mo, with 3, 6 and 12-mo overall survival rates of 89.7%, 61.1% and 34.5%, respectively[26]. An analysis of follow-up data from a further 32 patients with stages II-IV pancreatic cancer treated with percutaneous US and CT guided cryoablation between February 2009 and February 2010 found that their median overall survival was 12.6 mo, with 6, 12 and 24-mo survival rates of 82.8%, 54.7% and 27.3%, respectively (Figure 5)[16]. Although these patient numbers are too small to draw strong conclusions on the effectiveness of cryoablation in the treatment of pancreatic cancer, the data are encouraging.
Figure 5.
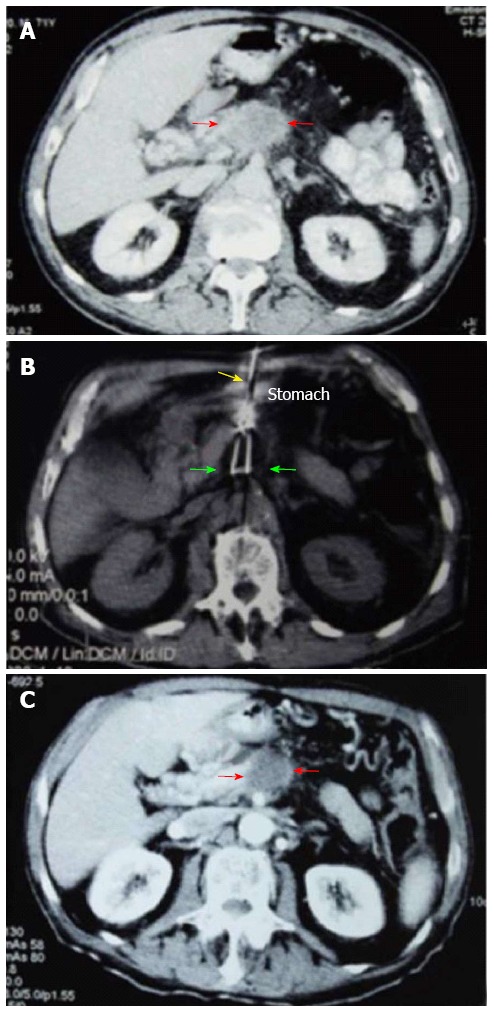
Comparisons between computed tomography images of pancreatic carcinoma before, during and 1.5 mo after cryoablation. A: The tumor at stage IV (red arrows) is 4.5 cm × 4.6 cm × 3.8 cm in size; B: The cryoprobe was inserted via the stomach approaching the tumor mass (yellow arrow); cryoablation was performed after the pancreatic tumor was completely encased within the ice ball (green arrows); C: The center of the tumor mass (red arrows) 1.5 mo post-cryoablation displays shrinkage with no enhancement of the tumor.
COMBINATION OF CRYOTHERAPY WITH OTHER TREATMENTS
A major limitation of cryoablation is incomplete destruction of cells at the border of the treated regions where the tissue temperature is greater than -20 °C. The irregular shape of the pancreas and infiltration of surrounding organs also give rise to complications during cryotherapy. For these reasons adjunctive approaches are required and percutaneous cryotherapy is usually combined with other treatments. Adjunctive therapy, including irradiation, chemotherapeutic drugs, apoptotic promoters and immunological potentiators, has been shown to increase the efficacy of cryosurgery in many types of cancer[27] and has been the subject of research in our institution in relation to pancreatic cancer.
Cryotherapy combined with iodine-125 seed implantation
Radioactive seed implantation has been used in the treatment of pancreatic cancer for over 20 years, however, the survival benefit has been limited in advanced cases[28,29]. Iodine-125 is an isotope that emits γ-radiation over short distances, resulting in the death of targeted cells. Brachytherapy using iodine-125 seed implantation has been successfully used in the treatment of prostate cancer, including metastatic and recurrent cancer[30,31]. Compared to other interventional procedures, this technique has the following advantages: radiation from the seeds is attenuated within a short distance of the target area, ensuring that the highest accumulative dose is confined to the tumor with minimal damage to neighboring organs[28]; radiation is applied continuously throughout the treatment, resulting in the protracted killing of tumor cells over several weeks or months, ultimately leading to apoptosis of tumor stem cells. In contrast, multiple applications of external radiotherapy can only target tumor cells at limited number of cell cycle phases; radiation from radioactive seeds in the treatment of pancreatic cancer is unaffected by respiratory movements, whereas absorption of radiation with traditional techniques is inhomogeneous; the metastatic potential of the cancer is decreased due to changes in the immunophenotype of tumor cells subjected to the low level of radioactivity from the seeds. Together, these advantages suggest that iodine-125 seed implantation may be an effective complementary therapy alongside cryosurgery in the treatment of pancreatic cancer.
A study of 67 patients with pancreatic cancer who had been treated with percutaneous cryosurgery combined with 125-iodine seed implantation and chemotherapy (Figure 6) showed that their median survival time was 11 mo, progression free survival was 5.5 mo and the 6-mo and 12-mo overall survival rates were 84.8% and 33.4%, respectively. There was no significant differences between patients with stages III and IV pancreatic cancer (Figure 7). In addition, 54 patients reported a > 50% decrease in their pain scores, the level of analgesia was decreased by > 50% in 50 cases, and the total response rate was 80.6%[32].
Figure 6.
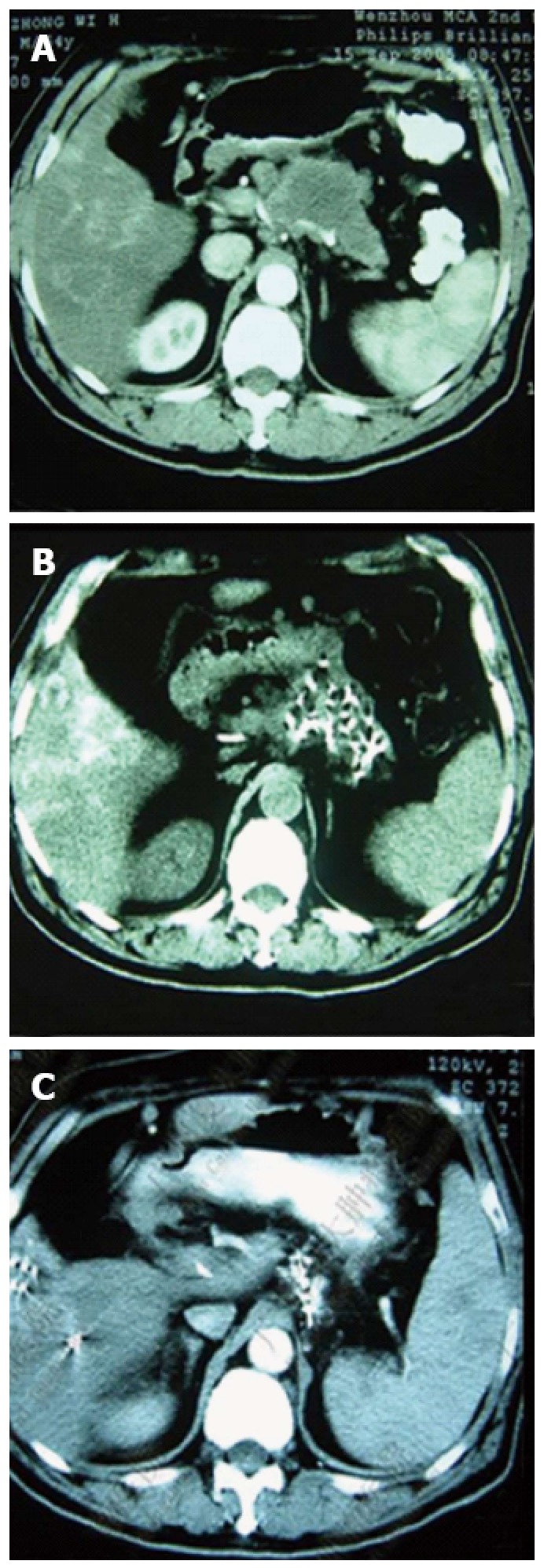
Pancreatic computed tomography scans before and after cryosurgery combined with 125-iodine seed implantation. A: Pancreatic lesions before treatment; B: Shrinkage of the pancreatic tumor one month post-treatment; C: Total shrinkage of the pancreatic tumor 6 mo post-treatment.
Figure 7.
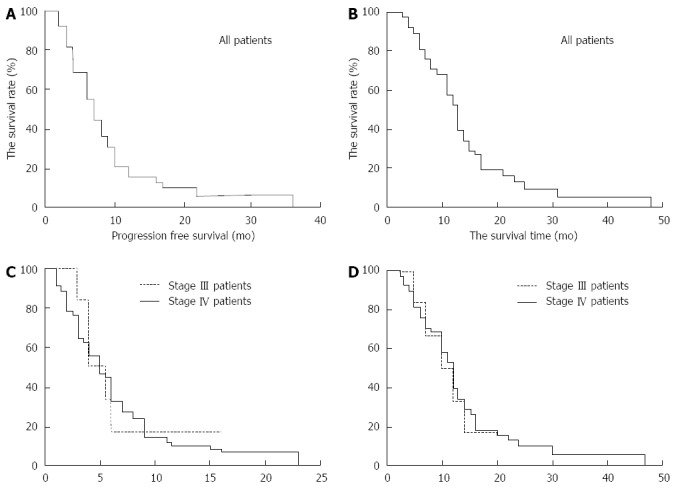
Survival plots of 67 patients with advanced pancreatic cancer after percutaneous cryoablation combined with 125-iodine seed implantation and chemotherapy. A: Progression-free survival curves for all 67 patients following treatment; B: Overall survival curves for all 67 patients following the combined treatment; C: Progression-free survival curves for patients with stages III and IV advanced pancreatic cancer post-treatment; D: Overall survival curves for patients with stages III and IV advanced pancreatic cancer post-treatment.
A retrospective analysis of 145 patients with stage IV pancreatic cancer treated at our hospital between October 2008 and August 2010 compared survival times between different treatments[33]. The patients included those who presented with primary cancer without metastases and those who presented with metastatic pancreatic cancer. The patients were categorized into the following treatment groups and sub-groups: cryoablation combined with 125-iodine seed implantation; 125-iodine seed implantation alone; those who were treated within two months of diagnosis; and those who were treated 2-14 mo after diagnosis. The results showed that the median survival time of patients who received the combined therapy was significantly longer than those treated with particle implantation alone (8 mo vs 4 mo, P = 0.001); similarly, the median survival times of patients with non-metastatic and metastatic pancreatic cancer who received combined treatment within two months of diagnosis were significantly higher than those who received treatment from 2-14 mo after diagnosis (13 mo vs 6 mo, P = 0.0034); whereas metastatic patients who received the delayed combination treatment showed no significant survival advantage compared to those treated with 125-iodine seed implantation alone (5 mo vs 3 mo, P = 0.0415). Further analyses demonstrated that repeated application of the combined treatment resulted in significantly longer median survival times than a single application (11 mo vs 7 mo, P = 0.0389); no such benefit was observed if seed implantation was administered alone (P = 0.990). In addition, patients who received repeated treatment within 2-14 mo also showed longer survival times, however, this may have been associated with pre-treatment with chemotherapy[33].
A follow-up study was conducted to determine overall survival rates in 49 patients with locally advanced pancreatic cancer who were treated at our hospital with combined cryosurgery and iodine-125 seed implantation (Figure 8). During a median follow-up period of 18 mo, the median overall survival time was 16.2 mo, with 6, 12, 24 and 36-mo overall survival rates of 94.9%, 63.1%, 22.8% and 9.5%, respectively. While in another study, 59 patients with locally advanced pancreatic cancer were treated percutaneous cryosurgery alone. The median survival was 8.4 mo. The overall survival rates at 3, 6 and 12 mo were 89.7%, 61.1% and 34.5%, respectively. These results confirmed that combined treatment could achieve longer overall median survival and higher 6-mo and 12-mo survival rates than cryosurgery alone[34].
Figure 8.
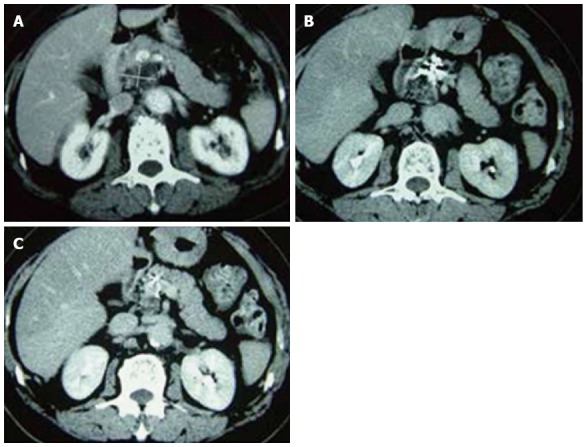
Pancreatic computed tomography scans of patients who received cryosurgery combined with 125-iodine seed implantation before and after treatment. A: Before treatment; B: 3 mo post-treatment; C: 12 mo post-treatment.
Together, the results of our analyses have demonstrated that combination of percutaneous cryosurgery with iodine-125 seed implantation is feasible, potentially safe and may be a promising option for the treatment of patients with locally advanced and unresectable pancreatic cancer, leading to longer survival times. The combined approach appears to achieve greater control of the tumor as the 125-iodine particles reduce damage to the target organ and surrounding normal tissue. The benefit was greatest where the anatomy was complex, such as those involving tumors at the head of the pancreas. This is an important consideration in the treatment of locally advanced pancreatic cancer.
Cryosurgery combined with immunotherapy
The local tumor microenvironment can inhibit immune cells, weakening the immune system and resulting in the rapid growth of tumors[35]. Immunotherapy with dendritic cells loaded with autologous tumor cells (antigen-loaded dendritic cells) has been shown to be important in preventing tumor recurrence and improving the quality of life in cancer patients. This recent development enables cytokine-induced killer cells (dendritic cell-cytokine induced killer, DC-CIK) to restore the number of T lymphocytes in the body that can directly kill tumor cells[35]. Cryotherapy not only promotes tumor cell necrosis but also enhances the anti-tumor immune response. As a result, immunotherapy can play an auxiliary role in cytoreductive surgery. In 2013 Niu et al[36] retrospectively assessed the effect of immunotherapy with comprehensive cryosurgery (ablation of intrapancreatic and extrapancreatic tumors) in 106 patients with metastatic pancreatic cancer. The patients were divided into the following treatment groups: cryoimmunotherapy (31 patients); cryotherapy (36 patients); immunotherapy (17 patients); and chemotherapy (22 patients). The results showed that the median overall survival times in the cryoimmunotherapy and cryotherapy groups (13 and 7 mo, respectively) were longer than those in the chemotherapy group (3.5 mo; both P = 0.001). The median overall survival time in the cryoimmunotherapy group was also significantly higher compared to both the cryotherapy group (P = 0.05) and immunotherapy group (5 mo; P = 0.001). Longer median overall survival times could be achieved by both cryoimmunotherapy and cryotherapy when multiple cryoablation procedures were administered compared to a single application (cryoimmunotherapy group: 15 mo vs 10 mo, P = 0.0048; cryotherapy group: 9 mo vs 7 mo, P = 0.041, respectively; Figure 9). In both groups, patients with normal immunologic function achieved longer median overall survival times than those with immune dysfunction (cryoimmunotherapy group: 14 mo vs 7.5 mo, P = 0.0001; immunotherapy group: 6 mo vs 2 mo, P = 0.0004, respectively; Figure 10).
Figure 9.
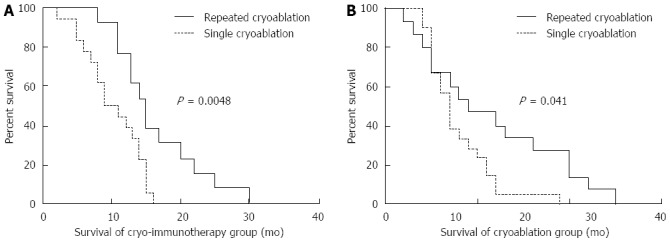
Correlation between overall survival and the number of cryoablation treatments in patients with pancreatic cancer. A: Comparisons of overall survival in the cryoimmunotherapy group between 13 patients who underwent repeated cryoablation and 18 patients who received a single cryoablation procedure; B: Comparisons of overall survival in the cryotherapy group between 15 patients who underwent repeated cryoablation and 21 patients who underwent a single cryoablation procedure. The analyses were performed by the Kaplan-Meier method with long-rank tests.
Figure 10.
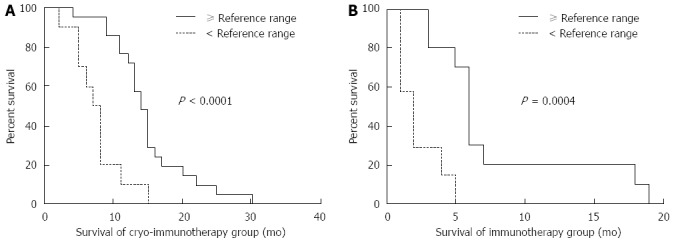
Correlation of overall survival with pretreatment immunologic indexes. A: Comparison between overall survival in 21 patients with immunologic indexes ≥ the reference range and 10 patients with immunologic indexes < the reference range in the cryoimmunotherapy group; B: Comparison between overall survival in 10 patients with immunologic indexes ≥ the reference range and 7 patients with immunologic indexes < the reference range in the immunotherapy group. The analyses were performed by the Kaplan-Meier method with long-rank tests.
Our findings have demonstrated that cryotherapy combined with immunotherapy can significantly improve the curative effect on metastatic pancreatic cancer compared to conventional chemotherapeutic techniques, suggesting that this approach may offer a novel therapy for improved treatment of patients with metastatic pancreatic cancer.
CONCLUSION
Patients with pancreatic cancer have a very poor prognosis, therefore new treatment modalities are urgently needed. Our experience in this field along with analyses of current treatment data has demonstrated that cryoablation is a feasible, potentially safe and promising option for the treatment of patients with locally advanced and unresectable pancreatic cancer. Percutaneous cryoablation, either alone or combined with 125-iodine seed implantation, was found to increase survival in patients with advanced or metastatic pancreatic cancer; further improvements in survival times could be achieved with repeated treatments or when cryotherapy was combined with immunotherapy. We also found that a plurality of probes or the use of chemical reagents, such as anhydrous alcohol injection, increased the area of freezing within the tumor; for patients with larger tumors, treatment combined with transcatheter arterial chemoembolisation was found to be effective. These observations need to be confirmed by further studies. For most patients with pancreatic cancer, cryosurgery can substitute conventional surgery. We now apply cryoablation as the standard approach in patients with unresectable pancreatic cancer. However, further research in a larger number of cases is necessary to support these encouraging results.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 20, 2015
First decision: June 23, 2015
Article in press: September 13, 2015
P- Reviewer: Moore W S- Editor: Yu J L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 5.Wilkowski R, Thoma M, Bruns C, Wagner A, Heinemann V. Chemoradiotherapy with gemcitabine and continuous 5-FU in patients with primary inoperable pancreatic cancer. JOP. 2006;7:349–360. [PubMed] [Google Scholar]
- 6.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for gallbladder cancer across the world. HPB (Oxford) 2008;10:327–331. doi: 10.1080/13651820802007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peddi PF, Lubner S, McWilliams R, Tan BR, Picus J, Sorscher SM, Suresh R, Lockhart AC, Wang J, Menias C, et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. JOP. 2012;13:497–501. doi: 10.6092/1590-8577/913. [DOI] [PubMed] [Google Scholar]
- 8.Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol. 2014;20:11142–11159. doi: 10.3748/wjg.v20.i32.11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 10.Gage AA, Baust JG. Cryosurgery - a review of recent advances and current issues. Cryo Letters. 2002;23:69–78. [PubMed] [Google Scholar]
- 11.Niu L, Chen J, Yao F, Zhou L, Zhang C, Wen W, Bi X, Hu Y, Piao X, Jiang F, et al. Percutaneous cryoablation for stage IV lung cancer: a retrospective analysis. Cryobiology. 2013;67:151–155. doi: 10.1016/j.cryobiol.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Xu KC, Niu LZ, He WB, Guo ZQ, Hu YZ, Zuo JS. Percutaneous cryoablation in combination with ethanol injection for unresectable hepatocellular carcinoma. World J Gastroenterol. 2003;9:2686–2689. doi: 10.3748/wjg.v9.i12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouraviev V, Polascik TJ. Update on cryotherapy for prostate cancer in 2006. Curr Opin Urol. 2006;16:152–156. doi: 10.1097/01.mou.0000193393.54598.9f. [DOI] [PubMed] [Google Scholar]
- 14.Berger A, Kamoi K, Gill IS, Aron M. Cryoablation for renal tumors: current status. Curr Opin Urol. 2009;19:138–142. doi: 10.1097/MOU.0b013e328323f618. [DOI] [PubMed] [Google Scholar]
- 15.Niu L, Mu F, Zhang C, Li Y, Liu W, Jiang F, Li L, Liu C, Zeng J, Yao F, et al. Cryotherapy protocols for metastatic breast cancer after failure of radical surgery. Cryobiology. 2013;67:17–22. doi: 10.1016/j.cryobiol.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Niu L, He L, Zhou L, Mu F, Wu B, Li H, Yang Z, Zuo J, Xu K. Percutaneous ultrasonography and computed tomography guided pancreatic cryoablation: feasibility and safety assessment. Cryobiology. 2012;65:301–307. doi: 10.1016/j.cryobiol.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Niu L, Wang Y, Yao F, Wei C, Chen Y, Zhang L, Chen J, Li J, Zuo J, Xu K. Alleviating visceral cancer pain in patients with pancreatic cancer using cryoablation and celiac plexus block. Cryobiology. 2013;66:105–111. doi: 10.1016/j.cryobiol.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Joosten JJ, Muijen GN, Wobbes T, Ruers TJ. In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: an experimental study. Cryobiology. 2001;42:49–58. doi: 10.1006/cryo.2001.2302. [DOI] [PubMed] [Google Scholar]
- 19.Mir LM, Rubinsky B. Treatment of cancer with cryochemotherapy. Br J Cancer. 2002;86:1658–1660. doi: 10.1038/sj.bjc.6600306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homasson JP, Pecking A, Roden S, Angebault M, Bonniot JP. Tumor fixation of bleomycin labeled with 57 cobalt before and after cryotherapy of bronchial carcinoma. Cryobiology. 1992;29:543–548. doi: 10.1016/0011-2240(92)90059-b. [DOI] [PubMed] [Google Scholar]
- 21.Kovach SJ, Hendrickson RJ, Cappadona CR, Schmidt CM, Groen K, Koniaris LG, Sitzmann JV. Cryoablation of unresectable pancreatic cancer. Surgery. 2002;131:463–464. doi: 10.1067/msy.2002.121231. [DOI] [PubMed] [Google Scholar]
- 22.Ben L, Ke D, Bo L. Analysis of Multiple Factors of Postsurgical Gastroparesis Syndrome after Pancreatico-duodenectomy and Cryotherapy for Pancreatic Cancer. Hua Xi Yixue. 2008;5:980–982. [Google Scholar]
- 23.Korpan NN. Basics of cryosurgery. Wein New York: Springer Verlag; 2001. pp. 151–157. [Google Scholar]
- 24.Xu KC, Niu LZ, Hu YZ, He WB, He YS, Li YF, Zuo JS. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol. 2008;14:1603–1611. doi: 10.3748/wjg.14.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu KC. Pancreatic cancer. In: Xu KC, Niu LZ, editors. Cryosurgery For Cancer. Shanghai: Shanghai Science-Technology-Education Pub; 2007. pp. 234–245. [Google Scholar]
- 26.Niu LZ, Li HB, Wen WF, HU Y, Wu BH. Feasibility and safety of percutaneous cryoablation for locally advanced pancreatic cancer. Zhonghua Yixianbing Zazhi. 2011;11:1–4. [Google Scholar]
- 27.Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59:229–243. doi: 10.1016/j.cryobiol.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peretz T, Nori D, Hilaris B, Manolatos S, Linares L, Harrison L, Anderson LL, Fuks Z, Brennan MF. Treatment of primary unresectable carcinoma of the pancreas with I-125 implantation. Int J Radiat Oncol Biol Phys. 1989;17:931–935. doi: 10.1016/0360-3016(89)90138-7. [DOI] [PubMed] [Google Scholar]
- 29.Takácsi-Nagy Z, Varga J, Poller I, Fodor J, Polgár C, Petrányi A, Major T, Németh G. Successful treatment of a T1 cancer of the pancreatic head with high dose rate brachytherapy and external radiotherapy. Hepatogastroenterology. 2002;49:844–846. [PubMed] [Google Scholar]
- 30.Kaye KW, Olson DJ, Payne JT. Detailed preliminary analysis of 125iodine implantation for localized prostate cancer using percutaneous approach. J Urol. 1995;153:1020–1025. [PubMed] [Google Scholar]
- 31.Holm HH, Juul N, Pedersen JF, Hansen H, Strøyer I. Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. 1983. J Urol. 2002;167:985–988; discussion 988-989. doi: 10.1016/s0022-5347(02)80320-2. [DOI] [PubMed] [Google Scholar]
- 32.Niu LZ, He LH, Zhou L, Yang ZZ, Zuo JS, Xu KC. [Percutaneous cryoablation and (125)I seed implantation combined with chemotherapy for advanced pancreatic cancer: report of 67 cases] Zhonghua Zhongliu Zazhi. 2012;34:940–944. doi: 10.3760/cma.j.issn.0253-3766.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Chen JB, Li JL, He LH, Liu WQ, Yao F, Zeng JY, Zhang Y, Xu KQ, Niu LZ, Zuo JS, et al. Radical treatment of stage IV pancreatic cancer by the combination of cryosurgery and iodine-125 seed implantation. World J Gastroenterol. 2012;18:7056–7062. doi: 10.3748/wjg.v18.i47.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu K, Niu L, Mu F, Hu Y. Cryosurgery in combination with brachytherapy of iodine-125 seeds for pancreatic cancer. Gland Surg. 2013;2:91–99. doi: 10.3978/j.issn.2227-684X.2013.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su X, Zhang L, Jin L, Ye J, Guan Z, Chen R. Coculturing dendritic cells with zoledronate acid efficiently enhance the anti-tumor effects of cytokine-induced killer cells. J Clin Immunol. 2010;30:766–774. doi: 10.1007/s10875-010-9434-1. [DOI] [PubMed] [Google Scholar]
- 36.Niu L, Chen J, He L, Liao M, Yuan Y, Zeng J, Li J, Zuo J, Xu K. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013;42:1143–1149. doi: 10.1097/MPA.0b013e3182965dde. [DOI] [PubMed] [Google Scholar]


