Abstract
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related death worldwide. Liver cancer is generally related to hepatitis B or C infection and cirrhosis. Usually, patients with HCC are asymptomatic and are diagnosed at late stages when surgical treatment is no longer suitable. Limited treatment options for patients with advanced HCC are a major concern. Therefore, there is an urge for finding novel therapies to treat HCC. Liver cancer is highly heterogeneous and involved deregulation of several signaling pathways. Wnt/β-catenin pathway is frequently upregulated in HCC and it is implicated in maintenance of tumor initiating cells, drug resistance, tumor progression, and metastasis. A great effort in developing selective drugs to target components of the β-catenin pathway with anticancer activity is underway but only a few of them have reached phase I clinical trials. We aim to review the role of β-catenin pathway on hepatocarcinogenesis and liver cancer stem cell maintenance. We also evaluated the use of small molecules targeting the Wnt/β-catenin pathway with potential application for treatment of HCC.
Keywords: Hepatocellular carcinoma, Wnt/β-catenin pathway, Liver cancer stem cells, Molecular therapy
Core tip: Several signaling pathways have been described to be deregulated in hepatocellular carcinoma (HCC). There are limited treatment options currently available in advanced liver cancer. Wnt/β-catenin pathway is frequently upregulated and has emerged as an alternative target in HCC. Our group has studied the role of β-catenin inhibition alone and in combination in HCC treatment. In this review we summarized the existing literature on the importance of Wnt/β-catenin pathway on hepatocarcinogenesis, tumor progression, relationship with liver stem cells and cancer therapeutics.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the third most common cause of cancer-related deaths worldwide[1]. Its prevalence differs greatly by geographical location reflecting variations in the main risk factors. Most cases of HCC (80%) arise in the Asian-Pacific and sub-Saharan African regions where the prevailing risk factor is chronic hepatitis B virus (HBV) infection. In Western countries, the incidence has been rapidly increasing due to infection with hepatitis C virus (HCV) and alcohol[2].
Early HCC is asymptomatic and a majority of the patients are diagnosed when the disease is advanced and they are no longer candidates for surgical curative therapy. Resection remains the treatment of choice for patients with well-preserved liver function; however, it is still associated with a high risk of post-operative complications and tumor recurrence. Among patients who have underlying cirrhosis, liver transplantation is considered the best therapeutic option in selected candidates but its use is limited by the shortage of potential donors. Other alternative treatments for HCC include radio-frequency ablation, microwave ablation, transcatheter arterial chemoembolization, radio embolization, and molecular targeted therapies/chemotherapy[2,3].
In past decades, there have been significant efforts from different groups to develop compounds to treat HCC. Sorafenib, a multikinase inhibitor, has been found to be active against HCC in several pre-clinical and clinical studies, slowing tumor progression and improving survival in patients with advanced HCC. It is the drug of choice in patients with advanced HCC that are unsuitable for other types of surgical, ablative and embolization interventions[4]. Tumor recurrence remains a strong limitation to any of the HCC treatments, hence understanding the molecular biology of HCC is crucial for the development of novel therapies.
Numerous signaling pathways have been found to be deregulated in HCC including the Ras/Raf/MAPK, PI3K/mTOR, Notch, HGF/c-MET, IGF, VEGF, PDGF, and Wnt/β-catenin pathway[4-6]. Wnt signaling is involved in several physiological and physio-pathological processes during embryonic development and carcinogenesis[7,8]. Wnt/β-catenin signaling plays a critical role in liver development, liver regeneration and liver zonation which is required for spatial separation of the diverse metabolic functions performed in the liver[7]. Aberrant activation of this signaling pathway has been found in several tumors from different origin. In HCC, β-catenin accumulation has been observed in about 10%-50% of tumors, and has been correlated with tumor progression and poor prognosis[9-11]. Based on these observations, interfering with the Wnt/β-catenin pathway might be a tempting target for liver cancer therapy. Several compounds have been developed to target this pathway and their potential in clinical trials is under study. Our group recently investigated the ability of FH-535, a dual inhibitor of β-catenin pathway and the peroxisome proliferator-activated receptor (PPAR), to inhibit HCC and liver cancer stem cells (LCSC) growth in vitro and its effectivity in a combination therapy with sorafenib[12,13].
The aim of this article is to review the literature on the role of Wnt/β-catenin signaling pathway as a potential molecular-targeted therapy in HCC.
WNT/β-CATENIN SIGNALING PATHWAY
The Wnt pathway diversifies into two main branches, canonical or β-catenin-dependent and non-canonical or β-catenin-independent. The canonical pathway involves three complexes: the ligand/receptor cell membrane complex, the cytosol β-catenin destruction complex, and the nuclear β-catenin/TCF/LEF transcription complex[14,15]. Wnt protein can bind to the heterodimeric receptor complex of a frizzled (Fz) and a single transmembrane lipoprotein receptor-related protein (LPR) 5 or 6 co-receptors. When Fz/LRP receptors are not engaged, β-catenin is clustered, phosphorylated and ubiquitin-labeled by the multiprotein “destruction complex” that includes the tumor suppressors axin and adenomatous polyposis coli (APC), the Ser/Thr kinases glycogen synthase kinase 3 (GSK-3) and casein kinase 1 (CK1), the protein phosphatase 2A, and the E3-ubiquitin ligase β-TrCP (Figure 1, right panel). Once phosphorylated, β-catenin is ubiquitinated and destroyed by the proteasome. Therefore, the destruction complex regulates the stability and availability of β-catenin, thus playing a key role in the modulation of the Wnt signaling cascade. Wnt/β-catenin signaling initiates by binding of the Wnt protein to the Fz/LPR receptor complex (Figure 1, left panel). As soon as Wnt binds Fz and LPR, the destruction complex is deactivated. As a consequence, non-phosphorylated active β-catenin accumulates in the cytoplasm and it translocates into the nucleus where it binds to the lymphoid enhancer factor/T-cell factor (LEF/TCF). This binding displaces the transcriptional inhibitor Groucho, and in a complex with TCF initiates transcription of target genes such as CD44, EpCAM, survivin, cyclin D1, glutamine synthetase, iNOS, and c-Myc, among others[7,8,16-19]. In normal cells, extracellular Wnt ligands can interact with a few secreted antagonists, including frizzled-related protein and Dickkopf family members, preventing the activation of this pathway.
Figure 1.
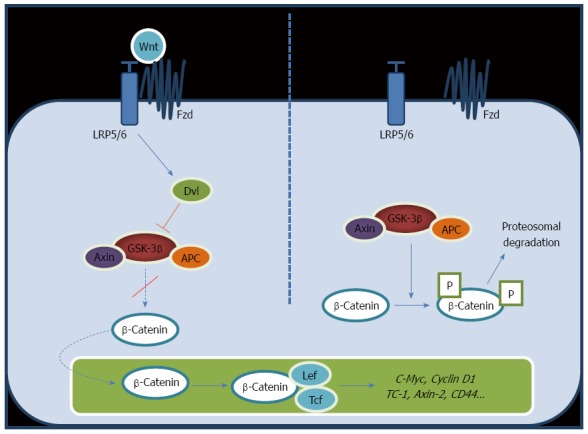
Wnt-β-catenin signaling pathway. Binding of Wnt protein initiates a cascade, which results in activation of β-catenin, its accumulation in the cytoplasm and translocation into the nucleus to enhance the transcription of target genes (Left); When Fz/LRP receptors are not engaged, CK1 and GSK3B sequentially phosphorylate Axin-bound β-catenin. Consequently, β-catenin is ubiquitinated and targeted for rapid destruction by the proteasome (Right).
The association of Wnt pathway and carcinogenesis was first described in patients with APC, where β-Catenin pathway is altered[20,21]. Interestingly, many additional associations between components of this pathway and disease, including hepatocarcinogenesis, have been revealed over the last two decades.
WNT/β-CATENIN ACTIVATION IN HEPATOCARCINOGENESIS
Aberrant activation of Wnt/β-Catenin signaling has been reported in a wide range of HCC patients. Nuclear accumulation of β-catenin is strongly associated with β-catenin mutations, a majority of which have been described to be missense mutations at exon 3, responsible for phosphorylation and destruction of β-catenin[22].
Mutations in β-catenin gene 1 (CTNNB1) have been reported in around 20%-40% of all HCC cases[6,23-26]. Cieply et al[27] compared the tumor features of HCC in the presence or absence of CTNNB1 gene mutations. Their results indicated a strong association between the presence of CTNNB1 mutations and increased tumor size, macrovascular and microvascular invasion, which supports the contribution of aberrant β-catenin signaling to tumor aggressiveness. The abnormal activity of β-catenin has been also linked with HCC in the setting of HCV and HBV infections. High incidence of CTNNB1 mutations in up to 40% of cases has been reported in patients with HCC and HCV infection. Previous studies have also correlated the presence of HCV core protein with increased expression of Wnt-1 in hepatoma cells[7].
Lachenmayer et al[11] proposed a molecular classification of HCC based on Wnt-pathway aberrations in HCC into two groups: CTNNB1 molecular class and Wnt-TGFβ molecular class. The authors found that Wnt-TGFβ class was associated with cytoplasmic β-catenin accumulation, vascular invasion, and an increased risk of early recurrence after surgical resection. A strong association between cytoplasmic β-catenin expression and poor histological differentiation, tumor size > 5 cm in diameter, and decreased disease-free survival has previously been reported[28]. Inagawa et al[29] demonstrated poor prognosis in HCC patients with nuclear β-catenin accumulation in high grade HCC tumors. Nuclear β-catenin accumulation in HCC has also been associated to Ki67, suggesting that β-catenin promotes tumor proliferation and progression[30].
The link between Wnt/β-catenin signaling pathway and cell cycle seems to play a crucial role during the genesis and development of HCC. Wang et al[31] reported that suppressing β-catenin gene may induce the changes in cyclin B1 and cyclin C protein expression. Liu et al[32] showed that human zinc finger protein 191 is a potential regulator of the β-catenin transcription, found to be significantly overexpressed in human HCC specimens and associated with growth of human HCC cells. Mutations have also been reported in the components of the degradation complex of β-catenin including AXIN1 in around 3%-16% and AXIN2 in around 3% of all HCC cases[33].
Three other Wnt genes (Wnt3, Wnt4 and Wnt5A), and three FZD genes (FZD3, FZD6 and FZD7) have been identified to be up-regulated in 60%-90% of human HCCs and more than 50% of the surrounding pre-neoplastic liver tissues, suggesting that their overexpression may be an early event in hepatocarcinogenesis[34].
Wnt/β-catenin signaling pathway has also been found to contribute to the regulation of HCC angiogenesis, infiltration, and metastasis through regulation of the expression of angiogenic factors such as MMP-2, MMP-9, VEGF-A, VEGF-C, and BFGF[35].
MOLECULAR MECHANISMS OF HCC
LCSC and β-catenin signaling
Hepatocarcinogenesis is a complex process that so far has not been completely elucidated. The Cancer Stem Cells (CSCs) hypothesis postulates that tumors are comprised of a heterogeneous population of cells, which includes a minor group of cellls characterized by their capacity of continuous self-renewal and differentiation, long-lasting survival, and transplantability. It has been suggested that presence of LCSCs within the tumors could explain HCC heterogeneity, metastasis, recurrence, and chemotherapeutic resistance[36-39].
Different cell markers, including EpCAM, CD133, CD90, CD44, CD24, and CD13, have been used to identify the LCSC subpopulation. Such markers are heterogeneous in expression and represent cells of different origins. EpCAM, CD44, and CD133 are the most frequently used markers for the enrichment of tumor-initiating cells from primary human cancer[38-40]. Yamashita et al[41] published that isolated HCC EpCAM+ cells from alpha-fetoprotein positive HCC cases are LCSCs, in which activation of Wnt/β-catenin signaling is a major feature. Other reports suggest that pharmacologic targeting of β-catenin could inhibit proliferation and invasiveness capacity of LCSCs producing downregulation of the expression of markers (CD44 and CD133) in vitro and in vivo[42].
Our group and others have studied LCSCs and differences in resistance patterns with HCC cell lines (Huh7, Hep3B, PLC) in vitro and in vivo by targeting the Wnt/β-catenin pathway[43-45]. Understanding the precise molecular basis of altered Wnt/β-catenin pathway should bring important advances in HCC biology with significant therapeutics associations.
MicroRNAs regulation of β-catenin pathway in HCC
Emerging evidence suggests the role of microRNAs (miRNAs) in the regulation of key biological properties of LCSCs[46]. During hepatocarcinogenesis miRNAs have been shown to have both tumor suppressive and oncogenic activity. Selected miRNAs such as miR-21, miR-224, miR-34a, miR-221/222, miR-106a, and miR-203, are upregulated in HCC[47].
Analyses of miRNA expression have shown the different level of expression of miR-122a, miR-125a, and miR-150 in HCC cells and normal human primary hepatocytes[48,49]. Augello et al[50] studied the expression of 664 mature miRNA in a cohort of 60 HCV-positive liver lesions and determined the genomic status of the miRNA chromosome 19 miRNA cluster (C19MC). Four miRNAs overexpressed in HCC belonged to C19MC and were significantly associated with microvascular invasion. Ji et al[51] found that the reduced expression of miR-26 correlated with the development of more aggressive forms of HCC. Interestingly, the same authors found that the expression of miR-26 is an independent predictor of survival in HCC patients. miRNA-181 has been shown to regulate the Wnt/β-catenin signaling pathway in a positive feedback loop, promoting the stem cell-like features of HCC cells[52]. Overall, the exploration of the molecular link between Wnt/β-catenin and miRNAs could increase the understanding of the intricate molecular regulation in LCSCs, facilitating the design of effective therapeutic strategies.
Targeting β-catenin pathway: Is there a role for combination therapy in HCC?
Sorafenib monotherapy is currently the standard of care for patients with advanced HCC[2,3]. However, in a double-blind, placebo-controlled trial, the median overall survival of patients on sorafenib group is prolonged by only 2.8 mo[3]. Therefore, there is a particular interest in the development of new and more effective strategies to treat HCC. Evidence from in vitro and in vivo studies indicates that combination therapy could be more effective. Our group has demonstrated additive and synergistic effect of targeting Ras/Raf/MAPK pathway in combination with other pathways important in HCC proliferation such as PI3K/AKT/mTOR and Wnt/β-catenin[53].
Based on recent studies indicating the important role of Wnt/β-catenin signaling in the maintenance of CSCs, there has been increasing interest in developing new compounds to inhibit this pathway (Tables 1 and 2). One of the first studies aimed at disrupting the interaction between β-catenin and TCF. About 7000 natural compounds were screened using an enzyme-linked immunosorbent assay[54]. Two fungal-derived compounds, PKF115-854 and CGP04909, showed the best results for their potency and specificity in antagonize the β-catenin-mediated cellular effects[54]. These compounds have been further tested as anticancer drugs for malignancies displaying frequent upregulation in the Wnt/β-catenin signaling such as human multiple myeloma[55], Acute Myelocytic Leukemia[56], Chronic Lymphocytic Leukemia[57] and HCC[58,59]. Two studies have assayed the therapeutic potential of CGP049090, PKF115-584 and PKF118-310 in HCC. All three compounds showed dose-dependent cytotoxicity against HCC cells with relative lower cytotoxicity to normal hepatocytes. In a HepG2 xenograft model, these compounds inhibited tumor growth associated with apoptosis and reduced levels of TCF4/β-catenin target genes[58]. Yamashita et al[59] demonstrated that the epithelial cell adhesion molecule (EpCAM, a hepatic stem cell marker) is a direct transcriptional target of the Wnt/β-catenin pathway in HCC. EpCAM-expressing HCC were more sensitive to the β-catenin/TCF4 antagonists, CGP049090, PKF115-584 and PKF118-310, than EpCAM-negative HCC.
Table 1.
Molecular therapies targeting Wnt/β-catenin pathway in hepatocellular carcinoma
| Compound | Target in vitro | Target in vivo |
| Sorafenib[4] | Decrease of TCF/LEF, β-catenin protein levels and Wnt-target genes mRNA levels[11] | Decrease tumor volume and increase survival of treated animals in HepG2 xenografts in nude mice[11] |
| sFZD7[66] | Block interaction between. FZD and Dvl. Decrease viability of HepG2, Hep40 and Huh7 cell lines. Reduced expression of c-Myc, Cyclin D1 and Survivin. The effect was potentiated in combination with Doxorubicin[66] | Inhibitory effect in Huh7 xenografts[66] |
| RHPDs[67] | Decrease viability of human HCC cell lines (Huh7 and HepG2) through degradation of β-catenin and activation of PKCδ in a TP53-independent manner[67] | Intratumor injection in SV40-TAg transgenic mouse model inhibited HCC progression[67] |
| BrMC[42] | Inhibition of CD133+ LCSCs proliferation, EMT and invasion in MHCC97 cell line, and decreased expression of beta-catenin in this LCSCs[42] | Inhibition of LCSCs proliferation in Balb/c-nu mice xenografts model[42] |
| SL1122-37[71] (Sorafenib derivative) | Inhibitory effect on the proliferation of HCC PLC/PRF/5 cells and the formation of angiogenesis of HUVECs[71] | |
| PMED-1[18] | Blocks β-catenin and CBP interaction. Suppression of down-stream effects in β-catenin signaling in HCC cell lines[18] | Decrease of Wnt signaling in transgenic zebrafish[18] |
| XAV939[61] | Inhibit Tankyrase 1 and 2 inducing degradation of β-catenin by stabilization of Axin. Antitumor activity against neuroblastoma[72], colon[73], breast[74] and lung[75] cancers, and HCC Decreased nuclear β-catenin levels, cell proliferation and colony formation in HepG2 and Huh7[76]. | Inhibited growth of HepG2 xenograft model of HCC[76]. Reduce tumor growth in a conditional APC mutant mouse model of colon cancer[73]. Repressed lung cancer formation in murine xenograft and transgenic syngeneic lung cancer models[75] |
| CGP049090 | Block TCF/LEF and β-catenin interaction. Decrease expression of c-myc, Cyclin D1 and Survivin in AML[56], CCL[57], MM[55] and HCC[58,59] cells. Induced apoptosis and cell cycle arrest at the G1/S phase. | Inhibitory effect in murine xenograft model of human MM[55], HepG2 xenograft model of HCC[58], JVM-3 subcutaneous xenograft model of CCL[57] |
| PKF115-854 | ||
| PKF118-310[54] | ||
| FH535[69] | Inhibition of the activation of β-catenin-regulated genes in the HCC cell lines Huh7, Hep3B and PLC and LCSC. Arrests the cell cycle from G1 to S-phase[69] | |
| FH535 and Sorafenib combination[12] | Synergistic inhibition of LCSC and Huh7 cell lines proliferation. Dose dependent inhibition of Cyclin D1, Survivin and Bcl2 expression[12] |
LEF/TCF: Lymphoid enhancer factor/T-cell factor; HCC: Hepatocellular carcinoma; LCSCs: Liver cancer stem cells.
Table 2.
Percentage inhibition of [3H]-thymidine incorporation in Huh-7 cells by FH535 analogs
| FH535 analog | C-2 | C-3 | C-4 | C-5 | C-6 | C-2' | C-3' | C-4' | C-5' | C-6' | [3H]-Thymidine incorporation ratio in Huh-7 cells at 10 μmol/L relative to DMSO | % Inhibition at 10 μmol/L | Ratio of % inhibition of analog to % inhibition by FH535 at 10 μmol/L | TOPFlash Assay (10 μmol/L) | TOPFlash Assay as a Percentage Decrease Relative to Control |
| Control | 100 ± 10 | 31.2 ± 4.5 | |||||||||||||
| 1a | Cl | H | H | Cl | H | CH3 | H | NO2 | H | H | 64 ± 3.7 | 36 | 1.0 | 8.5 ± 2.1 | 73 |
| 1b | Cl | H | H | Cl | H | CH3 | H | CO2CH3 | H | H | 82 ± 4.2 | 18 | 0.5 | ||
| 1c | Cl | H | H | Cl | H | CO2CH3 | H | F | H | H | 93 ± 5.6 | 7 | 0.2 | 12.1 ± 0.9 | 61 |
| 1d | Cl | H | H | Cl | H | CO2CH3 | H | Cl | H | H | 106 ± 14 | 0 | 0.0 | ||
| 1e | Cl | H | H | Cl | H | CH3 | H | CH2OH | H | H | 33 ± 4.8 | 67 | 1.9 | ||
| 1f | Cl | H | H | Cl | H | 1-(4-NO2)C10H6 | 52 ± 6.4 | 48 | 1.3 | ||||||
| 1g | Cl | H | H | H | Cl | CH3 | H | CO2CH3 | H | H | 71 ± 1 | 29 | 0.8 | ||
| 1h | Cl | H | H | H | Cl | CH3 | H | CH2OH | H | H | 27 ± 5.1 | 73 | 2.0 | 27.3 ± 3.5 | 13 |
| 1i | F | H | H | H | F | CH3 | H | CH2OH | H | H | 62 ± 1.5 | 38 | 1.1 | ||
| 1j | H | H | H | H | H | CH3 | H | CH2OH | H | H | 47 ± 2.3 | 53 | 1.5 | ||
| 1k | F | H | H | H | F | OC6H5 | H | H | H | H | 66 ± 3.2 | 34 | 0.9 | ||
| 1l | F | H | H | H | F | H | OCH2C6H5 | H | H | H | 57 ± 21 | 43 | 1.2 | ||
| 1m | Cl | H | H | H | Cl | H | H | COC6H5 | H | H | 67 ± 12 | 33 | 0.9 | ||
| 1n | F | H | H | F | H | 1-(4-NO2)C10H6 | 69 ± 3.51 | 31 | 0.9 | 11.2 ± 1.0 | 64 | ||||
| 1o | Cl | H | H | H | Cl | OC6H5 | H | H | H | H | 62 ± 10 | 38 | 1.1 | 17.2 ± 0.9 | 45 |
| 1p | Cl | H | H | H | Cl | H | OCH2C6H5 | H | H | H | 69 ± 6.9 | 31 | 0.9 | 14.8 ± 0.7 | 53 |
| 1q | F | H | H | H | F | H | H | COC6H5 | H | H | 67 ± 5.9 | 33 | 0.9 | ||
| 1r | Cl | H | H | H | Cl | 1-(4-NO2)C10H6 | 74 ± 27 | 26 | 0.7 | 16.6 ± 1.1 | 47 | ||||
| 1s | H | H | H | H | H | H | OCH2C6H5 | H | H | H | 71 ± 12 | 29 | 0.8 | ||
| 1t | H | H | H | H | H | H | H | COC6H5 | H | H | 62 ± 2.3 | 38 | 1.1 | 16.8 ± 0.9 | 46 |
| 1u | H | H | H | H | H | 1-(4-NO2)C10H6 | 58 ± 6.9 | 42 | 1.2 | 8.7 ± 0.1 | 72 | ||||
From Kril et al[70] with permission of Bioorg Med Chem Lett.
Thorne et al[60] identified the FDA-approved drug Pyrvinium as an inhibitor of the Wnt signaling by enhancing the activity of the destruction complex though the binding to CK1 and selectively potentiating CK1α kinase activity. Whereas protein kinases have proven to be effective drug targets in cancer, the specific role of inhibitors of CK1 have yet to be completely elucidated.
Other low molecular mass compounds such as XAV939 have been identified to prolong the half-life of Axin and promote β-Catenin degradation through inhibiting tankyrase[61-64].
Other strategy for attenuating the Wnt/β-catenin signaling pathway is by blocking the interaction between Fz receptors with their ligands. The effective interference of Fz and Wnt interaction has been achieved with monoclonal antibodies or with recombinant soluble fragment of Fz (sFz). OMP-18R5, is a novel monoclonal antibody that interacts with five Fz receptors to block canonical Wnt signaling. It has been used to inhibit the growth of several types of human cancers and has shown to be synergistic with other drugs such as taxol, irinotecan and gemcitabine[65].
Wei et al[66] found that a recombinant sFz7 peptide inhibited Wnt/β-Catenin signaling and decreased proliferation and tumorigenesis in some HCC cell lines. Nambotin et al[67] used small interfering peptides in HCC to block Fz7 function Wnt signaling and tumor progression.
Chrysin, a naturally distributed flavonoid, has been described to inhibit proliferation and induce apoptosis in a variety of cancer cells[68]. Quan et al[42] demonstrated that a synthetic analogue of Chrysin, 8-bromo-7-methoxychrysin, inhibited the stem cell-like properties of CD133+ cells derived from MHCC97 cell line in vivo and in vitro. They proposed that the mechanism may relate to a reduction levels of Akt and activation of GSK-3β[64].
Interestingly, some small molecules that inhibit the interaction between β-Catenin and CBP could induce cancer stem cells to differentiate, thereby eventually clearing the pool of cancer initiating cells.
Delgado et al[18] identified a small molecule inhibitor of β-catenin PMED-1 that blocks β-catenin signaling and β-catenin-CBP interactions in multiple cells lines (Hep3B, HepG2, Snu-398 and Snu-449), reducing their viability and proliferation on a transient mode. However, a major drawback of this study was the lack of in vivo studies in xenograft models due to the hydrophobic nature and relatively short half-life of PMED-1[65].
Sorafenib has also been investigated as a potential Wnt modulator in experimental models. Lachenmayer et al[11] found that Sorafenib reduced Wnt signaling and β-catenin expression in different cell lines in vitro and, in a xenograft model, xenograft nude mice treated with Sorafenib showed reduced HepG2 tumor growth. The small molecular agent FH535 has been shown to inhibit proliferation of HCC and hepatoblastoma cell lines[69]. Liu et al[19] also reported that this antiproliferative effect was associated with the reduced expression of β-catenin iNOS.
Our group studied the effects of combined therapy targeting Ras/Raf/MAPK and Wnt/β-catenin over HCC and LCSCs proliferation. We found that FH535 in combination with sorafenib caused synergistic inhibition of proliferation in both tumor cell types (Figure 2). FH535 demonstrated a dose-dependent inhibition of Cyclin D1, Survivin, Bcl2 and c-Myc expression[12]. It was found that co-transfected wild-type β-catenin expression vector increased nearly 15-fold the luciferase activity from TOPFlash reporter assay compared to cells co-transfected with the empty vector control[13]. We have also identified the FH535-mediated inhibition of β-Catenin-dependent gene expression in HCC cells. Our group has compared the activities induced by of XAV939 and FH535 on LCSCs and non-LCSC lines. Our results suggest that β-catenin/TCF/LEF inhibition site of FH535 may directly target the transcription promoter site. Further studies will determine the effects associated with FH535 ability to inhibit PPAR and to address the toxicity and efficacy for the treatment of HCC. Due to favorable results obtained using this compound alone and in combination with Sorafenib, our laboratory has recently developed an array of FH535 derivatives, some of which display a potent and selective β-catenin inhibitory activity in HCC cells[70].
Figure 2.
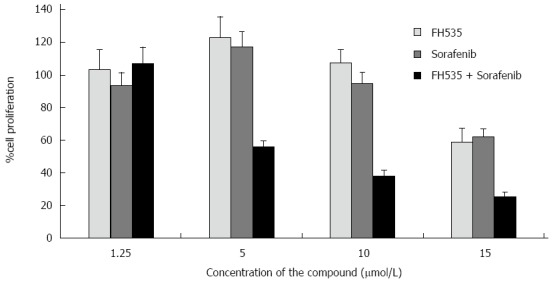
Synergistic inhibition of proliferation of liver cancer stem cells targeting β-catenin and Ras/Raf/MAPK pathways. FH535 and Sorafenib combination on inhibition of [3H]-Thymidine incorporation in liver cancer stem cells (CD133+, CD44+ and CD24+) Calculated combination index (CI) of less than 1. From Galuppo et al[12] with permission of Anticancer Research.
To date, there are two ongoing clinical phase I/II trials using compounds such as PRI-724 and OMP-18R5 targeting β-catenin signaling pathway for the treatment of solid tumors and myeloid malignancies, suggesting the potential use of β-catenin inhibition in the treatment of HCC.
CONCLUSION
The molecular mechanisms of early liver transformation in carcinogenesis are poorly elucidated. Hepatocarcinogenesis is a complex multistep process where tumors originate from either LCSC or mature hepatocytes, and the tissue undergoes chronic inflammation, apoptosis, unrestricted proliferation, and permanent liver remodeling. An ideal drug regimen would eliminate specifically different cancer cells, including those with stem cell properties. Combined therapy may be necessary to overcome the complex network of signaling pathways and ultimately inhibit the signaling events that control tumor growth and survival.
So far it remains unclear the specific Wnt-targets and downstream signals that contribute to Wnt signaling diversity on liver cancer. Evidence suggests that this pathway represents an important molecular target for HCC therapy as its mutation and activation are intrinsically involved with tumor initiation and development in at least one-third of HCC.
The increasing knowledge on adult stem cell biology and the general acceptance of the LCSCs hypothesis promise to bring revolutionary advances to HCC therapy. In this context, no effective compounds targeting β-catenin are yet approved for clinical use, but further investigation should be granted to identify selective inhibitors of this pathway and better define their efficacy and toxicity with the ultimate goal to be used either alone or in combination to treat patient with advanced HCC.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interest for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 31, 2015
First decision: November 13, 2015
Article in press: December 21, 2015
P- Reviewer: Cerwenka HR, Tomizawa M S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M. Current status of molecularly targeted therapy for hepatocellular carcinoma: clinical practice. Int J Clin Oncol. 2010;15:242–255. doi: 10.1007/s10147-010-0089-y. [DOI] [PubMed] [Google Scholar]
- 6.Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006;25:3778–3786. doi: 10.1038/sj.onc.1209547. [DOI] [PubMed] [Google Scholar]
- 7.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901–1915. doi: 10.2741/1933. [DOI] [PubMed] [Google Scholar]
- 10.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 11.Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, Hoshida Y, Villanueva A, Minguez B, Newell P, Tsai HW, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18:4997–5007. doi: 10.1158/1078-0432.CCR-11-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galuppo R, Maynard E, Shah M, Daily MF, Chen C, Spear BT, Gedaly R. Synergistic inhibition of HCC and liver cancer stem cell proliferation by targeting RAS/RAF/MAPK and WNT/β-catenin pathways. Anticancer Res. 2014;34:1709–1713. [PMC free article] [PubMed] [Google Scholar]
- 13.Gedaly R, Galuppo R, Daily MF, Shah M, Maynard E, Chen C, Zhang X, Esser KA, Cohen DA, Evers BM, et al. Targeting the Wnt/β-catenin signaling pathway in liver cancer stem cells and hepatocellular carcinoma cell lines with FH535. PLoS One. 2014;9:e99272. doi: 10.1371/journal.pone.0099272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 15.Cavard C, Colnot S, Audard V, Benhamouche S, Finzi L, Torre C, Grimber G, Godard C, Terris B, Perret C. Wnt/beta-catenin pathway in hepatocellular carcinoma pathogenesis and liver physiology. Future Oncol. 2008;4:647–660. doi: 10.2217/14796694.4.5.647. [DOI] [PubMed] [Google Scholar]
- 16.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oishi N, Yamashita T, Kaneko S. Molecular biology of liver cancer stem cells. Liver Cancer. 2014;3:71–84. doi: 10.1159/000343863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delgado ER, Yang J, So J, Leimgruber S, Kahn M, Ishitani T, Shin D, Mustata Wilson G, Monga SP. Identification and characterization of a novel small-molecule inhibitor of β-catenin signaling. Am J Pathol. 2014;184:2111–2122. doi: 10.1016/j.ajpath.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Li G, Liu D, Liu J. FH535 inhibits the proliferation of HepG2 cells via downregulation of the Wnt/β-catenin signaling pathway. Mol Med Rep. 2014;9:1289–1292. doi: 10.3892/mmr.2014.1928. [DOI] [PubMed] [Google Scholar]
- 20.Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 21.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 22.Harada N, Oshima H, Katoh M, Tamai Y, Oshima M, Taketo MM. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 23.Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787–3800. doi: 10.1038/sj.onc.1209556. [DOI] [PubMed] [Google Scholar]
- 24.Salahshor S, Woodgett JR. The links between axin and carcinogenesis. J Clin Pathol. 2005;58:225–236. doi: 10.1136/jcp.2003.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 26.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 27.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49:821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–145. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 29.Inagawa S, Itabashi M, Adachi S, Kawamoto T, Hori M, Shimazaki J, Yoshimi F, Fukao K. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res. 2002;8:450–456. [PubMed] [Google Scholar]
- 30.Nhieu JT, Renard CA, Wei Y, Cherqui D, Zafrani ES, Buendia MA. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol. 1999;155:703–710. doi: 10.1016/s0002-9440(10)65168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Xunsun JY, Yang D, Yang LL, Kong DX, Meng XW. The effect of cell cycle and expression of cyclin B1 and cyclin C protein in hepatocellular carcinoma cell line HepG2 and SMMC-7721 after of silencing β-catenin gene. Hepatogastroenterology. 2012;59:515–518. doi: 10.5754/hge11534. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Jiang S, Wang C, Jiang W, Liu Z, Liu C, Saiyin H, Yang X, Shen S, Jiang D, et al. Zinc finger transcription factor 191, directly binding to β-catenin promoter, promotes cell proliferation of hepatocellular carcinoma. Hepatology. 2012;55:1830–1839. doi: 10.1002/hep.25564. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, Nagorney DM, Burgart LJ, Roche PC, Smith DI, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002;21:4863–4871. doi: 10.1038/sj.onc.1205591. [DOI] [PubMed] [Google Scholar]
- 34.Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu B, Liu BR, DU YJ, Chen J, Cheng YQ, Xu W, Wang XH. Wnt/β-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol Lett. 2014;7:1175–1178. doi: 10.3892/ol.2014.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji J, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin Oncol. 2012;39:461–472. doi: 10.1053/j.seminoncol.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 38.Song W, Li H, Tao K, Li R, Song Z, Zhao Q, Zhang F, Dou K. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract. 2008;62:1212–1218. doi: 10.1111/j.1742-1241.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Hou Y, Zou Q, Ge R, Shen F, Wang Y. The critical role of CD133(+)CD44(+/high) tumor cells in hematogenous metastasis of liver cancers. Cell Res. 2012;22:259–272. doi: 10.1038/cr.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quan MF, Xiao LH, Liu ZH, Guo H, Ren KQ, Liu F, Cao JG, Deng XY. 8-bromo-7-methoxychrysin inhibits properties of liver cancer stem cells via downregulation of β-catenin. World J Gastroenterol. 2013;19:7680–7695. doi: 10.3748/wjg.v19.i43.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 44.Gedaly R, Galuppo R, Musgrave Y, Angulo P, Hundley J, Shah M, Daily MF, Chen C, Cohen DA, Spear BT, et al. PKI-587 and sorafenib alone and in combination on inhibition of liver cancer stem cell proliferation. J Surg Res. 2013;185:225–230. doi: 10.1016/j.jss.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newell P, Toffanin S, Villanueva A, Chiang DY, Minguez B, Cabellos L, Savic R, Hoshida Y, Lim KH, Melgar-Lesmes P, et al. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J Hepatol. 2009;51:725–733. doi: 10.1016/j.jhep.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 47.Chen XM. MicroRNA signatures in liver diseases. World J Gastroenterol. 2009;15:1665–1672. doi: 10.3748/wjg.15.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Masi A, Viganotti M, Antoccia A, Magrelli A, Salvatore M, Azzalin G, Tosto F, Lorenzetti S, Maranghi F, Mantovani A, et al. Characterization of HuH6, Hep3B, HepG2 and HLE liver cancer cell lines by WNT/β - catenin pathway, microRNA expression and protein expression profile. Cell Mol Biol (Noisy-le-grand) 2010;56 Suppl:OL1299–OL1317. [PubMed] [Google Scholar]
- 49.Xu J, Zhu X, Wu L, Yang R, Yang Z, Wang Q, Wu F. MicroRNA-122 suppresses cell proliferation and induces cell apoptosis in hepatocellular carcinoma by directly targeting Wnt/β-catenin pathway. Liver Int. 2012;32:752–760. doi: 10.1111/j.1478-3231.2011.02750.x. [DOI] [PubMed] [Google Scholar]
- 50.Augello C, Vaira V, Caruso L, Destro A, Maggioni M, Park YN, Montorsi M, Santambrogio R, Roncalli M, Bosari S. MicroRNA profiling of hepatocarcinogenesis identifies C19MC cluster as a novel prognostic biomarker in hepatocellular carcinoma. Liver Int. 2012;32:772–782. doi: 10.1111/j.1478-3231.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 51.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji J, Yamashita T, Wang XW. Wnt/beta-catenin signaling activates microRNA-181 expression in hepatocellular carcinoma. Cell Biosci. 2011;1:4. doi: 10.1186/2045-3701-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A, Evers BM. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:4951–4958. [PMC free article] [PubMed] [Google Scholar]
- 54.Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 55.Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD, Carrasco DE, Zheng M, He H, Tai YT, et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci USA. 2007;104:7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minke KS, Staib P, Puetter A, Gehrke I, Gandhirajan RK, Schlösser A, Schmitt EK, Hallek M, Kreuzer KA. Small molecule inhibitors of WNT signaling effectively induce apoptosis in acute myeloid leukemia cells. Eur J Haematol. 2009;82:165–175. doi: 10.1111/j.1600-0609.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 57.Gandhirajan RK, Staib PA, Minke K, Gehrke I, Plickert G, Schlösser A, Schmitt EK, Hallek M, Kreuzer KA. Small molecule inhibitors of Wnt/beta-catenin/lef-1 signaling induces apoptosis in chronic lymphocytic leukemia cells in vitro and in vivo. Neoplasia. 2010;12:326–335. doi: 10.1593/neo.91972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei W, Chua MS, Grepper S, So S. Small molecule antagonists of Tcf4/beta-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int J Cancer. 2010;126:2426–2436. doi: 10.1002/ijc.24810. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67:10831–10839. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- 60.Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 62.Behrens J, Jerchow BA, Würtele M, Grimm J, Asbrand C, Wirtz R, Kühl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 63.Kishida M, Koyama S, Kishida S, Matsubara K, Nakashima S, Higano K, Takada R, Takada S, Kikuchi A. Axin prevents Wnt-3a-induced accumulation of beta-catenin. Oncogene. 1999;18:979–985. doi: 10.1038/sj.onc.1202388. [DOI] [PubMed] [Google Scholar]
- 64.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 65.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei W, Chua MS, Grepper S, So SK. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer. 2011;10:16. doi: 10.1186/1476-4598-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nambotin SB, Lefrancois L, Sainsily X, Berthillon P, Kim M, Wands JR, Chevallier M, Jalinot P, Scoazec JY, Trepo C, et al. Pharmacological inhibition of Frizzled-7 displays anti-tumor properties in hepatocellular carcinoma. J Hepatol. 2011;54:288–299. doi: 10.1016/j.jhep.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 68.Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci. 2010;11:2188–2199. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Handeli S, Simon JA. A small-molecule inhibitor of Tcf/beta-catenin signaling down-regulates PPARgamma and PPARdelta activities. Mol Cancer Ther. 2008;7:521–529. doi: 10.1158/1535-7163.MCT-07-2063. [DOI] [PubMed] [Google Scholar]
- 70.Kril LM, Vilchez V, Jiang J, Turcios L, Chen C, Sviripa VM, Zhang W, Liu C, Spear B, Watt DS, et al. N-Aryl benzenesulfonamide inhibitors of [3H]-thymidine incorporation and β-catenin signaling in human hepatocyte-derived Huh-7 carcinoma cells. Bioorg Med Chem Lett. 2015;25:3897–3899. doi: 10.1016/j.bmcl.2015.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin Y, Lu Y, Wang R, Li W, Qu X. SL1122-37, a novel derivative of sorafenib, has greater effects than sorafenib on the inhibition of human hepatocellular carcinoma (HCC) growth and prevention of angiogenesis. Biosci Trends. 2013;7:237–244. [PubMed] [Google Scholar]
- 72.Tian XH, Hou WJ, Fang Y, Fan J, Tong H, Bai SL, Chen Q, Xu H, Li Y. XAV939, a tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma cell lines by inhibiting Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res. 2013;32:100. doi: 10.1186/1756-9966-32-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova O, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 2012;72:2822–2832. doi: 10.1158/0008-5472.CAN-11-3336. [DOI] [PubMed] [Google Scholar]
- 74.Bao R, Christova T, Song S, Angers S, Yan X, Attisano L. Inhibition of tankyrases induces Axin stabilization and blocks Wnt signalling in breast cancer cells. PLoS One. 2012;7:e48670. doi: 10.1371/journal.pone.0048670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Busch AM, Johnson KC, Stan RV, Sanglikar A, Ahmed Y, Dmitrovsky E, Freemantle SJ. Evidence for tankyrases as antineoplastic targets in lung cancer. BMC Cancer. 2013;13:211. doi: 10.1186/1471-2407-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma L, Wang X, Jia T, Wei W, Chua MS, So S. Tankyrase inhibitors attenuate WNT/β-catenin signaling and inhibit growth of hepatocellular carcinoma cells. Oncotarget. 2015;6:25390–25401. doi: 10.18632/oncotarget.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]


