Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common life-threatening hereditary disease in the USA resulting in chronic kidney disease and the need for dialysis and transplantation. Approximately 85% of cases of ADPKD are caused by a mutation in the Pkd1 gene that encodes polycystin-1, a large membrane receptor. The Pkd1 gene mutation results in abnormal proliferation in tubular epithelial cells, which plays a crucial role in cyst development and/or growth in PKD. Activation of the proliferative mammalian target of rapamycin (mTOR) signaling pathway has been demonstrated in polycystic kidneys from rodents and humans. mTOR inhibition with sirolimus or everolimus decreases cysts in most animal models of PKD including Pkd1 and Pkd2 gene deficient orthologous models of human disease. On the basis of animal studies, human studies were undertaken. Two large randomized clinical trials published in the New England Journal of Medicine of everolimus or sirolimus in ADPKD patients were very unimpressive and associated with a high side-effect profile. Possible reasons for the unimpressive nature of the human studies include their short duration, the high drop-out rate, suboptimal dosing, lack of randomization of “fast” and “slow progressors” and the lack of correlation between kidney size and kidney function in ADPKD. The future of mTOR inhibition in ADPKD is discussed.
Keywords: Everolimus, Mammalian target of rapamycin, Polycystic kidney, Polycystic kidney disease, Sirolimus
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common life-threatening hereditary disease in the USA. It affects about 1:400 to 1:1000 people. ADPKD occurs in all racial and ethnic groups. Most patients with ADPKD develop hypertension. Massive cystic disease may also lead to chronic pain and cyst infections. In clinical practice, detection of multiple renal cysts on ultrasound or CT scan is used to make the diagnosis of PKD. Precise determination of kidney and cyst volume on MRI scanning is used for clinical studies. Approximately 50% of people with ADPKD develop chronic kidney disease around age 50 years. ADPKD accounts for about 5% to 10% of end-stage renal failure in the USA, requiring dialysis and renal transplantation [1]. There is no effective treatment for ADPKD.
Approximately 85% of cases of ADPKD are caused by a mutation in the Pkd1 gene that encodes polycystin-1 (PC-1), a large membrane receptor, and approximately 15% caused by a mutation in the Pkd2 gene that encodes polycystin-2 (PC-2) a calcium channel that binds to PC-1. PC-1 and PC-2 have been localized to the cilia where they function as a mechanosensor that mediates flow-dependent calcium entry [2].
It has been determined that inhibitors of cyclic adenosine monophosphate (cAMP), cyclin-dependent protein kinase, tumor necrosis factor-α, sarcoma (Src) a proto-oncogene tyrosine-protein kinase, the renin-angiotensin-aldosterone system, and 3-hydroxy-3-methyl-glutaryl-CoA reductase reduce cyst formation and improve renal function in rat and mouse models of PKD, which are thus potential therapeutic targets in PKD. Current developments in the field of PKD research are very exciting. The results of studies in rat and mouse models of PKD have been translated to the bedside. Tolvaptan (a vasopressin V2-receptor antagonist) that inhibits cAMP, somatostatin, bosutinib (a Src/Abl tyrosine kinase inhibitor), angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and statins that reduce cyst formation and improve renal function in animal models of PKD are being tested in interventional studies in humans [3], [4]. It is likely that current or future interventional studies in patients with ADPKD will result in the discovery of an agent that can slow the growth of the polycystic kidneys and delay the onset of renal failure.
The mammalian target of rapamycin (mTOR) signaling pathway is the focus of the current review. Human and experimental data provide strong evidence that abnormal proliferation in tubular epithelial cells plays a crucial role in cyst development and/or growth in PKD [5]. Genetic manipulations that induce the proliferation of tubular epithelial cells in mice cause cysts to form in the kidney [6], [7]. The mTOR signaling pathway regulates cell growth and proliferation that are dysregulated in ADPKD. Sirolimus, an mTOR inhibitor, is an FDA-approved immunosuppressive drug and is a powerful antiproliferative [8]. In view of the importance of tubular cell proliferation in cyst formation and the antiproliferative effects of sirolimus, the hypothesis was developed that sirolimus would reduce cyst formation and disease progression in ADPKD via inhibition of tubular cell proliferation. In addition to inhibition of proliferation, mTOR inhibitors may also have a therapeutic effect in PKD by affecting vascular remodeling, angiogenesis, and fibrogenesis [9]. To understand the mechanism of action of mTOR inhibitors better, the mTOR signaling pathway will first be discussed in detail.
mTOR signaling pathway and PKD kidney
mTOR exists in association with two different complexes: mTORC1 and mTORC2. mTORC1 consists of mTOR and regulatory associated protein of mTOR (Raptor), while mTORC2 consists of mTOR and rapamycin-independent companion of mTOR (Rictor).
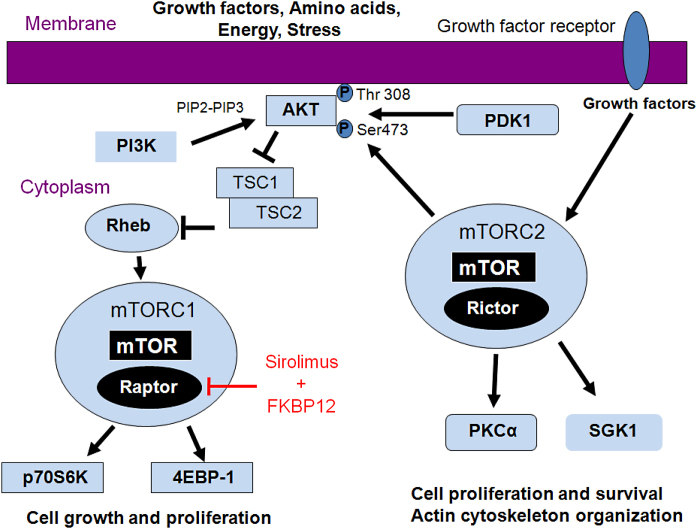
The mTORC1 pathway involves the following major players: insulin-like growth factor-I (IGF-1), serine/threonine kinase Akt (also known as protein kinase B), tuberous sclerosis complex 1 and 2 (TSC1/2), mTOR, and the serine/threonine kinase p70 S6 ribosomal protein kinase (p70S6K) [10], [11], [12] (Fig. 1). IGF-I is a major regulator of the mTOR pathway via signaling to PI3K/Akt/mTOR. Phosphoinositide-3-kinase (PI3K) converts the lipid phosphatidylinositol (4,5)-bisphosphate (PIP2) into phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which localizes Akt to the membrane. The TSC1 (hamartin) and TSC2 (tuberin) complex is inactivated by Akt-dependent phosphorylation. Inactivation of TSC2 results in activation of mTOR via the Ras-related small GTPase (Rheb). mTORC1 is a complex that is made up of mTOR and Raptor. mTOR phosphorylates both p70S6K and eukaryotic initiation factor 4E-binding protein (4E-BP1) via independent pathways. Increased p70S6K and 4E-BP1 act independently to promote cell proliferation (cell growth and cell cycle progression). The mode of action of sirolimus is to bind the cytosolic protein FK-binding protein 12 (FKBP12) in a manner similar to tacrolimus. While the tacrolimus-FKBP12 complex inhibits calcineurin, the sirolimus-FKBP12 complex inhibits the mTORC1 pathway. The binding of mTORC1 inhibitors to FKBP destabilizes the association between mTORC1 and raptor, preventing the downstream phosphorylation of p70S6Kinase [13].
Figure 1.
mTOR signaling. mTOR exists in association with two different complexes, mTORC1 and mTORC2. mTORC1 consists of mTOR and regulatory associated protein of mTOR (Raptor), while mTORC2 consists of mTOR and rapamycin-independent companion of mTOR (Rictor). In the mTORC1 pathway, PI3K converts PIP2 into PIP3, which localizes Akt to the membrane. The TSC1 (hamartin) and TSC2 (tuberin) complex is inactivated by Akt-dependent phosphorylation. Inactivation of TSC2 results in activation of mTOR via the GTPase, Rheb. mTOR phosphorylates both p70 S6 kinase (p70S6K) and 4E-BP1 via independent pathways that promote cell proliferation. The mode of action of sirolimus is to bind the cytosolic protein FK-binding protein 12 (FKBP12) to destabilize the association between mTORC1 and raptor, preventing the downstream phosphorylation of p70S6K. In the mTORC2 pathway, there is downstream signaling to the AGC kinases Akt, PKCα, and SGK1. Phosphorylation of Akt at Serine 473 by mTORC2 primes Akt for further phosphorylation at Threonine 308.
mTORC2 consists of mTOR and Rictor [14], [15], [16] (Fig. 1). Upstream signaling of mTORC2 is not yet well described. mTORC2 activity may be stimulated directly or indirectly by growth factors [17]. In IGF-1 stimulated cells, mTORC2 signaling is increased [18], [17], [19]. The best characterized downstream substrates of mTORC2 are the AGC kinases Akt, protein kinase C-alpha (PKCα) and serum glucocorticoid-induced protein kinase 1 (SGK1). Phosphorylation of Akt at Serine473 by mTORC2 primes Akt for further phosphorylation at Threonine308 in the catalytic domain. Loss of Rictor results in complete loss of Akt Serine473 phosphorylation. Knockout of mTORC2 does not affect mTORC1 suggesting that mTORC2 activates a pool of Akt that is not upstream of mTORC1 [15]. Akt regulates cell survival, proliferation, and growth. PKCα, another mTORC2 substrate, is ubiquitously expressed in many tissues and plays a role in actin cytoskeleton organization, cell proliferation, and tumor progression [20]. SGK1 is a recently discovered mTORC2 substrate [16]. The phosphorylation of SGK1 on Serine422 and SGK1 activity are inhibited in fibroblasts from mice lacking mTORC2 but still possessing mTORC1 activity. SGK1 regulates cancer cell proliferation.
PC-1 and PC-2 have been localized to the cilia, but the connection between the cilia and mTOR signaling was not previously known. A recent study demonstrates that primary cilia regulate mTOC1 activity and cell size via the tumor suppressor protein liver kinase B1 [21]. In this study it was demonstrated that bending of the cilia by flow is required for mTOR downregulation and cell size control. It has recently been demonstrated that abnormalities of the cilia mediate increased mTOR activity in PKD. In 3-week-old cilia knockout mice there was a large increase in mTOR activity [22].
An important question is whether there is mTOR activation in human PKD kidneys. Expression of mTOR pathway molecules was determined in paraffin-embedded liver and kidney samples from autosomal recessive polycystic kidney disease (ARPKD) patients and controls [23]. mTOR was strongly expressed in renal cyst-lining cells and bile ducts from ARPKD specimens. S6K immunostaining was strong in smaller tubules and weak both in larger renal cysts and in the bile duct epithelium. In controls, mTOR and S6K were expressed in distal tubule segments. 4E-BP1-immunostaining was restricted to noncystic tubules in ARPKD. Tuberin/TSC2 immunostaining was negative in all specimens. In another study, human kidney specimens from ARPKD and normal control children were evaluated for Akt, mTOR, and S6K [24]. Phosphorylated Akt as well as activated mTOR and its downstream effector S6K were strongly expressed in cystic epithelia of all kidney specimens but not in control tissues. These studies suggest that the activation of mTOR signaling in human PKD kidneys and provide a rationale for human studies of mTOR inhibitors.
Animal studies of mTOR inhibition in PKD (Table 1)
Table 1.
mTOR inhibition in animal models of PKD
| Drug dose | Model | Duration of treatment | Outcome | Blood levels (ng/mL) | Side effects | Ref |
|---|---|---|---|---|---|---|
| Sirolimus 0.2 mg/kg/d IP | Male Han:SPRD | Age 4–8 wk | Decreased kidney enlargement and cyst volume. Improved kidney function. | Not reported | Reduced body weight by 22% | [25] |
| Sirolimus 2 mg/kg/d orally | Male Han:SPRD | 3 mo | Decreased kidney enlargement and cyst volume. Improved kidney function. | 0.5–1.9 | No change in body weight | [26] |
| Everolimus 3 mg/kg/d orally | Male Han:SPRD | 5 wk | Decreased kidney enlargement and cyst volume. Improved kidney function. | 5–7 | Impaired weight gain | [27] |
| Sirolimus 5 mg/kg/d IP | orpk rescue mouse | Age 150–178 d | Decrease in cyst volume. | Not reported | [28] | |
| Sirolimus 5 or 1.67 mg/kg/d IP | bpk mouse | Age 7–21 d | Decrease in cyst volume. Normalization of kidney function. | Not reported | [28] | |
| Sirolimus 0.2 mg/kg/d IP | Male Han:SPRD | Age 1–12 mo | Normalized kidney volume, renal function, blood pressure and heart weight. | 6.6–6.9. | Reduced body weight by 11% | [29] |
| Sirolimus 0.2 mg/kg/d IP | Female Han:SPRD rat | Age 4–12 wk | No effect on kidney and cyst volume. | 5.9 | Reduced body weight by 15% | [30] |
| Sirolimus 5 mg/kg/d IP. | Pkd1 knockout | Age 28–49 d | Reduced cyst growth. Preserved renal function. | Not reported | [31] | |
| Sirolimus 0.5 mg/kg/d IP | Pkd2 knockout | Age 4–16 wk | Reduced kidney size and cyst volume. No effect on kidney function. | 22 | No change in body weight | [32] |
| Everolimus 3 mg/kg orally | Male Han:SPRD | 4–9 wk treatment (pulse). 4–16 wk treatment (continuous). | Both regimens reduced cyst volume and improved kidney function. | 4.7–6.2 | Impaired weight gain | [33] |
| Sirolimus 2 mg/kg orally | pck rat | 4, 8, or 12 wk | No effect on liver and kidney cysts | 0.6 | Reduced weight gain | [34] |
| Low dose (10 mg/kg) vs. High dose (100 mg/kg) orally | Pkd1 knockout mice | Early vs. late treatment | Low dose did not affect renal cysts. Early treatment was better than late treatment. | 3 vs. 30–60 | Not reported | [35] |
Rapamycin (sirolimus) is a macrolide that was first discovered as a product of the bacterium Streptomyces hygroscopicus in a soil sample from Easter Island, an island also known as Rapa Nui, hence the trade name rapamycin. Sirolimus was originally developed as an antifungal agent. However, this was abandoned when it was discovered that it had potent immunosuppressive and antiproliferative properties. Sirolimus is now FDA-approved for the prevention of organ transplantation rejection. Everolimus and temsirolimus are FDA-approved for the treatment of renal cell cancer.
In 2005, it was demonstrated that sirolimus decreases kidney and cyst enlargement and prevents the loss of kidney function in the male Han:SPRD rat model of ADPKD [25]. Two subsequent studies in 2006 and 2007 in male Han:SPRD rats demonstrated that sirolimus or everolimus decreased kidney and cyst enlargement and ameliorated the loss of kidney function [26], [27]. In 2006 the first studies in mice were reported. Sirolimus decreased cyst volume in the Oak Ridge polycystic kidney (orpk) ciliary defect model of ARPKD and bpk mouse model of ARPKD [28]. As ADPKD patients would probably require life-long therapy with mTOR inhibitors, male Han:SPRD rats were treated with sirolimus up to age 1 year [29]. Chronic sirolimus therapy normalized kidney volume, renal function, blood pressure and heart weight and reduced cyst density by 72% [29]. Next, sirolimus was tested in female Han:SPRD rats that have a milder form of PKD [30]. Rapamcyin treatment for 9 weeks had no effect on kidney size and cyst volume density in female Cy/+ rats with PKD [30]. The lack of effect of sirolimus in females was despite the same dose, similar blood levels and a similar degree of sirolimus-induced weight loss as reported in male rat studies. In the female Han:SPRD rats, sirolimus resulted in an increase in the pro-proliferative p-Akt Serine 473 [30]. The first studies of sirolimus in Pkd1 or Pkd2 knockout mouse models were reported in 2010. Sirolimus 5 mg/kg/day reduced cyst growth and preserved renal function in mice with PKD resulting from a conditional inactivation of Pkd1 [31]. Sirolimus 0.5 mg/kg/day reduced cyst growth, but had no effect on renal function in Pkd2WS25/- mice an orthologous model of ADPKD caused by a mutation in the Pkd 2 gene [32]. Next, pulse versus continuous everolimus treatment was compared in the male Han:SPRD rat [33]. Both pulse and continuous treatment reduced cyst volume and improved kidney function. This study suggested that pulse mTOR inhibition may be as good as continuous treatment but with a lower side effect profile [33]. In the autosomal recessive polycystic kidney (pck) rat model, sirolimus had no significant effect on of renal and liver cysts [34]. However, in this study, blood levels were very low (0.6 ng/mL) [34]. In summary of the animal studies, mTOR inhibition decreases cysts in most animal models including Pkd1 and Pkd2 gene deficient orthologous models of human disease.
There are other agents besides sirolimus and everolimus that may inhibit mTOR in PKD. Curcumin, principal curcuminoid of the popular Indian spice turmeric, inhibits cystogenesis by simultaneous interference of multiple signaling pathways including mTOR [35]. Metformin, a widely-used drug, stimulates AMP-activated protein kinase, resulting in inhibition of the cystic fibrosis transmembrane conductance receptor and mTOR and slowing of renal cystogenesis [36]. Slowing of progression of PKD by 2-hydroxyestradiol in male Han:SPRD rats is associated with downregulation of p21 and mTOR expression [37]. mTOR is hyperactivated in Pkd1 null mice due to failure of the hepatocyte growth factor (HGF) receptor c-Met to be properly ubiquinated and subsequently degraded after stimulation by HGF [38]. A c-Met pharmacological inhibitor resulted in inhibition of mTOR activity and reduced cystogenesis [38]. Thus there are other drugs besides the sirolimus-derivatives that may inhibit mTOR and inhibit multiple pathways in PKD with fewer side effects.
Clinical studies of mTOR inhibition in PKD (Table 2)
Table 2.
mTOR inhibition in human ADPKD
| Drug dose levels (ng/ml) | Study design | Duration oftreatment | No of patients | Outcome | Adverse events | Ref |
|---|---|---|---|---|---|---|
| Sirolimus | Retrospective, Kidney transplant patients Sirolimus vs. no sirolimus | 24–40 mo | 7 | Reduction in kidney volume | Not reported | [28] |
| Sirolimus (mean levels: 14.3) | Retrospective, Kidney transplant patients Sirolimus vs. Tacrolimus | 19 mo | 16 | Reduction in liver cyst volume, trend towards reduction in kidney cyst volume | Increased LDL | [39] |
| Sirolimus 3 mg/d (trough levels: 10–15, SIRENA study) | Retrospective crossover study, Mean baseline eGFR 76 mL/min | 1 y | 21 | TKV increased less and cyst volume stable on sirolimus | Increased total and LDL cholesterol and triglycerides. aphthous ulcers, acne, edema | [40] |
| Everolimus 5 mg/d (trough levels: 3–8) | Randomized double blind trial, Mean baseline eGFR 53–56 mL/min | 2 y | 433 | Slowed increase in TKV at 1 y Did not slow progression of eGFR | Anemia, leukopenia, thrombocytopenia, stomatitis, hyperlipidemia, acne, angioedema | [41] |
| Sirolimus 2 mg/d (mean levels: 4.1–4.9) | Randomized open label trial, Mean baseline eGFR 91–92 mL/min | 18 mo | 100 | No change in TKV or eGFR | Mucositis, diarrhea | [42] |
TKV, total kidney volume.
As a result of the positive studies in rodent models of PKD, human studies were undertaken. Three initial small studies in humans suggested that sirolimus could reduce cystic disease [28], [39], [40]. Thus larger randomized control studies were undertaken and the results have recently been published [41], [42]. In a randomized double blind trial, 433 ADPKD patients with a mean baseline estimated glomerular filtration rate (eGFR) of 53 to 56 mL/min/1.73 m2 were randomized to receive everolimus at 5 mg/day or placebo for 2 years [41]. The primary end point was total kidney volume (TKV) on MRI scan. The increase in TKV between baseline and 1 year was lower in the everolimus group versus the placebo (P=0.02). The increase in TKV between baseline and 2 years was lower in the everolimus group versus the placebo, but did not reach statistical significance (P=0.06). The increase in cyst volume was not statistically different between the groups. The increase in parenchymal volume was less in the everolimus group. The mean decrement in eGFR was more in the everolimus group but did not reach statistical significance different between the groups. Drug specific adverse events were higher in the everolimus group. The dropout rate was 33% in the everolimus-treated group. The results of the study are summarized in Table 3.
Table 3.
Everolimus in patients with ADPKD [41]
| Everolimus | Placebo | P | |
|---|---|---|---|
| Increase in TKV—Year 1 (mL) | 102 | 157 | 0.02 |
| Increase in TKV—Year 2 (mL) | 230 | 301 | 0.06 |
| Baseline eGFR (chronic kidney disease Stage 3) | 53 | 56 | |
| Decrease in eGFR (mL/min/1.73m2)-year 2 | 8.7 | 7.7 | 0.15 |
| Any serious adverse event (%) e.g anemia, leukopenia, thrombocytopenia, stomatitis, hyperlipidemia, acne, angioedema. | 80 | 50 | 0.002 |
The second study was an 18-month open-label, randomized, controlled trial in which 100 patients with a mean baseline eGFR of 91 to 92 mL/min/1.73 m2, received either sirolimus (target dose 2 mg/day) or placebo. The median increase in TKV was not different between the groups. The GFR did not differ between the groups. Sirolimus treated patients had a higher incidence of gastrointestinal side effects. A suboptimal dose of sirolimus was used as the achieved dose was 25% lower than the intended dose of 2 mg/d. The mean serum sirolimus level was 4.1 to 4.9 ng/mL. The results of the study are summarized in Table 4.
Table 4.
Sirolimus in patients with ADPKD [42]
| Sirolimus | Placebo | P | |
|---|---|---|---|
| Increase in TKV 1 (mL) | 99 | 97 | Not significant |
| Baseline eGFR (No chronic kidney disease) | 92 | 91 | |
| Decrease in eGFR (mL/min/1.73 m2) | 0.2 | 3.5 | 0.07 |
| Any serious adverse event (%): | 50 | 50 | |
| Gastrointestinal side effects e.g. mucositis, diarrhea. | 94 | 52 |
Why were the human studies on mTOR inhibition in ADPKD unimpressive?
The human studies suggest that the use of mTOR inhibitors in ADPKD patients may be more complicated than was expected [43]. There are multiple reasons for the unimpressive effect of mTOR inhibitors in the human studies:
-
(1)
The relationship between kidney and cyst volume and the decline in GFR is complex. Kidney size does not correlate with eGFR decline. PKD kidneys are massive before there is a significant decline in GFR. Thus GFR may not change even if TKV changes significantly. A 2-year period may be too short to detect changes in eGFR. Also sirolimus may have effects on glomerular hemodynamics that may influence GFR.
-
(2)
In the everolimus study there was a 33% drop-out rate [41]. The high dropout rate may have decreased the power of the trial to detect significant differences between the groups [44].
-
(3)
In the sirolimus study, the dose of sirolimus was suboptimal with blood levels of 4.1 to 4.9 ng/mL [42].
-
(4)
Most rats and mouse models develop early and severe disease while PKD is slower and more chronic in humans. The slow progression of ADPKD in humans may make it difficult to find differences in a short period of time.
-
(5)
It is possible that the dose of an mTOR inhibitor required to inhibit mTOR in tubular cells may be higher than the dose required to inhibit mTOR in blood mononuclear cells [43]. In this regard, biopsy of a transplanted kidney from a transplant patient on sirolimus failed to demonstrate mTOR inhibition in tubular cells in the kidney [45]. However, the side effect profile of everolimus and sirolimus in PKD patients may limit the dose that patients can tolerate.
-
(6)
In the everolimus study, the mean baseline eGFR was 53 to 56 mL/min/1.73 m2. In these patients, the disease may have been too advanced for therapy to yield a functional benefit [43]. Processes such as chronic interstitial fibrosis, that may be independent of mTOR, may cause the GFR decline in the later stages of ADPKD [43].
-
(7)
The rate of progression of total kidney volume before treatment was not known, so that there may not have been a balanced randomization between slow and fast progressors [46].
-
(8)
Increased phosphorylation of pAkt Serine 473, a marker of mTORC2 signaling has been demonstrated in PKD in rodents [30], [31]. The rapalogues, sirolimus, and everolimus, bind to FKBP12, which directly binds and inhibits mTORC1, not mTORC2. Sirolimus does not directly target mTORC2-dependent Akt-induced proliferation [47]. Target of rapamycin kinase inhibitors (TORKs) selectively bind to the ATP-binding site in the mTOR catalytic domain and thereby block both mTORC1 and 2. It is possible that more complete suppression of the mTOR signaling network by the new TORKs will give a better antiproliferative response than sirolimus in PKD. The potential clinical use of TORKs is demonstrated by five Phase I/II clinical studies of the TORK AZD8055 (AstraZeneca) in patients with various advanced solid tumors (see clinicaltrials.gov).
The future of mTOR inhibition in PKD
mTOR signaling and mTOR inhibition in animals and humans requires further study. In animal models of PKD, mTOR kinase inhibitors or TORKs that inhibit both mTORC1 and 2 need to be tested. In humans, it is possible that high dose pulse treatment, starting in adolescence or in combination with other therapies that reduce cyclic AMP levels or reduce fluid secretion, may be needed in the future. mTOR inhibitors with a better safety profile need to be developed.
Conflict of interest
None.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants DK074835 and DK07483503S1 to Charles L. Edelstein.
References
- 1.Gabow P.A. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 2.Nauli S.M., Alenghat F.J., Luo Y., Williams E., Vassilev P., Li X., Elia A.E., Lu W., Brown E.M., Quinn S.J., Ingber D.E., Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 3.Belibi F., Edelstein C.L. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs. 2010;19:315–328. doi: 10.1517/13543781003588491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang M., Ong A.C. Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clin Pract. 2012;120:c25–c35. doi: 10.1159/000334166. [DOI] [PubMed] [Google Scholar]
- 5.Wilson P.D. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 6.Trudel M., D'Agati V., Costantini F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 1991;39:665–671. doi: 10.1038/ki.1991.80. [DOI] [PubMed] [Google Scholar]
- 7.Schaffner D.L., Barrios R., Massey C., Bañez E.I., Ou C.N., Rajagopalan S., Aguilar-Cordova E., Lebovitz R.M., Overbeek P.A., Lieberman M.W. Targeting of the rasT24 oncogene to the proximal convoluted tubules in transgenic mice results in hyperplasia and polycystic kidneys. Am J Pathol. 1993;142:1051–1060. [PMC free article] [PubMed] [Google Scholar]
- 8.Fingar D.C., Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 9.Torres V.E., Boletta A., Chapman A., Gattone V., Pei Y., Qian Q., Wallace D.P., Weimbs T., Wüthrich R.P. Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin J Am Soc Nephrol. 2010;5:1312–1329. doi: 10.2215/CJN.01360210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao X., Zhang Y., Arrazola P., Hino O., Kobayashi T., Yeung R.S., Ru B., Pan D. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 11.Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 12.Burnett P.E., Barrow R.K., Cohen N.A., Snyder S.H., Sabatini D.M. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oshiro N., Yoshino K., Hidayat S., Tokunaga C., Hara K., Eguchi S., Avruch J., Yonezawa K. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells. 2004;9:359–366. doi: 10.1111/j.1356-9597.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang J., Manning B.D. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Foster K.G., Fingar D.C. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285:14071–14077. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 18.Sparks C.A., Guertin D.A. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–3744. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoang B., Frost P., Shi Y., Belanger E., Benavides A., Pezeshkpour G., Cappia S., Guglielmelli T., Gera J., Lichtenstein A. Targeting TORC2 in multiple myeloma with a new mTOR kinase inhibitor. Blood. 2010;116:4560–4568. doi: 10.1182/blood-2010-05-285726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martiny-Baron G., Fabbro D. Classical PKC isoforms in cancer. Pharmacol Res. 2007;55:477–486. doi: 10.1016/j.phrs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Boehlke C., Kotsis F., Patel V., Braeg S., Voelker H., Bredt S., Beyer T., Janusch H., Hamann C., Gödel M., Müller K., Herbst M., Hornung M., Doerken M., Köttgen M., Nitschke R., Igarashi P., Walz G., Kuehn E.W. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol. 2010;12:1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell P.D., Fitzgibbon W., Sas K., Stenbit A.E., Amria M., Houston A., Reichert R., Gilley S., Siegal G.P., Bissler J., Bilgen M., Chou P.C., Guay-Woodford L., Yoder B., Haycraft C.J., Siroky B. Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol. 2011;22:839–848. doi: 10.1681/ASN.2010050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker J.U., Saez A.O., Zerres K., Witzke O., Hoyer P.F., Schmid K.W., Kribben A., Bergmann C., Nurnberger J. The mTOR pathway is activated in human autosomal-recessive polycystic kidney disease. Kidney Blood Press Res. 2010;33:129–138. doi: 10.1159/000314380. [DOI] [PubMed] [Google Scholar]
- 24.Fischer D.C., Jacoby U., Pape L., Ward C.J., Kuwertz-Broeking E., Renken C., Nizze H., Querfeld U., Rudolph B., Mueller-Wiefel D.E., Bergmann C., Haffner D. Activation of the AKT/mTOR pathway in autosomal recessive polycystic kidney disease (ARPKD) Nephrol Dial Transplant. 2009;24:1819–1827. doi: 10.1093/ndt/gfn744. [DOI] [PubMed] [Google Scholar]
- 25.Tao Y., Kim J., Schrier R.W., Edelstein C.L. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease (PKD) J Am Soc Nephrol. 2005;16:46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 26.Wahl P.R., Serra A.L., Le Hir M., Molle K.D., Hall M.N., Wüthrich R.P. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 27.Wu M., Wahl P.R., Le Hir M., Wackerle-Men Y., Wuthrich R.P., Serra A.L. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney Blood Press Res. 2007;30:253–259. doi: 10.1159/000104818. [DOI] [PubMed] [Google Scholar]
- 28.Shillingford J.M., Murcia N.S., Larson C.H., Low S.H., Hedgepeth R., Brown N., Flask C.A., Novick A.C., Goldfarb D.A., Kramer-Zucker A., Walz G., Piontek K.B., Germino G.G., Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zafar I., Belibi F.A., He Z., Edelstein C.L. Long-term rapamycin therapy in the Han:SPRD rat model of polycystic kidney disease (PKD) Nephrol Dial Transplant. 2009;24:2349–2353. doi: 10.1093/ndt/gfp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belibi F., Ravichandran K., Zafar I., He Z., Edelstein C.L. mTORC1/2 and rapamycin in female Han:SPRD rats with polycystic kidney disease. Am J Physiol Renal Physiol. 2011;300:F236–F244. doi: 10.1152/ajprenal.00129.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shillingford J.M., Piontek K.B., Germino G.G., Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol. 2010;21:489–497. doi: 10.1681/ASN.2009040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zafar I., Ravichandran K., Belibi F., Doctor R.B., Edelstein C.L. Sirolimus attenuates disease progression in an orthologous mouse model of human autosomal dominant polycystic kidney disease. Kidney Int. 2010;78:754–761. doi: 10.1038/ki.2010.250. [DOI] [PubMed] [Google Scholar]
- 33.Wu M., Arcaro A., Varga Z., Vogetseder A., Le Hir M., Wuthrich R.P., Serra A.L. Pulse mTOR inhibitor treatment effectively controls cyst growth but leads to severe parenchymal and glomerular hypertrophy in rat polycystic kidney disease. Am J Physiol Renal Physiol. 2012;297:F1597–F1605. doi: 10.1152/ajprenal.00430.2009. [DOI] [PubMed] [Google Scholar]
- 34.Renken C., Fischer D.C., Kundt G., Gretz N., Haffner D. Inhibition of mTOR with sirolimus does not attenuate progression of liver and kidney disease in PCK rats. Nephrol Dial Transplant. 2011;26:92–100. doi: 10.1093/ndt/gfq384. [DOI] [PubMed] [Google Scholar]
- 35.Novalic Z., van der Wal A.M., Leonhard W.N., Keohl G., Breuning M.H., Geissler E.K., de Heer E., Peters D.J. Dose-dependent effects of sirolimus on mTOR signaling and polycystic kidney disease. J Am Soc Nephrol. 2012;23:842–853. doi: 10.1681/ASN.2011040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takiar V., Nishio S., Seo-Mayer P., King J.D., Jr., Li H., Zhang L., Karihaloo A., Hallows K.R., Somlo S., Caplan M.J. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA. 2011;106:2462–2467. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson S., Oyama T.T., Lindsley J.N., Schutzer W.E., Beard D.R., Gattone V.H., Komers R. 2-Hydroxyestradiol slows progression of experimental polycystic kidney disease. Am J Physiol Renal Physiol. 2012;302:F636–F645. doi: 10.1152/ajprenal.00265.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin S., Taglienti M., Nauli S.M., Contrino L., Takakura A., Zhou J., Kreidberg J.A. Failure to ubiquitinate c-Met leads to hyperactivation of mTOR signaling in a mouse model of autosomal dominant polycystic kidney disease. J Clin Invest. 2010;120:3617–3628. doi: 10.1172/JCI41531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian Q., Hui D., King B.F., Kumar S., Dean P.G., Cosio F.G., Torres V.E. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol. 2008;19:631–638. doi: 10.1681/ASN.2007050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perico N., Antiga L., Caroli A., Ruggenenti P., Fasolini G., Cafaro M., Ondei P., Rubis N., Diadei O., Gherardi G., Prandini S., Panozo A., Bravo R.F., Carminati S., De Leon F.R., Gaspari F., Cortinovis M., Motterlini N., Ene-Iordache B., Remuzzi A., Remuzzi G. Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol. 2010;21:1031–1040. doi: 10.1681/ASN.2009121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walz G., Budde K., Mannaa M., Nurnberger J., Wanner C., Sommerer C., Kunzendorf U., Banas B., Hörl W.H., Obermüller N., Arns W., Pavenstädt H., Gaedeke J., Büchert M., May C., Gschaidmeier H., Kramer S., Eckardt K.U. Everolimus in patients with autosomal dominant polycystic kidney disease. New Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 42.Serra A.L., Poster D., Kistler A.D., Krauer F., Raina S., Young J., Rentsch K.M., Spanaus K.S., Senn O., Kristanto P., Scheffel H., Weishaupt D., Wüthrich R.P. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. New Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 43.Watnick T., Germino G.G. mTOR inhibitors in polycystic kidney disease. New Engl J Med. 2010;363:879–881. doi: 10.1056/NEJMe1006925. [DOI] [PubMed] [Google Scholar]
- 44.Perico N., Remuzzi G. Do mTOR inhibitors still have a future in ADPKD? Nar Rev Nephrol. 2010;6:696–698. doi: 10.1038/nrneph.2010.153. [DOI] [PubMed] [Google Scholar]
- 45.Canaud G., Knebelmann B., Harris P.C., Vrtovsnik F., Correas J.M. Pallet N, Heyer CM, Letavernier E, Bienaimé F, Thervet E, Martinez F, Terzi F, Legendre C: Therapeutic mTOR inhibition in autosomal dominant polycystic kidney disease: What is the appropriate serum level? Am J Transplant. 2010;10:1701–1706. doi: 10.1111/j.1600-6143.2010.03152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponticelli C., Locatelli F. Autosomal dominant polycystic kidney disease and mTOR inhibitors:the narrow road between hope and disappointment. Nephrol Dial Transplant. 2010;25:3809–3812. doi: 10.1093/ndt/gfq527. [DOI] [PubMed] [Google Scholar]
- 47.Shor B., Gibbons J.J., Abraham R.T., Yu K. Targeting mTOR globally in cancer; thinking beyond rapamycin. Cell Cycle. 2009;8:3831–3837. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]