Abstract
Background
Currently, the dosage of tacrolimus used after transplantation is based on the patient's body weight. However, there is a low correlation between body weight and body composition in kidney transplant recipients. In this study, we evaluate the pharmacokinetics of tacrolimus according to body composition in 18 Korean kidney transplant recipients with stable graft function.
Methods
Body composition parameters were calculated using bioelectrical impedance analysis. Pharmacokinetic profiles were determined 0, 1, 2, 3, and 4 hours after treatment with tacrolimus and were compared between high- and low-level median body composition groups. The values of C0, C1, C2, C3, and C4 were used in determining an abbreviated area under the curve (AUC) for tacrolimus.
Results
The mean body mass index (BMI) and body composition values were as follows: BMI, 24.3 kg/m2; lean mass, 49.8 kg; and fat mass, 17.4 kg. There were no statistical differences in pharmacokinetic profiles between groups with different BMIs. However, the C0 and C4 in the high-fat group were significantly elevated compared with those of the low-fat group (P=0.024 and 0.031, respectively). Furthermore, the C0, C2, C3, and C4 and the AUC were significantly different between the two lean mass groups (P=0.007, 0.038, 0.047, 0.015, and 0.015, respectively). Other variables, such as waist circumference and arm muscle circumference, did not differentiate between the pharmacokinetic profiles of tacrolimus.
Conclusion
Taken together, these data suggest that tacrolimus dose monitoring based on body composition may provide adequate dosage leading to favorable long-term outcomes.
Keywords: Adipose tissue, Body composition, Muscles, Pharmacokinetics, Tacrolimus
Introduction
Calcineurin inhibitors, such as cyclosporine and tacrolimus, have a long history as immunosuppressants in the kidney transplant field [1], [2]. The use of these inhibitors has improved short-term outcomes in kidney transplant patients [3], and this improvement is primarily associated with fewer rejection episodes [4]. However, these short-term improvements have not led to parallel improvements in long-term outcomes and, accordingly overall graft survival remains suboptimal. One of reasons is chronic nephrotoxicity, which is the Achilles’ heel of calcineurin inhibitors [5].
Calcineurin inhibitors are characterized by a narrow therapeutic index and high inter-individual pharmacokinetic variations. Owing to these challenging characteristics, other regimens, such as using low doses of drugs or not using drugs, have been introduced [6], [7], but these regimens are not widely accepted. Nonetheless, calcineurin inhibitors are still widely used in clinical practice; therefore, it is important to determine the factors that affect drug concentration, to aid in the prevention of chronic nephrotoxicity, as well as graft rejection. Several factors such as age, disease status, lipoprotein concentrations, and genetic variations have been found in pharmacokinetic studies [8].
After kidney transplantation, kidney recipients show a change in body composition, including an increase in fat mass and a decrease in lean mass [9]. The metabolic adverse events of steroid and calcineurin inhibitor treatment can lead to these changes and contribute to obesity [10]. Tacrolimus, a calcineurin inhibitor, is highly lipophilic, and the partition coefficient of tacrolimus in n-octanol is 1000-fold greater than in water. This lipophilicity is associated with the distribution of tacrolimus mainly in fat-rich organs, such as adipose tissue, liver, kidneys, and lymph nodes [11].
In this study, we hypothesized that the blood level and distribution of tacrolimus would be influenced by the patient's body composition, and we aimed to verify this hypothesis in Korean kidney transplant recipients.
Methods
Patients
The institutional review board at the Seoul National University Hospital approved this study (no. H-1005-038-318). Inclusion criteria were as follows: patients’ age ≥20 years old; transplanted at least 6 months previously; serum creatinine concentration <2.0 mg/dL; change in serum creatinine level <30% within 1 month; and receiving triple immunosuppressive agents (steroid, mycophenolate mofetil, and tacrolimus). The dosage of tacrolimus was adjusted to keep the morning trough levels in the range of 5–10 ng/mL. We excluded patients who had the following histories: multiorgan transplantation or previous history of kidney transplantation; nephrotoxicity or other serious adverse events induced by tacrolimus; acute kidney injury within 1 month of the trial; taking other drugs or foods known to affect tacrolimus pharmacokinetics (such as antiepileptics, antimycotics, cholestyramine, and grape juice); gastrointestinal disease (which can affect the absorption of tacrolimus); current evidence of infection; and treatment history for rejection or infection within 1 month of the trial.
Between November 2010 and August 2011, 18 patients were enrolled in this study. All patients were of Korean origin. We recorded clinical parameters, such as age at transplantation, sex, and the cause of end-stage renal disease. Donor factors, including age at transplantation, sex, and donor source (living relative, living nonrelative, and cadaveric), were also evaluated.
On the study day, each patient was admitted to the clinical trials center in the fasting state. Next, blood samples were obtained before the morning dose of tacrolimus (C0, serum creatinine, blood urea nitrogen, hemoglobin, albumin, total cholesterol, triglyceride, high- and low-density lipoprotein cholesterols, and high-sensitivity C-reactive protein) and after dosing (C1, C2, C3, C4). The whole-blood concentrations of tacrolimus were determined by mass spectrometry. The area under the curve (AUC) for the concentration versus time curve of tacrolimus was calculated using the linear trapezoidal rule. We used an abbreviated AUC (AUC0–4), which had provided good correlation with the complete AUC in the monitoring of tacrolimus [12], [13], [14]. The dose-adjusted concentrations and AUC of tacrolimus were used throughout the study.
Body compositions, including lean mass (kg) and fat mass (kg), were measured using bioelectrical impedance analysis (BIA; Inbody 720; Biospace Company, Ltd., Seoul, Korea). We also measured weight (kg), height (cm), and circumferences (cm) of the waist, hip, arm, and arm muscles. BMI was calculated as the weight divided by the square of the height (m).
Statistical analysis
All analyses and calculations were performed using SPSS software (SPSS version 16.0, Chicago, IL, USA). Data are presented as the mean±standard deviation (SD) for continuous variables and as a proportion for categorical variables. If the distribution of the data was skewed, the median [interquartile range (IQR)] was used. Correlations between BMI and body composition (muscle mass and fat mass) were evaluated using Pearson's correlation method. We divided the patients into two groups according to the median value for body composition. Next, the blood concentrations and the AUCs of the two groups were compared using the Mann-Whitney U test. A P value of less than 0.05 was considered to be significant.
Results
Baseline characteristics
In this study, 18 kidney recipients were recruited from Seoul National University Hospital. The baseline characteristics of study subjects are described in Table 1. The mean age of testing was 44 years. Of the 18 patients, 7 had received grafts from deceased donors. There were no ABO-incompatible transplantations. All of the participants had stable graft function, and serum creatinine concentrations ranged from 0.91 mg/dL to 1.82 mg/dL. There was no evidence of possible infectious or inflammatory diseases. Furthermore, 15 patients had high-sensitivity C-reactive protein concentrations of less than 0.1 mg/dL, whereas the other three patients did not. The causes of patients’ end-stage renal disease included diabetes mellitus (five), glomerulonephritis (four), autosomal dominant polycystic kidney disease, drug-related disease, and kidney donor. Of all of the patients, six recipients did not know the original cause of end-stage renal disease.
Table 1.
Baseline characteristics of the study participants
| Lean mass |
Fat mass |
Total (n=18) | |||
|---|---|---|---|---|---|
| High level group (n=9) | Low level group (n=9) | High level group (n=9) | Low level group (n=9) | ||
| Age (y) | 45±11.1 | 43±13.4 | 44±13.7 | 44±11.0 | 44±12.0 |
| Recipient's male sex (%) | 100.0 | 55.6 | 88.9 | 66.7 | 77.8 |
| Deceased donor (%) | 55.6 | 22.2 | 33.3 | 44.4 | 38.9 |
| Donor's age (y) | 31±7.3 | 33±9.3 | 35±8.6 | 29±6.8 | 32±8.2 |
| Donor's male sex (%) | 75.0 | 55.6 | 50.0 | 77.8 | 64.7 |
| HLA mismatching | 3±1.8 | 3±1.9 | 3±1.8 | 3±1.9 | 3±1.8 |
| Serum biochemical marker | |||||
| Creatinine (mg/dL) | 1.3±0.17 | 1.2±0.29 | 1.3±0.17 | 1.2±0.29 | 1.2±0.23 |
| Glucose (mg/dL) | 107±18.9 | 120±83.4 | 105±20.0 | 121±82.9 | 113±59.1 |
| Bilirubin (mg/dL) | 1.0±0.41 | 0.7±0.24 | 1.0±0.43 | 0.7±0.24 | 0.9±0.36 |
| Total cholesterol (mg/dL) | 168±37.6 | 164±28.0 | 175±25.8 | 158±37.0 | 166±32.2 |
| Triglyceride (mg/dL) | 174±53.8 | 140±42.9 | 168±44.8 | 146±55.9 | 157±50.4 |
| HDL cholesterol (mg/dL) | 46±9.2 | 51±15.1 | 48±8.8 | 48±15.9 | 48±12.5 |
| LDL cholesterol (mg/dL) | 101±36.0 | 95±25.3 | 105±30.1 | 90±30.4 | 98±30.3 |
| High-sensitivity CRP (mg/dL)⁎ | 0.01 (0.01–0.08) | 0.06 (0.01–0.08) | 0.01 (0.01–0.04) | 0.06 (0.01–1.00) | 0.01 (0.01–0.07) |
| From operation to test (mo)⁎ | 40 (10–57) | 56 (34–100) | 26 (10–61) | 56 (43–99) | 46 (16–74) |
CRP, C-reactive protein; HDL, high-density lipoprotein; HLA, human leukocyte antigen; LDL, low-density lipoprotein.
Data are expressed as the median (interquartile range) when the distribution of data was skewed.
Body composition parameters
All of the patients underwent the BIA for assessing body compositions (Table 2). The mean BMI was 24.3 kg/m2. Of all total patients, a BMI of more than 25.0 kg/m2 was measured in eight patients, and a BMI greater than 30.0 kg/m2 was measured in one patient. We analyzed the correlation between BMI and body composition parameters such as lean mass and fat mass and found a positive correlation (P<0.05). Among those parameters, fat mass had a stronger correlation with BMI than did lean mass (R=0.875 and 0.706, respectively). Body weight had significant correlations with body composition parameters except waist-to-hip ratio. Correlation of body weight with fat mass was weaker than correlation with lean mass (R=0.782 and 0.947, respectively). Waist circumference had a stronger correlation with fat mass (R=0.757) than lean mass (R=0.613). However, arm muscle circumference was better correlated with lean mass (R=0.917) than with fat mass (R=0.619).
Table 2.
Body composition parameters estimated by bioelectrical impedance analysis
| Lean mass |
Fat mass |
Total (n=18) | |||
|---|---|---|---|---|---|
| High level group (n=9) | Low level group (n=9) | High level group (n=9) | Low level group (n=9) | ||
| Body weight (kg) | 77.3±7.87 | 57.2±6.01 | 76.1±9.07 | 58.3±7.96 | 67.2±12.37 |
| Height (cm) | 172.1±4.81 | 159.6±5.10 | 168.9±8.25 | 162.8±6.98 | 165.8±8.05 |
| Body mass index (kg/m2) | 26.0±1.97 | 22.5±3.02 | 26.6±1.72 | 22.0±2.15 | 24.3±3.06 |
| Lean mass (kg) | 57.2±6.03 | 42.5±4.40 | 55.2±8.10 | 44.4±6.81 | 49.8±9.15 |
| Proportion of lean mass (%) | 74.1±3.21 | 74.5±5.88 | 72.4±4.02 | 76.2±4.56 | 74.3±4.60 |
| Fat mass (kg) | 20.1±3.50 | 14.7±4.45 | 20.9±3.27 | 13.8±3.04 | 17.4±4.77 |
| Proportion of fat mass (%) | 26.0±3.21 | 25.5±5.93 | 27.6±4.01 | 23.8±4.59 | 25.7±4.63 |
| Waist circumference (cm) | 90.1±5.88 | 81.8±5.02 | 89.9±5.97 | 82.0±5.32 | 85.9±6.82 |
| Hip circumference (cm) | 97.7±4.87 | 90.9±4.83 | 98.0±5.00 | 90.6±4.10 | 94.3±5.86 |
| Waist-to-hip ratio | 0.92±0.033 | 0.90±0.042 | 0.92±0.033 | 0.91±0.044 | 0.91±0.038 |
| Arm circumference (cm) | 24.6±1.61 | 21.5±1.80 | 24.6±1.62 | 21.6±1.85 | 23.1±2.29 |
| Arm muscle circumference (cm) | 31.2±2.04 | 27.6±2.32 | 31.6±1.80 | 27.3±1.80 | 29.4±2.82 |
Tacrolimus concentration according to body composition
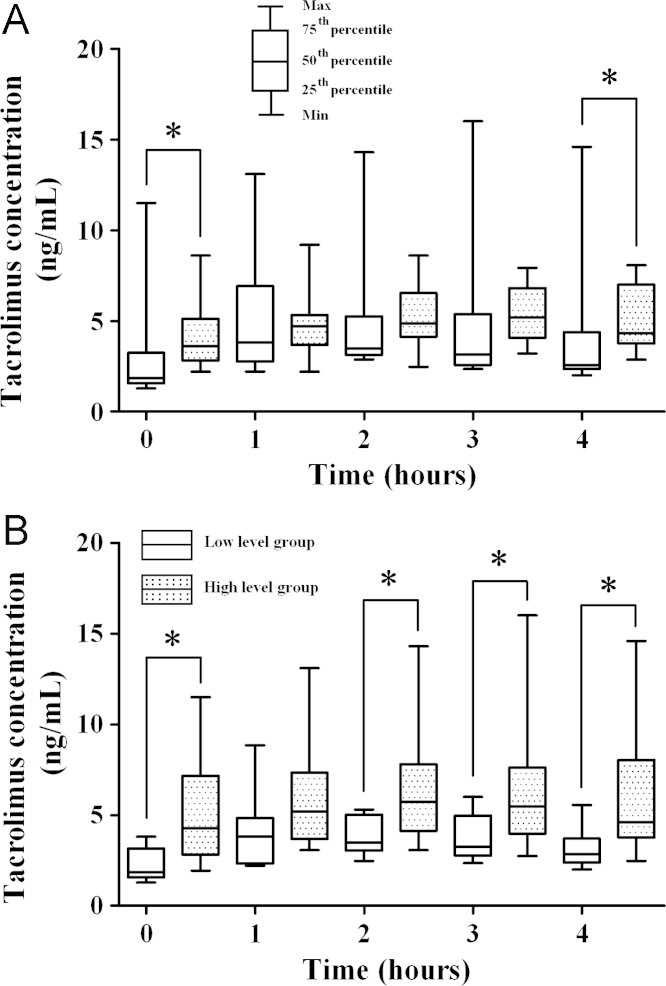
We evaluated the blood concentrations of tacrolimus after dividing the patients into two groups according to median body-composition values. When considering BMI, the tacrolimus concentrations from 0 to 4 hours were not different between the high and the low level groups (P>0.05 all comparisons). However, the concentrations at 0 and 4 hours were different between the groups with the high and low of fat-mass values (C0, P=0.024; C4, P=0.031). This is shown in Fig. 1. In the lean mass analysis, the concentrations at all times except 1 hour (C1) were different (C0, P=0.007; C2, P=0.038; C3, P=0.047; C4, P=0.015). This is shown in Fig. 1. However, tacrolimus concentrations did not significantly vary with waist and arm muscle circumference, unlike with lean and fat mass values [waist circumference; all P values were greater than 0.05; arm muscle circumference, all P values were greater than 0.05 except C0 (P=0.031)].
Figure 1.
Concentrations of tacrolimus after classifying patients into groups according to median fat-mass (A) and lean-mass (B). The tacrolimus concentrations of the two groups are compared using the Mann-Whitney U test. *P<0.05.
The differences between the AUC of the body composition groups are shown in Table 3. The AUC was significantly different according to lean-mass value but not between the groups classified using other parameters.
Table 3.
Area under the concentration–time curve for tacrolimus in the high and the low level groups of body composition parameters
| Parameter | High level group (n=9) | Low level group (n=9) | P |
|---|---|---|---|
| Body mass index | 20.1 (14.35–24.59) | 17.0 (11.07–19.05) | 0.200 |
| Lean mass | 21.2 (17.34–25.93) | 12.3 (10.86–18.45) | 0.015 |
| Fat mass | 20.1 (17.34–24.59) | 12.3 (10.98–19.05) | 0.122 |
| Waist | 18.5 (14.06–24.15) | 17.5 (11.07–22.07) | 0.453 |
| Hip | 19.3 (17.19–23.62) | 14.9 (10.87–20.96) | 0.237 |
| Waist-to-hip ratio | 18.4 (11.71–23.59) | 17.5 (11.07–22.82) | 0.691 |
| Arm circumference | 20.1 (17.34–24.59) | 12.3 (10.86–19.05) | 0.070 |
| Arm muscle circumference | 20.1 (14.35–24.59) | 17.0 (11.07–19.05) | 0.200 |
Discussion
The present study evaluated the relationship between the pharmacokinetic profiles of tacrolimus and body composition parameters. The results showed that BMI did not differentiate between the tacrolimus concentrations, but the lean mass and fat mass did differentiate between tacrolimus concentrations. Furthermore, the AUC for tacrolimus was different only when the patients were classified according to lean-mass group. These data may suggest that the body composition should be considered in tacrolimus dose monitoring.
Tacrolimus is a representative immunosuppressive drug that has emerged as a valuable therapeutic alternative to cyclosporine after solid organ transplantation [15]. Tacrolimus not only has a positive effect on short-term graft function but may also have a negative effect on long-term function. When tacrolimus is maintained at high levels in the blood, several negative effects are observed, such as nephrotoxicity, neurotoxicity, diabetes, gastrointestinal disturbance, hypertension, and infections [15], [16]. Because of these toxicities, the tacrolimus level should be adjusted and maintained within a narrow therapeutic window. However, there is considerable inter- and intraindividual variability in the pharmacokinetic profile of tacrolimus, and the adjustment of tacrolimus levels is not easy. Thus, factors related to the pharmacokinetics of tacrolimus have been heavily studied. Tacrolimus is a highly lipophilic drug [17], and the distribution of tacrolimus is predominantly in the fat-rich organs, such as adipose tissue. However, studies on the relationship between tacrolimus levels and body composition have not been conducted. The current study is the first to demonstrate that body composition is one of several factors that should be considered in determining the tacrolimus level.
Previously, some studies have shown that being overweight is a risk factor for high blood concentrations of calcineurin inhibitors [18], [19], [20], [21]. Although their study was not designed to measure pharmacokinetics, Rodrigo and colleagues [18] showed that a transplant recipient group with a high BMI was associated with tacrolimus levels higher than 15 ng/mL. With respect to pharmacokinetic design, cyclosporine has been more intensively studied with respect to body weight. In most studies, patients with a higher BMI have been found to require less cyclosporine to maintain therapeutic trough levels [19], [20], [21]. However, other studies have found the opposite results [22]. The current study showed that BMI level is not related to tacrolimus concentrations. The variable results in previous studies may have arisen from the shortcoming of measuring BMI. Although BMI is used for indicating obesity, BMI does not differentiate lean mass from fat tissue [23]. Our previous research has already shown that there is only a weak correlation between BMI and lean mass [24], and the correlation was lesser in the disease groups. Furthermore, some studies did not find a negative correlation between BMI and mortality, and this finding has been termed the “obesity paradox.” This paradoxical phenomenon is observed mainly in disease states and the elderly [25]. Transplant recipients also have different body compositions, owing to immunosuppressive agents [26]; therefore, BMI may not be a perfect substitute for fat mass or other body composition parameters. Therefore, the effect of BMI on pharmacokinetics in transplant recipients could vary according to their body composition.
In the current study, fat mass was correlated with the concentrations of tacrolimus at certain time points (C0 and C4) of tacrolimus. Because tacrolimus is highly lipophilic, an individual's fat mass composition may affect the pharmacokinetics of this drug. In other words, the factor determining the tacrolimus level is the proportion of fat mass, not the BMI or body weight. Lean mass was also shown to be correlated with the concentrations of tacrolimus at certain time points (C0, C2, C3, and C4) and the AUC of tacrolimus. The reason for this correlation is not clear, but one possibility is a difference in liver volume. Patients with high lean-mass values have larger liver volumes [27], and tacrolimus accumulates in the liver tissue. These relationships may affect the tacrolimus level. Evidence from some studies suggests that lean mass is a better predictor of the appropriate drug dosage than total body weight, although the mechanism underlying this is not fully established [28]. However, calculating dosage on the basis of lean mass is known to be more accurate for drugs with weak or moderate lipophilicity [29]. This issue should be further delineated in future studies.
Although our results are informative, this study has some limitations. First, we did not check variations in genes that might affect the pharmacokinetics of tacrolimus, such as cytochrome P-450 gene [30]. Second, we used the abbreviated AUC (AUC0-4) instead of the complete AUC. However, the abbreviated AUC has been identified to adequately monitor and guide tacrolimus dosing and may be quicker and more inexpensive in clinical practice than the complete AUC. Third, the number of study participants is relatively small, although the study is designed for pharmacokinetics. Therefore, the statistical power in the correlation analysis may not be strong enough to detect statistical significance.
In summary, body composition parameters were found to be associated with the blood level of tacrolimus in kidney-transplant recipients. This trend was stronger when considering lean mass than other body composition parameters. The BMI was not associated with the blood level or the AUC of tacrolimus. Body weight is commonly used to calculate tacrolimus dosage. However, total body-weight and BMI values cannot be easily substituted for lean-mass and fat-mass values in kidney-transplant recipients. Measuring body composition can more precisely determine tacrolimus levels in kidney transplant recipients. In the future, well-designed research and intervention studies will be required to identify the importance of these correlations.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by a grant from Seoul National University Hospital (1220100114).
References
- 1.Calne R.Y., White D.J., Thiru S., Evans D.B., McMaster P., Dunn D.C., Craddock G.N., Pentlow B.D., Rolles K. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;2:1323–1327. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 2.Spencer C.M., Goa K.L., Gillis J.C. Tacrolimus. An update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs. 1997;54:925–975. doi: 10.2165/00003495-199754060-00009. [DOI] [PubMed] [Google Scholar]
- 3.Palestine A.G., Austin HA 3rd, Balow J.E., Antonovych T.T., Sabnis S.G., Preuss H.G., Nussenblatt R.B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4:1289–1295. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 4.Mayer A.D., Dmitrewski J., Squifflet J.P., Besse T., Grabensee B., Klein B., Eigler F.W., Heemann U., Pichlmayr R., Behrend M., Vanrenterghem Y., Donck J., van Hooff J., Christiaans M., Morales J.M., Andres A., Johnson R.W.G., Short C., Buchholz B., Rehmert N., Land W., Schleibner S., Forsythe J.L.R., Talbot D., Neumayer H.H., Hause I., Ericzon B.G., Brattstrom C., Claesson K., Muhlbacher F., Pohanka E. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation. 1997;64:436–443. doi: 10.1097/00007890-199708150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Palestine A.G., Austin H.A., 3rd, Balow J.E., Antonovych T.T., Sabnis S.G., Preuss H.G., Nussenblatt R.B. Renal histopathologic alterations in patients treated with cyclosporine for uveitis. N Engl J Med. 1986;314:1293–1298. doi: 10.1056/NEJM198605153142005. [DOI] [PubMed] [Google Scholar]
- 6.Ekberg H., Tedesco-Silva H., Demirbas A., Vítko S., Nashan B., Gürkan A., Margreiter R., Hugo C., Grinyó J.M., Frei U., Vanrenterghem Y., Daloze P., Halloran P.F. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 7.Oberbauer R. Improved renal function in de novo renal transplant patients on sirolimus maintenance therapy following discontinuation of cyclosporine. Ther Drug Monit. 2005;27:7–9. doi: 10.1097/00007691-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet. 1993;24:472–495. doi: 10.2165/00003088-199324060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Habedank D., Kung T., Karhausen T., von Haehling S., Doehner W., Schefold J.C., Hasper D., Reinke S., Anker S.D., Reinke P. Exercise capacity and body composition in living-donor renal transplant recipients over time. Nephrol Dial Transplant. 2009;24:3854–3860. doi: 10.1093/ndt/gfp433. [DOI] [PubMed] [Google Scholar]
- 10.Dmitrewski J., Krentz A.J., Mayer A.D., Buckels J.A., Barnes A.D., Smith J., Nattrass M. Metabolic and hormonal effects of tacrolimus (FK506) or cyclosporin immunosuppression following renal transplantation. Diabetes Obes Metab. 2001;3:287–292. doi: 10.1046/j.1463-1326.2001.00132.x. [DOI] [PubMed] [Google Scholar]
- 11.Boland J., Atkinson K., Britton K., Darveniza P., Johnson S., Biggs J. Tissue distribution and toxicity of cyclosporin A in the mouse. Pathology. 1984;16:117–123. doi: 10.3109/00313028409059087. [DOI] [PubMed] [Google Scholar]
- 12.Pisitkun T., Eiam-Ong S., Chusil S., Praditpornsilpa K., Pansin P., Tungsanga K. The roles of C4 and AUC0-4 in monitoring of tacrolimus in stable kidney transplant patients. Transplant Proc. 2002;34:3173–3175. doi: 10.1016/s0041-1345(02)03684-9. [DOI] [PubMed] [Google Scholar]
- 13.Uchida K., Tominaga Y., Haba T., Katayama T., Matsuoka S., Sato T., Goto N., Takeda A., Morozumi K., Takagi H. Usefulness of monitoring of AUC(0-4h) during the induction period of immunosuppressive therapy with tacrolimus after renal transplantation. Transplant Proc. 2002;34:1736–1737. doi: 10.1016/s0041-1345(02)03002-6. [DOI] [PubMed] [Google Scholar]
- 14.Wong K.M., Shek C.C., Chau K.F., Li C.S. Abbreviated tacrolimus area-under-the-curve monitoring for renal transplant recipients. Am J Kidney Dis. 2000;35:660–666. doi: 10.1016/s0272-6386(00)70013-8. [DOI] [PubMed] [Google Scholar]
- 15.Plosker G.L., Foster R.H. Tacrolimus: a further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs. 2000;59:323–389. doi: 10.2165/00003495-200059020-00021. [DOI] [PubMed] [Google Scholar]
- 16.Henry M.L. Cyclosporine and tacrolimus (FK506): a comparison of efficacy and safety profiles. Clin Transplant. 1999;13:209–220. doi: 10.1034/j.1399-0012.1999.130301.x. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki K. Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug Metab Pharmacokinet. 2007;22:328–335. doi: 10.2133/dmpk.22.328. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigo E., de Cos M.A., Sánchez B., Ruiz J.C., Piñera C., Fernández-Fresnedo G., Palomar R., Pérez-Ceballos M.A., Cotorruelo J.G., Zubimendi J.A., de Francisco A.L., Arias M. High initial blood levels of tacrolimus in overweight renal transplant recipients. Transplant Proc. 2005;37:1453–1454. doi: 10.1016/j.transproceed.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 19.Flechner S.M., Kolbeinsson M.E., Tam J., Lum B. The impact of body weight on cyclosporine pharmacokinetics in renal transplant recipients. Transplantation. 1989;47:806–810. doi: 10.1097/00007890-198905000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Kasap B., Soylu A., Turkmen M., Kavukcu S., Bora S., Gulay H. Effect of obesity and overweight on cyclosporine blood levels and renal functions in renal adolescent recipients. Transplant Proc. 2006;38:463–465. doi: 10.1016/j.transproceed.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 21.Orofino L., Pascual J., Quereda C., Burgos J., Marcen R., Ortuno J. Influence of overweight on survival of kidney transplant. Nephrol Dial Transplant. 1997;12:855. doi: 10.1093/ndt/12.4.855. [DOI] [PubMed] [Google Scholar]
- 22.Hortal L., Fernandez A., Losada A., Lorenzo M., Baamonde E., Plaza C., Gallego R., Vega N., Palop L. Study of the cyclosporine concentration at 2 hours in stable renal transplant patients and relation to body mass index. Transplant Proc. 2001;33:3110–3111. doi: 10.1016/s0041-1345(01)02326-0. [DOI] [PubMed] [Google Scholar]
- 23.Mafra D., Guebre-Egziabher F., Fouque D. Body mass index, muscle and fat in chronic kidney disease: questions about survival. Nephrol Dial Transplant. 2008;23:2461–2466. doi: 10.1093/ndt/gfn053. [DOI] [PubMed] [Google Scholar]
- 24.Han S.S., Heo N.J., Na K.Y., Chae D.W., Kim Y.S., Kim S., Chin H.J. Age- and gender-dependent correlations between body composition and chronic kidney disease. Am J Nephrol. 2010;31:83–89. doi: 10.1159/000258660. [DOI] [PubMed] [Google Scholar]
- 25.Han S.S., Kim K.W., Kim K.I., Na K.Y., Chae D.W., Kim S., Chin H.J. Lean mass index: a better predictor of mortality than body mass index in elderly Asians. J Am Geriatr Soc. 2010;58:312–317. doi: 10.1111/j.1532-5415.2009.02672.x. [DOI] [PubMed] [Google Scholar]
- 26.Keogh J.B., Tsalamandris C., Sewell R.B., Jones R.M., Angus P.W., Nyulasi I.B., Seeman E. Bone loss at the proximal femur and reduced lean mass following liver transplantation: a longitudinal study. Nutrition. 1999;15:661–664. doi: 10.1016/s0899-9007(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 27.Nawaratne S., Brien J.E., Seeman E., Fabiny R., Zalcberg J., Cosolo W., Angus P., Morgan D.J. Relationships among liver and kidney volumes, lean body mass and drug clearance. Br J Clin Pharmacol. 1998;46:447–452. doi: 10.1046/j.1365-2125.1998.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan D.J., Bray K.M. Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet. 1994;26:292–307. doi: 10.2165/00003088-199426040-00005. [DOI] [PubMed] [Google Scholar]
- 29.De Baerdemaeker L.E., Mortier E.P., Struys M.M. Pharmacokinetics in obese patients. Continuing Education in Anaesthesia, Critical Care & Pain. 2004;4:152–155. [Google Scholar]
- 30.Hesselink D., van Schaik R., van der Heiden I., van der Werf M., Gregoor P., Lindemans J., Weimar W., van Gelder T. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clinical Pharmacology & Therapeutics. 2003;74:245–254. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]