Abstract
Nephrotic syndrome (NS) rarely occurs after hematopoietic stem cell transplantation (HSCT) as a late manifestation of graft-versus-host disease (GVHD). Herein, we report a case of HSCT-associated membranous nephropathy in a female patient with aplastic anemia. The patient received an allogeneic HSCT from her human leukocyte antigen-identical brother following myeloablative conditioning chemotherapy. NS occurred 21 months after HSCT without any concurrent features of chronic GVHD. The patient was treated with prednisolone and cyclosporine after renal biopsy confirmed membranous nephropathy, and achieved complete remission. Our report contradicts previous assumptions that concomitant chronic GVHD is responsible for the development of NS, suggesting that NS can develop as a new, independent manifestation of GVHD.
Keywords: Graft-versus-host disease, Membranous nephropathy, Nephrotic syndrome
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an established treatment for hematologic malignancy, and more than 15,000 procedures are performed worldwide each year [1]. After HSCT, chronic graft-versus-host disease (cGVHD) is the most common cause of morbidity and mortality. Indeed, the incidence of cGVHD is reported to be 60–80% during long-term follow-up [2], and the incidence has recently been increasing because of the extensive use of unrelated donor transplants, older donor age, increased use of donor leukocyte infusion, and peripheral blood stem cell transplantation (PBSCT) [3]. Symptoms of cGVHD can vary depending on the site of involvement, which may include the skin, eyes, oropharynx, or respiratory and gastrointestinal tracts. However, renal involvement associated with GVHD, particularly glomerulopathy, is very rare. In general, renal injury after HSCT occurs due to hemodynamic compromise, medications, radiation, or thrombotic microangiopathy [4], which manifests as tubulointerstitial nephropathy. Cases of nephrotic or nephritic syndrome after HSCT have recently been reported, and these glomerulopathies are presumably related to cGVHD. Herein, we report a case of membranous nephropathy (MN) in a patient who underwent HSCT 21 months before this unusual nephrotic syndrome (NS) developed.
Case report
A 39-year-old female patient was admitted to our hospital due to generalized edema and fatigue. The patient was diagnosed with aplastic anemia 3 years previously, and had no history of diabetes or hypertension. Most importantly, she had undergone allogeneic PBSCT from her human leukocyte antigen-identical brother 21 months before admission following myeloablative conditioning chemotherapy with cyclophosphamide and anti-thymoglobulin. Grade IV acute gastrointestinal GVHD accompanied by diarrhea developed 12 days after transplantation despite GVHD prophylaxis with cyclosporine, methotrexate, and steroids, for which a continuous maintenance regimen of cyclosporine and prednisolone resulted in resolution. The patient also suffered from cytomegalovirus colitis 4 months after transplantation, and recovered after a 2-week administration of gancyclovir while cyclosporine was discontinued and prednisolone was tapered to 5 mg/day. Eighteen months after cytomegalovirus infection, the patient suddenly developed generalized edema and gained 5 kg of body weight over a 2-week period.
At this time physical examination revealed 3+ pitting edema of the lower extremities. Initial laboratory tests showed the following values: hemoglobin, 10.5 g/dL; platelets, 320×109/L; serum albumin, 2.2 g/dL; total cholesterol, 402 mg/dL; low-density lipoprotein cholesterol, 248 mg/dL; serum creatinine, 0.77 mg/dL; random urine protein-to-creatinine ratio (UPCR), 7.85 g/g; and 24-hour urinary protein excretion, 5.03 g/day. Hepatitis B surface antigen, hepatitis C antibody and anti-nuclear antibody titers were undetectable, and serum concentrations of C3, C4, and immunoglobulins G, A, and M were within reference range.
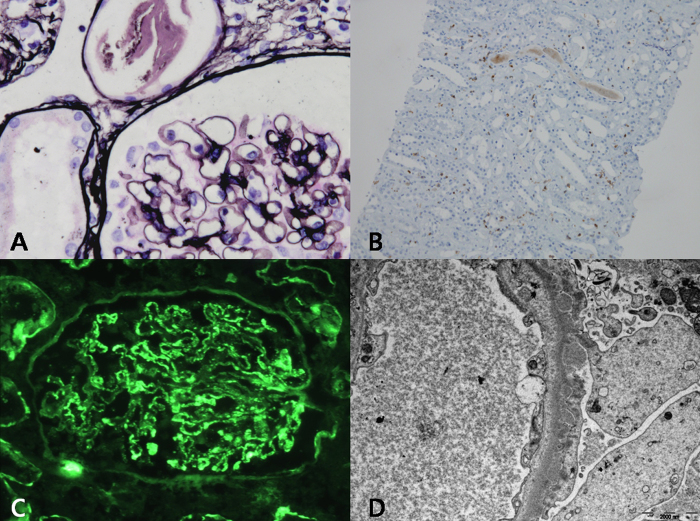
Renal biopsy was performed 3 days after admission. On light microscopy (Fig. 1A), eight nonsclerotic glomeruli were normocellular without mesangial expansion. The glomerular basement membrane was not thickened and double contours or subepithelial spikes were not noted. The interstitium was moderately infiltrated by mononuclear inflammatory cells, which immunohistochemical staining confirmed as CD3+ T cells (Fig. 1B). Immunofluorescence studies showed a granular pattern of IgG (2+) (Fig. 1C). Electron microscopy demonstrated numerous nodular electron-dense deposits that were mainly located in the subepithelial space along with diffusely effaced epithelial foot processes (Fig. 1D).
Figure 1.
Pathologic findings in a patient with membranous nephropathy as a manifestation of graft versus host disease. (A) Light microscopy shows normal appearance of glomeruli without thickened basement membrane (original magnification ×400). (B) Immunohistochemical staining identifies CD3+, suggesting infiltration of T cells in the interstitium (original magnification ×100). (C) Immunofluorescence staining shows granular pattern of IgG (2+) deposition along the peripheral capillary wall. (D) Electron microscopy shows numerous nodular electron-dense deposits in the subepithelial space.
These pathologic findings were consistent with Grade II MN. As such, oral prednisolone at a dose of 1 mg/kg was immediately started, and an 8-week treatment resulted in partial remission with a UPCR of 1.9 g/g. Because the patient was intolerant to the side effects of corticosteroid treatment, prednisolone was tapered. Two weeks later, however, the random UPCR increased to 7.58 g/g; thus, cyclosporine at a dose of 5 mg/kg was added. Complete remission was achieved as demonstrated by a UPCR of 0.23 g/g 4 months after the combined treatment with cyclosporine and 10 mg/day of low-dose prednisolone.
Discussion
Acute kidney injury (AKI) is a common complication in patients with allo-HSCT. In general, AKI can be attributed to preexisting renal disease, underlying malignancy, previous chemotherapies, irradiation, and various nephrotoxic agents such as antimicrobials, antifungals, and antivirals [5]. Accordingly, acute tubular necrosis, hemolytic uremic syndrome, thrombotic microangiopathy, and radiation nephritis are the most common forms of AKI after HSCT. However, glomerulopathy such as NS or nephritis rarely occurs as a manifestation of cGVHD.
The notion that graft-versus-host reaction is directly related to glomerular injury has recently been gaining acceptance. However, the clinical characteristics of HSCT-associated glomerulopathy are not well defined. Several studies conducted comprehensive analyses of patients with postHSCT NS [1], [6], [7], [8], [9]. One of these reports showed that nine of 889 patients who underwent allo-HSCT from 1994 to 2002 developed NS, with an overall incidence of 1% [8]. When only sibling transplant patients with or without cGVHD were taken into account, the incidence of NS was 1.8% and 0.6%, respectively [8], suggesting that it is not a common complication. Glomerular diseases tend to occur in allogeneic PBSCT patients from unrelated donors who received nonmyeloablative therapy shortly after immunosuppression was decreased or stopped [9], [10], and the median time elapsed to the onset of NS from cessation of immunosuppression was 1.5 months [9]. Regarding pathologic features, MN (61%) was the most common type of NS, followed by minimal change disease (22%) [7]. Approximately 70% of patients had concomitant cGVHD at the onset of NS and, in particular, 75–93% of patients with postHSCT MN had recent or current cGVHD [1], [9]. In addition, development of NS appears to be a late event after HSCT because NS was diagnosed 15.5–24 months after HSCT [8].
In line with these findings, our patient developed NS 21 months after allogeneic PBSCT, whereby MN was confirmed by renal biopsy. Unlike previous observations, the patient did not show other features of cGVHD before the onset of NS, although she did experience acute GVHD 12 days after HSCT. However, a prior episode of acute GVHD is unlikely to be related to the development of glomerular disease considering its late onset after HSCT [9]. Despite the high incidence of cGVHD, only a small portion of patients with cGVHD developed glomerular disease, and this low incidence is no different than that of the whole HSCT population [8], [11]. Furthermore, cGVHD was not evident in approximately 30% of patients who presented with NS [9], suggesting that NS can develop irrespective of GVHD. In this regard, it is not clear whether preexisting cGVHD can indeed contribute to the development of glomerular disease. Nevertheless, in this case, immunohistochemical studies clearly identified T cells in the interstitium. Because complete donor chimerism was confirmed, these T cells originated from the donor and were presumed to be associated with kidney injury. This finding may be nonspecific because kidney injury could be caused by other conditions such as drugs or infections. However, considering the fact that the patient had no evidence of kidney injury associated with such conditions before the onset of NS, this pathologic finding partly explains the presence of GVHD in the kidney.
Our patient developed NS while 5 mg/day of prednisolone was maintained. Previous observations showed that NS usually occurred shortly after immunosuppressive medications were stopped or their dose was reduced [9]. However, it is uncertain whether cessation or reduction of immunosuppression may play a role in the development of NS. In fact, 40% of patients that have NS may develop glomerular disease while on immunosuppressive agents [9].
Although controversy remains over the role of preexisting cGVHD in postHSCT NS, it has been suggested that immune complex, which is formed as a result of GVHD, can deposit within the glomeruli and cause damage. In particular, autoantibody formation has been detected in both human and experimental cGVHD [12]. In addition, B-cell dysregulation with a high prevalence of autoantibodies to cellular antigens and minor histocompatibility antigens has been demonstrated in patients with cGVHD [12], [13], [14], [15]. Besides B-cell dysregulation, alloreactive T cells are also implicated in HSCT-associated NS. In fact, cGVHD is considered an immune reaction between donor CD4+/Th2 cells and the recipient's minor histocompatibility complex antigens. Furthermore, preHSCT conditioning chemotherapy or irradiation can cause renal damage, which renders hiding autoantigen exposed or altered, ultimately leading to selective activation of alloreactive donor CD4+ cells and the production of immune complex [1]. The pathogenesis of HSCT-associated NS cannot be explained by a single factor, and is more likely to be attributed to the combination of B cell dysregulation, alloreactive T cells, preconditioning chemotherapy, etc.
Treatment of HSCT-associated NS has not yet been defined, but, similar to idiopathic NS, corticosteroids and cyclosporine have been most commonly used [7]. Other immunosuppressive agents such as cyclophosphamide, mycofenolate mofetil, and rituximab have also been attempted to induce remission. Overall responsiveness to these drugs appears favorable: according to a previous report, 27% and 62% of patients with MN attained complete and partial remission, respectively, and 90% of patients with minimal change disease achieved complete remission[7]. Likewise, in our case, cyclosporine in addition to corticosteroids resulted in complete resolution of proteinuria, and the patient has been in complete remission.
In conclusion, we present a case of postHSCT MN as a rare manifestation of GVHD. However, NS occurred without other features of cGVHD while the patient was on low-dose corticosteroids. Our report contradicts previous assumptions that concomitant GVHD is responsible for the development of NS, suggesting that NS can develop as a new, independent manifestation of GVHD.
Conflict of interest
None.
References
- 1.Terrier B, Delmas Y, Hummel A, Presne C, Glowacki F, Knebelmann B, Combe C, Lesavre P, Maillard N, Noel LH, Patey-Mariaud de Serre N, Nusbaum S, Radford I, Buzyn A, Fakhouri F. Post-allogeneic haematopoietic stem cell transplantation membranous nephropathy: clinical presentation, outcome and pathogenic aspects. Nephrol Dial Transplant. 2007;22:1369–1376. doi: 10.1093/ndt/gfl795. [DOI] [PubMed] [Google Scholar]
- 2.Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant. 2001;28:121–129. doi: 10.1038/sj.bmt.1703111. [DOI] [PubMed] [Google Scholar]
- 3.Flowers ME, Parker PM, Johnston LJ, Matos AV, Storer B, Bensinger WI, Storb R, Appelbaum FR, Forman SJ, Blume KG, Martin PJ. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100:415–419. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- 4.Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol. 2006;17:1995–2005. doi: 10.1681/ASN.2006020118. [DOI] [PubMed] [Google Scholar]
- 5.Abboud I, Peraldi MN, Hingorani S. Chronic kidney diseases in long-term survivors after allogeneic hematopoietic stem cell transplantation: monitoring and management guidelines. Semin Hematol. 2012;49:73–82. doi: 10.1053/j.seminhematol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Romagnani P, Lazzeri E, Mazzinghi B, Lasagni L, Guidi S, Bosi A, Cirami C, Salvadori M. Nephrotic syndrome and renal failure after allogeneic stem cell transplantation: novel molecular diagnostic tools for a challenging differential diagnosis. Am J Kidney Dis. 2005;46:550–556. doi: 10.1053/j.ajkd.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Brukamp K, Doyle AM, Bloom RD, Bunin N, Tomaszewski JE, Cizman B. Nephrotic syndrome after hematopoietic cell transplantation: do glomerular lesions represent renal graft-versus-host disease? Clin J Am Soc Nephrol. 2006;1:685–694. doi: 10.2215/CJN.00380705. [DOI] [PubMed] [Google Scholar]
- 8.Reddy P, Johnson K, Uberti JP, Reynolds C, Silver S, Ayash L, Braun TM, Ratanatharathorn V. Nephrotic syndrome associated with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:351–357. doi: 10.1038/sj.bmt.1705446. [DOI] [PubMed] [Google Scholar]
- 9.Hu SL. The role of graft-versus-host disease in haematopoietic cell transplantation-associated glomerular disease. Nephrol Dial Transplant. 2011;26:2025–2031. doi: 10.1093/ndt/gfq645. [DOI] [PubMed] [Google Scholar]
- 10.Hingorani S. Chronic kidney disease after pediatric hematopoietic cell transplant. Biol Blood Marrow Transplant. 2008;14:84–87. doi: 10.1016/j.bbmt.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Colombo AA, Rusconi C, Esposito C, Bernasconi P, Caldera D, Lazzarino M, Alessandrino EP. Nephrotic syndrome after allogeneic hematopoietic stem cell transplantation as a late complication of chronic graft-versus-host disease. Transplantation. 2006;81:1087–1092. doi: 10.1097/01.tp.0000209496.26639.cb. [DOI] [PubMed] [Google Scholar]
- 12.Bruijn JA, Hogendoorn PC, Corver WE, van den Broek LJ, Hoedemaeker PJ, Fleuren GJ. Pathogenesis of experimental lupus nephritis: a role for anti-basement membrane and anti-tubular brush border antibodies in murine chronic graft-versus-host disease. Clin Exp Immunol. 1990;79:115–122. doi: 10.1111/j.1365-2249.1990.tb05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miklos DB, Kim HT, Zorn E, Hochberg EP, Guo L, Mattes-Ritz A, Viatte S, Soiffer RJ, Antin JH, Ritz J. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graze PR, Gale RP. Chronic graft versus host disease: a syndrome of disordered immunity. Am J Med. 1979;66:611–620. doi: 10.1016/0002-9343(79)91171-9. [DOI] [PubMed] [Google Scholar]
- 15.Ruggenenti P, Chiurchiu C, Abbate M, Perna A, Cravedi P, Bontempelli M, Remuzzi G. Rituximab for idiopathic membranous nephropathy: who can benefit? Clin J Am Soc Nephrol. 2006;1:738–748. doi: 10.2215/CJN.01080905. [DOI] [PubMed] [Google Scholar]