Abstract
Purpose
The incidence of non-melanoma skin cancer (NMSC) has been increasing over the past 30 years. Basal cell carcinoma and squamous cell carcinoma are the two most common subtypes of NMSC. The aim of this study was to estimate tumour control, toxicity, and aesthetic events in elderly patients treated with high-dose-rate (HDR) brachytherapy (BT) using Valencia applicator.
Material and methods
From January 2012 to May 2015, 57 lesions in 39 elderly eligible patients were enrolled. All the lesions had a diameter ≤ 25 mm (median: 12.5 mm) and a depth ≤ 4 mm. The appropriate Valencia applicator, 2 or 3 cm in diameter was used. The prescribed dose was 40 Gy in 8 fractions (5 Gy/fraction) in 48 lesions (group A), and 50 Gy in 10 fractions (5 Gy/fraction) in 9 lesions (group B), delivered 2/3 times a week. The biological effective dose (BED) was 60 Gy and 75 Gy, respectively.
Results
After median follow-up of 12 months, 96.25% lesions showed a complete response and only two cases presented partial remission. Radiation Therapy Oncology Group – European Organization for Research and Treatment of Cancer (RTOG/EORTC) G 1-2 acute toxicities were observed in 63.2% of the lesions: 56.3% in group A and 77.7% in group B. Late G1-G2 toxicities was observed in 19.3% of the lesions: 18.8% in group A and 22.2% in group B, respectively. No G3 or higher acute or late toxicities occurred. In 86% of the lesions, an excellent cosmetic result was observed (87.5% in group A and 77.8% in group B). Six lesions had a good cosmetic outcome and only 2.3% presented a fair cosmetic impact.
Conclusions
The treatment of NMSC with HDR-BT using Valencia surface applicator is effective with excellent and good cosmetics results in elderly patients. The hypofractionated course appears effective and no statistical differences were observed between the two groups analysed.
Keywords: HDR brachytherapy, skin cancer, skin brachytherapy, Valencia applicator
Purpose
The incidence of skin cancer has been increasing over the past 30 years and currently 2-3 million new cases are diagnosed worldwide every year. Non-melanoma skin cancer (NMSC) is the most common skin malignancy (95%) and in recent years its incidence has been increasing rapidly, even in young populations [1, 2]. The development of NMSC is due to a combination of environmental, genetic, and phenotypic factors [3, 4]. Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the two most common subtypes: about 75-80% of all NMSC are characterized by the presence of BCC, 15-20% of these malignancies present SCCs, while 1% show a mixed phenotype [5]. There are different treatment options for NMSC such as surgery, cryotherapy, laser therapy (recommended only for shallow and early SCC), topical chemotherapy, photodynamic therapy, and radiotherapy (RT). Surgical excision is the most frequent treatment due to its low rates of recurrence, reported less than 5% [6, 7, 8, 9, 10]. In addition, RT is often used to treat NMSC and – specifically – different techniques can be used such as superficial X-rays, electron beams, megavoltage photons, and low-dose-rate (LDR) or high-dose-rate (HDR) brachytherapy (BT). Usually, the treatment options are chosen based on the institutional resources and the specialist's experiences. The introduction of new devices usable with the equipment of HDR-BT and the commercialization of electronic BT has attracted considerable interest in the BT treatment of small skin tumours. The Valencia applicator (Nucletron, an Elekta company, Stockholm, Sweden) is a new superficial device used in BT to treat skin lesions, and has been projected to be used with the HDR afterloader microSelectron (Nucletron, an Elekta company, Stockholm, Sweden) [11, 12, 13, 14]. The design of Valencia applicators is based on Leipzig applicators, adding to them a flattening filter to improve the dose rate distributions’ homogeneity and limit the penumbra. Regarding the dimensions, there are two sizes of Valencia applicators: 2 cm (VH2) and 3 cm (VH3) in diameter. The use of Valencia applicators is recommended for superficial tumours (less than 4 mm depth) with a maximum diameter of 25 mm due to guaranteeing adequate tumour coverage. The reinforced shielding at the back of the skin radiation applicator adds to patient safety during treatment, and plastic caps on the applicator help to avoid over-dosage and assist with correct applicator positioning [11, 12, 13]. The design of this applicator allows us to focus the radiation on the target while normal tissue irradiation is minimized, leading to safer treatment and a decrease in side effects in normal tissue [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24].
The aim of this study was to retrospectively estimate tumour control, toxicity, and cosmetic events in two case series of elderly patients, affected by NMSC treated with HDR BT using a Valencia applicator.
Material and methods
Patients’ eligibility
We retrospectively evaluated 57 lesions in 39 elderly patients treated with HDR-BT using a Valencia applicator. All the patients presented NMSC (confirmed by histological examination) and were treated at the Department of Radiotherapy, University of Pisa. All the enrolled patients were older than 70 years. Patients aged less than 70 years with a diagnosis of melanoma were excluded from the study; patients showing diameter lesions greater than 25 mm and a depth of more than 4 mm by clinical and imaging evaluation were also excluded. In this study, NMSC patients who were surgically treated or who had relapses or recidivisms were also included.
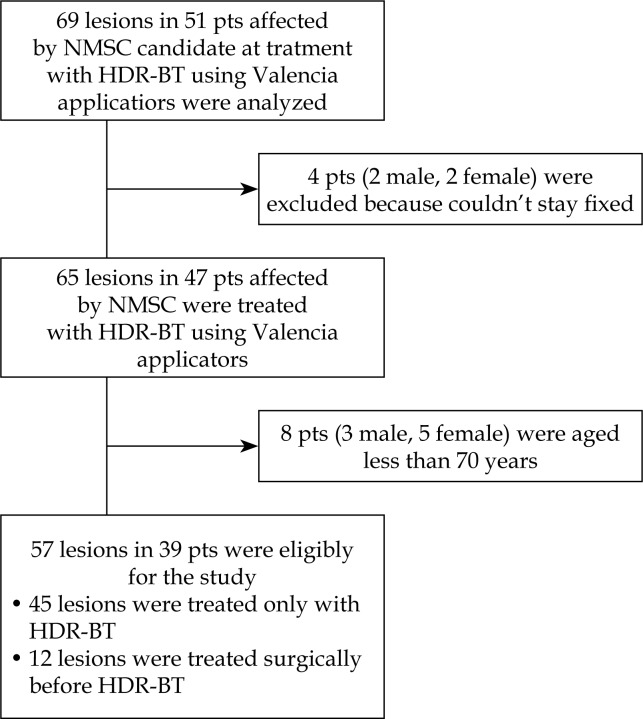
Furthermore, any patients who were unable to collaborate and stayed fixed during the treatment were excluded from the study. The cohort's characteristics are shown in Table 1 and a consort flow diagram is shown in Figure 1.
Table 1.
Patients and lesion characteristics
| Demographics | Number | % |
|---|---|---|
| Patient characteristics | ||
| Age (years) | ||
| Median | 84 | |
| Minimum | 70 | |
| Maximum | 96 | |
| Sex | ||
| Male | 24 | 61 |
| Female | 15 | 39 |
| Histology | ||
| Basal cell carcinoma | 44 | 77.2 |
| Squamous cell carcinoma | 12 | 21.1 |
| Kaposi's sarcoma | 1 | 1.7 |
| Lesion diameter (mm) | ||
| Minimum | 3 mm | |
| Maximum | 25 mm | |
| Median | 12.5 mm | |
| Lesion location | ||
| Head and Neck | 46 | 80.7 |
| Scalp | 18 | 31.6 |
| Face | 15 | 26.3 |
| Nose | 8 | 14 |
| Ear | 3 | 5.3 |
| Neck | 2 | 3.5 |
| Trunk | 7 | 12.3 |
| Extremity | 4 | 7 |
Fig. 1.
Consort flow diagram
Treatment procedure
All the lesions for the selected cases were limited to a maximum depth of 4 mm and a diameter equal to or less than 25 mm. This limitation was necessary to keep the skin dose at acceptable levels because the percentage depth dose of the 192Ir Valencia applicators has a gradient of about 10% per mm [14]. The planning target volume was defined as a BCC and SCC macroscopic lesion (gross tumour volume) adding an adequate margin of 5 mm. According to Brodland et al.'s [8] data and based on the other HDR-BT studies [18, 19, 20, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36], a margin of 5 mm for BCC and SCC lesions appears to be adequate. The maximal diameter able to be treated using Valencia applicators is 25 mm, since the largest surface applicator has an inner diameter of 30 mm. This is possible due to improvements in the lateral homogeneity and flatness of the Valencia applicator compared to the Leipzig applicators. The values of the penumbra (80-20%) are significantly improved from the Leipzig (6.2-9.5 mm) to the Valencia applicators (1.9 mm). As such, the useful treatment area of the Valencia applicator is larger and this allows a 5 mm margin for microscopic diseases and set-up errors in lesions with a diameter ≤ 25 mm [11, 12, 14]. The gross tumour volume (GTV) was generally visually assessed; however, ultrasound imaging or a CT scan were, in a few cases (n = 7 lesions), used to determine the real depth and lesion dimensions. An appropriate Valencia applicator – 2 or 3 cm of diameter – was chosen based on the diameter lesion for an optimal dose rate distribution to the GTV [14, 21]. The treatment dose prescription was 40 Gy in eight fractions (5 Gy for each fraction daily) in 48 lesions (group A) and 50 Gy in 10 fractions (5 Gy for each fraction daily) in nine lesions (group B). The total dose was chosen based on the lesion dimensions, age, and performance status. The dose prescription was delivered as two/three fractions a week, with a minimum interval of 48 hours between fractions. The treatment characteristics are shown in Table 2.
Table 2.
Treatment characteristics
| Treatment characteristics | Number of lesions | BCC | SCC and Kaposi's sarcoma |
|---|---|---|---|
| Total dose (Gy; BED α/β:10) | |||
| 50 Gy (BED75) | 9 (16%) | 6 | 3 |
| 40 Gy (BED60) | 48 (84%) | 38 | 10 |
| Applicator size (mm) | |||
| Valencia 20 | 28 (49%) | 20 | 8 |
| Valencia 30 | 29 (51%) | 24 | 5 |
BED – biological effective dose, BCC – basal cell carcinoma, SCC – squamous cell carcinoma
The Biological Effective Dose (BED) was (BED α/β:10) 60 Gy in group A and (BED α/β:10) 75 Gy in group B. All the patients were immobilized during the treatment and a skin marker delineating the outside applicator circumference was used in some patients to ensure reproducible treatment conditions. The immobilization was achieved in some cases (such as patients with head and neck lesions) using an articulated arm device provided by Nucletron; in less difficult cases, the methods of immobilization were tape or a thermoplastic mask. The treatment was effectuated under the direct supervision of the radiation oncologist for accurate applicator positioning and dose delivery. As a precaution, it was recommended for all patients to not wear any make-up and in addition they were continuously monitored during treatment by video camera and audio connection with a treatment room to ensure the immobility of the patient.
End points
The end points chosen for this study were analysis of efficacy, safety, toxicity, and cosmetic outcomes in elderly patients treated with HDR-BT using Valencia applicators. Acute and chronic toxicities were evaluated in both groups according to the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) scales by clinical evaluation during and after treatment. The cosmetic results were estimated at each follow-up visit based on the radiation therapy oncology group scale (Table 3) [25].
Table 3.
Cosmetic rating scale [25]
| Excellent | No changes to slight atrophy or pigment change or slight hair loss or no changes to slight induration or loss of subcutaneous fat |
| Good | Patch atrophy, moderate telangiectasia, and total hair loss; moderate fibrosis but asymptomatic; slight field contracture with less than 10% linear reduction |
| Fair | Marked atrophy and gross telangiectasia; severe induration or loss of subcutaneous tissue; field contracture greater than 10% linear measurement |
| Poor | Ulceration or necrosis |
Results
Patient and treatment characteristics
Between January 2012 and May 2015, 57 lesions in a total of 39 patients affected by NMSC were treated with HDR-BT using a Valencia surface applicator. Of them, 25 patients (61%) were male and 14 (39%) were female. Twelve lesions were treated as a supplementary therapy after surgery treatment (Figure 1). The median age of the patients treated was 84 years (DS ± 7.84) with a wide range from 70 to 96 years. Most of lesions (77.2%) histologically were BCC: 21.1% were SCC and one lesion was Kaposi's sarcoma. In addition, 46 lesions (80.7%) were located on the head and the neck: seven lesions (12.3%) on the trunk, and four lesions (7%) were found on the extremities. The median diameter of the lesion was 12.5 mm (range: 3-25) and all lesions had a depth of less than 4 mm. Table 1 describes the patient and lesion characteristics. During the treatment, we used 29 applicators with a diameter of 3 cm and 28 applicators with a diameter of 2 cm, as shown in Table 2. The median follow-up was 12 months (range: 3-29 months).
Efficacy
After 12 months median follow-up, 55 lesions (96.5%) completely regressed and only two lesions persisted: one lesion was histologically diagnosed as SCC, located in the trunk, and received a total dose of 50 Gy delivered in 10 fractions. The second lesion was instead diagnosed as BCC, and it was located in the face, treated with a total dose of 40 Gy in eight fractions. In both lesions, the depth of the lesion was less than 4 mm and the tumour diameter was less than 25 mm. No recurrences or disease persistence were detected during the follow-up and there was no difference between the two groups (Table 4).
Table 4.
Results
| All lesions | Group A (40 Gy in 8 faction) |
Group B (50 Gy in 10 fraction) |
|
|---|---|---|---|
| Response to treatment | |||
| Complete response | 55 (96.25%) | 47 | 8 |
| Partial response | 2 (3.5%) | 1 (BCC) | 1 (SCC) |
| Recurrence | 0 | 0 | 0 |
| Acute toxicity | 36 (63.2%) | 29 (60.4%) | 7 (77.7%) |
| Grade 1 | 33 (58%) | 27 (56.3%) | 6 (66.7) |
| Grade 2 | 3 (5.3%) | 2 (4.1%) | 1 (11.1%) |
| Grade 3 | 0 | 0 | 0 |
| Late toxicities | 11 (19.3%) | 9 (18.8%) | 2 (22.2%) |
| Grade 1 | 10 (17.5%) | 8 (16.7%) | 2 (22.2%) |
| Grade 2 | 1 (1.9%) | 1 (2.1%) | 0 |
| Grade 3 | 0 | 0 | 0 |
| Cosmetic results | |||
| Excellent | 49 (86%) | 42 (87.5%) | 7 (77.8%) |
| Good | 7 (12.3% | 5 (10.4%) | 2 (22.2%) |
| Fair | 1 (1.7%) | 1 (2.1%) | 0 |
| Poor | 0 | 0 | 0 |
Adverse events and cosmetic results
The treatment was well tolerated in all cases. The most common early side-effects were erythema, rash dermatitis, and pruritus, which occurred in 63.2% of the patients. The highest skin acute toxicity was Grade 1 RTOG/EORTC [25] and occurred in 58% of the lesions: 56.3% of the lesions in group A and 66.7% of the lesions in group B. Only three (5.3%) lesions had Grade 2 toxicities: 4.1% in group A and 11.1% in group B. All the cases of G1-G2 acute toxicity were resolved with topical treatment. No statistically differences were observed between the two groups analysed regarding acute toxicities (p = 0.269).
On the subject of late toxicities, there were 11 cases of G1-G2 late toxicities: G1 was observed in 16.7% of the lesions in group A and 22.2% of the lesions in group B. Only one case of G2 late toxicity in group A was observed. They were all resolved with adequate local treatment and no statistical differences existed between the two groups (p = 0.404). There were no Grade 3 or higher acute or late toxicities.
The cosmetic results were evaluated at each follow-up visit based on the radiation therapy oncology group scale (Table 3) [25]. An excellent cosmetic result was observed in 86% of lesions: 87.5% in group A and 77.8% in group B (Table 4 results; Figure 2).
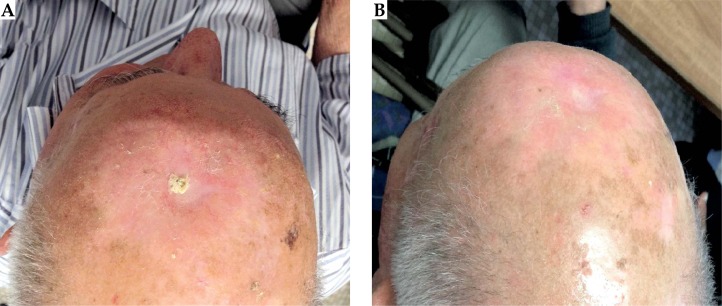
Fig. 2.
An example of complete response (B) after nine months of treatment of NMSC with HDR-BT using a Valencia applicator
About 12.3% of the lesions had good cosmetic results (moderate atrophy in three patients and moderate asymptomatic fibrosis in another four): five (10.4%) in group A and two (22.2%) in group B.
Only one patient 1.7% (in group A) presented a fair cosmetic result. There was no correlation between the two groups and excellent cosmetic results (p = 0.458). Finally, there were no cases of poor cosmetic results.
With univariate statistical analysis of local control prognostic factors, only the total dose prescribed was statistically significant (p = 0.001), leading to improve local control. This means that with increasing the total dose – with an equal dose for fractions – local control probability is improved. Other prognostic factors at regression univariate analysis, such as lesion dimensions, histology, and surgery did not lead to improved disease local control (Table 5).
Table 5.
Univariate analysis of local control prognostic factors
| Factor | Intercept | B* | Wald test | p value |
|---|---|---|---|---|
| Lesion dimension (3-25 mm) | 11,663 | –0,31 | 0,034 | 0,854 |
| Histology (SCC, SCC, Kaposi's sarcoma) | 11,949 | –1,656 | 2,015 | 0,156 |
| Surgery (yes, no) | 8,484 | 2,044 | 1,062 | 0,303 |
| Total dose (40 Gy, 50 Gy) | 10,021 | 7,979 | 10,799 | 0,001 |
Regression coefficient; BCC – basal cell carcinoma, SCC – squamous cell carcinoma
The statistical analyses data were performed with SPSS version 22 (SPSS Inc. SPSS® Chicago, IL, USA). All the variables were described by statistical characteristics: categorical data were described by frequency and percentage, whereas continuous data were described by mean and range. The study of local control prognostic factors such as lesion dimensions, histology type, surgery, and total dose was performed through the use of a univariate generalized linear model. The results of the regression model were calculated by a Wald test and expressed using the regression coefficients. Differences were considered significant at p < 0.05.
Discussion
Surgery is often the primary treatment for NMSC lesions for the low rates of recurrence reported [6, 7, 8, 9, 10]. However, surgical treatment is an invasive procedure and in elderly patients is not always feasible because of comorbidities, performance status, or lesion location (near the eyes, nose, and on facial skin). Typically, RT is the treatment of choice in this class of patients since surgery might be accompanied with functional or cosmetic deficits. The development of new devices for small skin tumour treatment and the introduction of commercial electronic BT, have attracted considerable interest for BT as a skin cancer treatment. Despite the new technologies available, few studies have focused on the treatment of NMSC with HDR-BT. Köhler et al. [13] in 1999 described the outcome of 520 lesions treated with HDR-BT using Leipzig applicators. The dose prescribed was 30-40 Gy in 5-10 fractions, and after 10 years follow-up local control was 92% of the cases; only G1-G2 late and acute toxicities were observed. In the study was included Kaposi's sarcoma, melanomas, and skin metastases. One year later, Guix et al. [24] reported the results of 236 NMSC lesions treated with HDR-BT using custom-made surface moulds. At five years median follow-up, local control was 98%. In addition, Gauden et al. [31] published the data of 236 lesions using Leipzig applicators. The total dose prescribed was 36 Gy in 12 fractions and the local control was 98% after 36 months follow-up. No G3 or higher late or acute toxicities were observed. Recently, Bhatnagar et al. [32] and Tormo et al. [36] published the results of a hypofractionated course (using Valencia applicators and HDR electronic BT with surface applicators, respectively), which resulted in excellent local control, cosmetic results, and very low-grade toxicities after a median follow-up of 47 months and 12 months, respectively. Table 6 describes some previous studies of HDR-BT for NMSC.
Table 6.
Summary of some previous studies of high-dose-rate brachytherapy (HDR-BT) for non-melanoma skin cancer (NMSC)
| Study | Modality | No. of lesions | Lesion type (number) | Dose Gy | Fractions | Follow-up (median) | Recurrence rate | Cosmetic results | Toxicities |
|---|---|---|---|---|---|---|---|---|---|
| Köhler et al. [13] | HDR-BT using Leipzig applicator | 520 | SCC BCC Kaposi's sarcoma Melanoma Lymphomas |
30-40 | 6-4 | 10 years | 8% | – | G1-G2 |
| Guix et al. [24] | HDR-BT using custom-made surface molds | 136 | BCC (102) SCC (34) |
60-65 | 33-36 | 5 years | 2% | Excellent | G1-2 |
| Ghaly et al. [18] | HDR-BT using Leipzig applicator | 21 | SCC BCC |
40 | 8 | 18 months | No recurrence (2 lesions persisted) | Excellent | G1-G2 |
| Gauden et al. [31] | HDR BT using Leipzig applicator | 236 | BCC (121) SCC (115) |
36 | 12 | 66 months | 2% | Poor 5.5% Fair 6.5% Good 26% Excellent 62% |
G1-G2 |
| Bhatnagar et al. [32] | HDR electronic brachytherapy using surface applicators | 171 | BCC (91) SCC (70) Lymphoma (3) Merkel cell (2) Basosquamous (1) Not available (4) |
40 | 8 | 12 months | No recurrence | Excellent 92.9% Good 7.1% |
G1-G2 |
| Tormo et al. [36] | HDR-BT using Valencia applicator | 45 | BCC (45) | 42 | 6-7 | 47 months | 2.2% | Excellent | G1 |
| Current study | HDR-BT using Valencia applicator | 57 | BCC (44) SCC (12) Kaposi's sarcoma (1) |
40-50 | 8-10 | 12 months | No recurrence (2 lesions persisted) | Excellent 86% Good 12.6% Fair 1.7% |
G1-G2 |
HDR-BT – high-dose-rate brachytherapy, BCC – basal cell carcinoma, SCC – squamous cell carcinoma
We showed in our study that the treatment of 57 NMSC lesions with HDR-BT using Valencia surface applicator with doses of 50 Gy and 40 Gy in 10 and eight fractions is effective and safe in elderly patients. In this study, the BED was evaluated and in particular the BED values were BED 60 in group A and BED 75 in group B. Biological effective dose is an inherent part of the linear quadratic (LQ) model of radiation effects, and estimates the true biological dose delivered by a particular combination of dose per fraction and total dose to a given tissue characterized by a specific a/b ratio. It is calculated by the equation BED = nd [1 + d(α/β)], where n = the number of fractions, d = the dose/fraction, and α/β = radio-sensitivity coefficients at the dose at which the linear and quadratic components (for early or late cell damage, respectively) of cells killed are equal [33, 34].
The a/b ratios vary based on the tumour type. For example, squamous cell cancers with high cell proliferation are characterized by 10-30 α/β ratio, while breast cancer shows lower values (4-5 Gy) [34] as well in prostate cancer (0.8-2.5 Gy) [35] and melanoma malignancies [34]. For NMSC, the alpha/beta ratios are approximately 10 Gy [34]. From the previous equation, it is evident that the BED will increase proportionally to the dose per fraction and inversely proportional to the α/β ratio. If the total dose is kept constant, the BED will increase if the dose per fraction is increased [33, 34, 35]. For these reasons, it is important to perform BED calculations before clinical decisions since different histological classes of cancers have different a/b ratios, leading to different clinical responses, despite the total dose not change. The hypofractionated course (40-50 Gy in 8-10 fractions delivered two/three time a week with a minimum interval of 48 hours between fractions) appears to be effective with very good local control, excellent cosmetic results, and acceptable toxicities in elderly patients. No recurrences after 12 months follow-up have been observed at the time of the analysis, and overall, the treatment was very well tolerated with no evidence of Grade 3 or higher toxicities.
The limitation of this study compared with studies of more established treatments for NMSC was the relatively short follow-up and small number of patients due to the age of the patients (mean age 84 years) aa well as comorbidities. In particular, patients exhibited a low life expectancy and important comorbidities such as cardiovascular and pulmonary complications (due to age rather than therapy), which did not allow a long follow-up in all patients. Non-melanoma skin cancer patients will continue to be followed and additional patients will be enrolled for further study of the outcomes using HDR-BT.
Conclusions
In our study, the treatment of NMSC with HDR-BT using Valencia surface applicator was effective and safe in elderly patients. After 12 months follow-up, no recurrences were observed and the treatment was very well tolerated with no Grade 3 or higher acute or late toxicities. In addition, we found excellent and good cosmetics results. Valencia applicators provide a simple, safe, quick, and easy alternative for skin cancer treatment compared with more invasive methods, such as surgery or cryotherapy, in this subset of patients. Overall, the hypofractionated course appears effective with very good local disease control; moreover, this cost effective therapy shows high compliance and a feasible outpatient treatment regimen, essential in elderly patients. No statistical differences were observed between the two groups analysed regarding efficacy, acute toxicities, late toxicities, and cosmetic results.
Acknowledgments
The authors declare that an abstract with preliminary data (a total of 52 lesions) has been accepted as a poster (abstract number 3 316) at 18th ECCO – 40th ESMO European Cancer Congress held in Vienna, Austria, 25th – 29th September 2015.
Disclosure
Authors report no conflict of interest.
References
- 1.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 2.Eisemann N, Waldmann A, Geller AC, et al. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol. 2014;134:43–50. doi: 10.1038/jid.2013.304. [DOI] [PubMed] [Google Scholar]
- 3.Kricker A, Armstrong BK, English DR, et al. Does intermittent sun exposure cause basal cell carcinoma. A case-control study in Western Australia. Int J Cancer. 1995;60:489–494. doi: 10.1002/ijc.2910600411. [DOI] [PubMed] [Google Scholar]
- 4.Rosso S, Zanetti R, Martinez C, et al. The multicentre south European study ‘Helios’. I: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer. 1996;73:1440–1446. doi: 10.1038/bjc.1996.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katalinic A, Kunze U, Schafer T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer) Br J Dermatol. 2003;149:1200–1206. doi: 10.1111/j.1365-2133.2003.05554.x. [DOI] [PubMed] [Google Scholar]
- 6.Caresana G, Giardini R. Dermoscopy-guided surgery in basal cell carcinoma. J Eur Acad Dermatol Venereol. 2010;24:1395–1399. doi: 10.1111/j.1468-3083.2010.03652.x. [DOI] [PubMed] [Google Scholar]
- 7.Smeets NW, Kuijpers DI, Nelemans P, et al. Mohs’ micrographic surgery for treatment of basal cell carcinoma of the face – results of a retrospective study and review of the literature. Br J Dermatol. 2004;151:141–147. doi: 10.1111/j.1365-2133.2004.06047.x. [DOI] [PubMed] [Google Scholar]
- 8.Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992;27:241–248. doi: 10.1016/0190-9622(92)70178-i. [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane L, Waters A, Evans A, et al. Seven years’ experience of Mohs micrographic surgery in a UK centre, and development of a UK minimum dataset and audit standards. Clin Exp Dermatol. 2013;38:262–269. doi: 10.1111/ced.12108. [DOI] [PubMed] [Google Scholar]
- 10.Chren MM, Torres JS, Stuart SE, et al. Recurrence after treatment of non melanoma skin cancer: a prospective cohort study. Arch Dermatol. 2011;147:540–546. doi: 10.1001/archdermatol.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Calatayud J, Granero D, Ballester F, et al. A dosimetric study of the Leipzig applicators. Int J Radiat Oncol Biol Phys. 2005;62:579–584. doi: 10.1016/j.ijrobp.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Niu H, Hsi WC, Chu JC, et al. Dosimetric characteristics of the Leipzig surface applicators used in the high dose rate brachy radiotherapy. Med Phys. 2004;31:3372–3327. doi: 10.1118/1.1812609. [DOI] [PubMed] [Google Scholar]
- 13.Köhler-Brock A, Prager W, Pohlmann S, et al. The indications for and results of HDR afterloading therapy in diseases of the skin and mucosa with standardized surface applicators (the Leipzig Applicator) Strahlenther Onkol. 1999;175:170–174. [PubMed] [Google Scholar]
- 14.Granero D, Perez-Calatayud J, Ballester F, et al. Radiation leakage study for the Valencia applicators. Phys Med. 2013;29:60–64. doi: 10.1016/j.ejmp.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Granero D, Pérez-Calatayud J, Gimeno J, et al. Design and evaluation of a HDR skin applicator with flattening filter. Med Phys. 2008;35:495–503. doi: 10.1118/1.2825622. [DOI] [PubMed] [Google Scholar]
- 16.Kowalik L, Lyczek J, Sawicki M, et al. Individual applicator for brachytherapy for various sites of superficial malignant lesions. J Contemp Brachytherapy. 2013;5:45–49. doi: 10.5114/jcb.2013.34340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang IM, Lin SY, Lin LC, et al. Alternative effective modality of Leipzig applicator with an electron beam for the treatment of superficial malignancies. Nuc Inst Meth A. 2003;508:460–466. [Google Scholar]
- 18.Ghaly M, Birnes R, Musmacher J, et al. HDR brachytherapy with standardized surface applicators (the Leipzig applicator) as an alternative, radiotherapy treatment for superficial malignant skin lesions. Int J Radiat Oncol Biol Phys. 2006;66:S719–S720. [Google Scholar]
- 19.Svoboda V, Kovarik J, Morris F. High dose-rate microselectron molds in the treatment of skin tumors. Int J Radiat Oncol Biol Phys. 1995;31:967–972. doi: 10.1016/0360-3016(94)00485-4. [DOI] [PubMed] [Google Scholar]
- 20.Ghaly M, Zinkin H, Dannenberg M, et al. HDR brachytherapy with Standardized Surface Applicators in the Treatment of Superficial Malignant Skin Lesions. Int J Radiat Oncol Biol Phys. 2008;72:S505–S506. [Google Scholar]
- 21.Khan L, Choo R, Breen D, et al. Recommendations for CTV margins in radiotherapy planning for non melanoma skin cancer. Radiother Oncol. 2012;104:263–266. doi: 10.1016/j.radonc.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Lovett RD, Perez CA, Shapiro SJ, et al. External radiation of epithelial skin cancer. Int J Radiat Oncol Biol Phys. 1990;19:235–242. doi: 10.1016/0360-3016(90)90529-s. [DOI] [PubMed] [Google Scholar]
- 23.Alam M, Nanda S, Mittal BB, et al. The use of brachytherapy in the treatment of nonmelanoma skin cancer: a review. J Am Acad Dermatol. 2011;65:377–388. doi: 10.1016/j.jaad.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Guix B, Finestres F, Tello J, et al. Treatment of skin carcinomas of the face by high dose rate brachytherapy and custom made surface molds. Int J Radiat Oncol Biol Phys. 2000;47:95–102. doi: 10.1016/s0360-3016(99)00547-7. [DOI] [PubMed] [Google Scholar]
- 25.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 26.Fabrini MG, Perrone F, De Liguoro M, et al. High dose rate brachytherapy in a large squamous cell carcinoma of the hand. Brachytherapy. 2008;7:270–275. doi: 10.1016/j.brachy.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Ballester-Sánchez R, Pons-Llanas O, Llavador-Ros M, et al. Depth determination of skin cancers treated with superficial brachytherapy: ultrasound vs. histopathology. J Contemp Brachytherapy. 2015;6:356–361. doi: 10.5114/jcb.2014.47860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montero A, Hernanz R, Capuz AB, et al. High-dose-rate (HDR) plesiotherapy with custom-made moulds for the treatment of non-melanoma skin cancer. Clin Transl Oncol. 2009;11:760–764. doi: 10.1007/s12094-009-0439-2. [DOI] [PubMed] [Google Scholar]
- 29.Maroñas M, Guinot JL, Arribas L, et al. Treatment of facial cutaneous carcinoma with high dose rate contact brachytherapy with customized molds. Brachytherapy. 2011;10:221–227. doi: 10.1016/j.brachy.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Kanikowski M. HDR brachytherapy of skin cancer in material of Greater Poland Cancer Center. J Contemp Brachytherapy. 2009;1:197. (Abstract) [PMC free article] [PubMed] [Google Scholar]
- 31.Gauden R, Pracy M, Avery AM, et al. HDR brachytherapy for superficial non-melanoma skin cancers. Radiat Oncol. 2013;57:212–217. doi: 10.1111/j.1754-9485.2012.02466.x. [DOI] [PubMed] [Google Scholar]
- 32.Bhatnagar A. Nonmelanoma skin cancer treated with electronic brachytherapy: results at 1 year. Brachytherapy. 2013;12:134–140. doi: 10.1016/j.brachy.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Jones B, Dale RG, Deehan C, et al. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol (R Coll Radiol) 2001;13:71–81. doi: 10.1053/clon.2001.9221. [DOI] [PubMed] [Google Scholar]
- 34.Jones B, Dale RG. Mathematical models of tumour and normal tissue response. Acta Oncol. 1999;38:883–893. doi: 10.1080/028418699432572. [DOI] [PubMed] [Google Scholar]
- 35.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 36.Tormo A, Celada F, Rodriguez S, et al. Non-melanoma skin cancer treated with HDR Valencia applicator: clinical outcomes. J Contemp Brachytherapy. 2014;6:167–172. doi: 10.5114/jcb.2014.43247. [DOI] [PMC free article] [PubMed] [Google Scholar]