Abstract
Purpose
Palliation of dysphagia is a challenge in advanced esophageal cancer. The addition of external beam radiotherapy (EBRT) to intraluminal brachytherapy (ILBT) has shown significant improvement in dysphagia relief and symptom scores. The aim of the present study was to evaluate the efficacy of combined use of ILBT and EBRT.
Material and methods
The medical records of 148 patients with advanced/metastatic esophageal cancer were screened from January 2008 to April 2014, and 74 patients were found eligible for the analysis. All patients received two fractions of 8 Gy each of ILBT, followed by EBRT. Patients were assessed for the symptom scores of dysphagia, odynophagia, regurgitation, and chest pain and weight was recorded periodically.
Results
For a median follow-up of 6 months, the median OS was 9.5 months (95% CI: 7.5-10.5). The median dysphagia free survival was 6 months (95% CI: 4.8-7.1). The scores for dysphagia significantly improved after completion of 1st ILBT (p = 0.000), 2nd ILBT (p = 0.000), and at 3 months after EBRT compared to ILBT (p = 0.02). Overall 47% had improvement in dysphagia scores and 35% maintained the improvement of scores till last follow up. There was significant improvement in weight after completion of ILBT (p = 0.001) and at 3 months after completion of EBRT (p = 0.00). Twenty nine (39%) patients required nasogastric (NGT) insertions and 12 (16%) needed hospitalization for supportive care. 36.4% had complications in the form of stricture (27%), fistula (5.4%), and bleeding (4%).
Conclusions
Palliative radiotherapy is an effective alternative for palliation of dysphagia with improvement in symptom scores being evident and sustained. The results of this clinical audit were comparable with those from the trial setting.
Keywords: esophageal cancer, intraluminal brachytherapy, EBRT, palliative treatment
Purpose
Esophageal cancer is the eighth most common cancer worldwide and the sixth most common cause of death [1]. The incidence is higher in developing countries in Asia and in Africa. In India, it is reported to be the sixth most common cancer among males and eighth most common cancer among females [1]. About 50-80% of patients have locally advanced disease at presentation or have distant metastasis. Majority of them are in poor general condition. The prognosis is poor with a median survival ranging from 4-6 months [2].
Dysphagia is the most common debilitating symptom seen at presentation and remains up to patient's death [3]. Effective palliation of dysphagia is the primary goal of treatment to ensure adequate quality of remaining life. Various modalities have been used for palliation of dysphagia like bypass surgery [4], laser [5], photodynamic therapy [6], stent placement [7], chemotherapy [8], external beam radiotherapy (EBRT) [9], intraluminal brachytherapy (ILBT) [10, 11], and chemoradiation [12]. External beam radiotherapy alone or in combination with ILBT, or ILBT alone have all been tried. These have given results at par with other modalities with median overall survival (OS) ranging from 5 to 9 months [13]. Studies have examined whether the addition of EBRT could further improve the outcomes of palliative ILBT. The IAEA pilot trial by Sur et al. compared HDR ILBT vs. HDR ILBT plus EBRT to estimate any benefit in dysphagia relief. There was improvement in dysphagia relief but this was not statistically significant [14]. The results of the phase III IAEA trial by Rosenblatt et al. showed significant improvement in dysphagia relief and symptom scores with addition of EBRT to ILBT [15]. However, the results of a trial may often not be reproducible in the general population.
The aim of the present study was to evaluate whether the palliative RT schedule described by Rosenblatt et al. could be used in the clinic and to evaluate its efficacy, duration of palliation, and its complications in terms of need for any other interventions/hospitalizations after palliative radiotherapy (RT).
Material and methods
Study design
We conducted a retrospective review of patients with advanced/metastatic esophageal cancer who were referred for palliative radiotherapy. The medical records of 148 patients treated from January 2009 to April 2014 were screened, and 74 patients who received ILBT and EBRT were found eligible for the analysis. The baseline investigations for purpose of staging were routine blood tests, endoscopy to ascertain extent of disease, computed tomography (CT) thorax and abdomen, and biopsy for confirmation of diagnosis. Positron emission tomography (PET) – CT was also done in the majority of patients as a part of the initial workup. Patients were staged using the TNM system.
The decision for palliative RT was made after evaluation in a multidisciplinary tumor board comprising of radiation oncologists, surgical oncologists, and medical oncologists. The patients were considered for palliative RT if they had poor general condition, advanced age, loco regionally advanced disease not amenable to curative treatment, or metastatic disease when the predominant symptom was dysphagia/odynophagia.
All patients were treatment naïve. Suitability for ILBT was assessed on endoscopy and barium swallow. Patients with a stenotic growth, disease within 2 cm of the cricopharynx or involving the gastroesophageal junction, and those with a fistula were excluded. Only those patients who were eligible for ILBT followed by EBRT and received the same were evaluated.
The patients were assessed prior to commencement of radiation. Weight and symptom scores for dysphagia, odynophagia, regurgitation, and pain (DORP) were recorded. All patients underwent ILBT followed by EBRT. The treatment regimen was: 2 fractions of HDR-ILBT (8 Gy each), 1 week apart. Each ILBT session consisted of placement of the ILBT applicator of 6-8 mm diameter in the lumen across the lesion under endoscopic guidance. X-ray imaging was done to confirm catheter position and determine dummy source positions. The treatment length included the tumor with 2 cm margin craniocaudally. The lesion was localized with information from the diagnostic endoscopy, Barium swallow, and CT scan. The prescribed dose was 8 Gy at 1 cm from the active mid-source or mid dwell position without optimization (equal dwell times) [16]. This was followed by EBRT to a dose of 30 Gy/10 fractions/2 weeks or 20 Gy/5 fractions/1 week within 1-2 weeks of completion of 2nd ILBT. The treatment was delivered with anterior and posterior portals with dose prescribed to the midline. The upper and lower borders were 3 cm margins superior and inferior to the initial tumor extension. Radiation treatment methods and margins were as per the trial by Rosenblatt et al. [15].
All patients were followed up at 3 monthly intervals and regularly till death. A complete general physical examination, symptom scoring, weight, and performance status were recorded at each visit. Endoscopy was done at first follow-up at 3 months and a Barium swallow at subsequent visits. Patients were assessed for the symptoms scores of dysphagia, odynophagia, regurgitation, and chest pain at baseline, after 1st ILBT, after 2nd ILBT, 3 months after completion of EBRT (first follow-up visit and at the event: death/symptom progression/need for intervention). Scores were determined by the physicians interviewing the patients. The definition of scores used to measure dysphagia, odynophagia, regurgitation, and chest-back pain was as per the trial by Rosenblatt et al. [15] (Table 1).
Table 1.
Definition of scores for the symptoms
| Symptoms | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Dysphagia | Able to eat normal diet | Able to swallow some solids | Able to swallow only semisolid foods | Able to swallow liquids only | Unable to swallow anything/complete obstruction |
| Odynophagia | No pain on swallowing | Mild pain | Moderate pain | Severe pain | – |
| Regurgitation | None | Infrequent | Frequent | Constant | – |
| Chest pain | None | Relieved by non-narcotics | Relieved by narcotics | Not relieved by narcotics | – |
During follow-up, local and distant failures were recorded with their dates of occurrence. The duration of relief was recorded. Other endpoints assessed were: need for a nasogastric tube (NGT), weight loss/gain, need and timing of other interventions like serial dilatation, NGT/stent placement/chemotherapy for progression of dysphagia/hospitalizations for supportive care for dysphagia progression.
Statistical methods
The end points for the study were duration of dysphagia relief, overall survival (OS), and dysphagia free survival (DFS). Dysphagia relief was defined as duration of improvement (decrease) in dysphagia score when compared to baseline scores from completion of 1st ILBT to the time of symptom progression or need for intervention. The follow-up time was calculated from the date of diagnosis to the event of interest. For OS, death due to any cause was considered an event and for DFS, time to worsening of dysphagia scores, progression or death due to disease, whichever occurred first. SPSS version 21 was used for all statistical analyses. Survival curves for OS and DFS were estimated using the Kaplan-Meier method, and statistical differences were evaluated using the 2-sided log-rank test. The Cox regression model was used to estimate the hazard ratio (HR), and the corresponding 95% confidence interval (CI) for univariate and multivariate analyses. The reported p values were 2-tailed with p value of < 0.05 being considered significant. χ2 test was used to document the change of symptoms scores and t test was used to compare the change in weight.
Results
Among 148 patients with advanced esophageal cancer receiving palliative radiotherapy from January 2008 to April 2014, 74 (54%) were eligible for the study.
The age of these 74 patients ranged between 37 to 88 years with a median age of 65 years. Sixty five (87.8%) patients had a KPS of ≥ 70. Mean baseline weight was 44.37 kg (range: 23-70 kg) (Table 2).
Table 2.
Baseline characteristics of patients
| N (%) | |
|---|---|
| Age | |
| < 65 years | 36 (48.6) |
| ≥ 65 years | 38 (51.35) |
| Gender | |
| Male | 40 (54.1) |
| Female | 34 (45.9) |
| Karnofsky Performance Scale (KPS) | |
| < 70 | 9 (12.2) |
| 70 | 37 (50) |
| 80 | 24 (32.4) |
| 90 | 4 (5.4) |
Forty four (59.5%) and 26 (35.1%) patients had grade 2 and 3 dysphagia, respectively at baseline. Grade 1 and 2 odynophagia was seen only in 23 (31.1%) and 18 (24.3%) patients. Forty patients (54.1%) had mild pain relieved with non-narcotics and none had moderate or severe pain. Nine patients (12.2%) had cachexia (5-10 kg weight loss) and 19 (25.7%) had < 5 kg weight loss (Table 3).
Table 3.
Baseline symptoms of patients
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| Dysphagia | – | 2 (2.7%) | 44 (59.5%) | 26 (35.1%) | 2 (2.7%) |
| Odynophagia | 30 (40.5%) | 23 (31.1%) | 18 (24.3%) | 3 (4.1%) | – |
| Regurgitation | 39 (52.7%) | 34 (45.9%) | 1 (1.4%) | – | – |
| Pain | 34 (45.9%) | 40 (54.1%) | – | – | – |
| Vomiting | 3 (4.1%) | ||||
| Median weight (SD) | 44 kg (10), range 23-70 | ||||
| Weight loss | Nil; 46 (62.2%) | 1-5 kg; 19 (25.7%) | 5-10 kg; 9 (12.2%) | ||
| Hoarseness of voice | 6 (8.1%) | ||||
| Anorexia | 4 (5.4%) | ||||
| Neck swelling | 3 (4.05%) | ||||
| Cough | 2 (2.7%) | ||||
| Fatigue | 3 (4.1%) | ||||
Middle 1/3 of esophagus was the most common site of involvement (77%) followed by lower 1/3 (21.6%). The median lesion length was 6 cm (range: 2-15 cm). Majority were T3 lesions (79.7%) followed by T4 (18.9%). Forty patients (54.1%) had metastatic disease. Histologically poorly differentiated squamous cell carcinoma was the most common (Table 4).
Table 4.
Disease characteristics of patients
| Site | Upper 1/3 | Middle 1/3 | Lower 1/3 | |
| 1 (1.4%) | 57 (77%) | 16 (21.6%) | ||
| Lesion length | Range: 2-15 cm (median = 6) | |||
| TNM staging | ||||
| T | T1 | T2 | T3 | T4 |
| – | 1 (1.4%) | 59 (79.7%) | 14 (18.9%) | |
| N | N0 | N1 | N2 | N3 |
| 7 (9.5%) | 41 (55.4%) | 17 (23%) | 9 (12.2%) | |
| M | M0 | M1 | ||
| 34 (45.9%) | 40 (54.1%) | |||
| Metastatic sites | ||||
| Lung | 14 | |||
| Liver | 7 | |||
| Bone | 4 | |||
| Lymph nodes | 14 | |||
| Adrenal | 2 | |||
| Peritoneum | 1 | |||
| Muscle | 1 | |||
| Stage | III A | III B | III C | IV |
| 18 (24.32%) | 6 (8.1%) | 10 (13.5%) | 40 (54.1%) | |
| Morphology | Ulcero-proliferative | Ulcero-infiltrative | ||
| 58 (78.4%) | 16 (21.6%) | |||
| Histology | NOS SCC | MD SCC | PD SCC | Adenocarcinoma |
| 27 (36.5%) | 14 (18.9%) | 31 (41.9%) | 2 (2.7%) | |
NOS – not otherwise specified, MD – median, PD SCC – poorly differentiated squamous cell carcinoma
Thirty (40.5%) patients needed nasogastric tube prior to start of treatment. The mean time taken to initiate ILBT from diagnosis was 32.11 days (range: 3-180 days). All patients received 2 fractions of ILBT. The average length treated was 10.9 cm (range: 7-18 cm). The time between 2 fractions of ILBT ranged from 5-14 days (median 7 days) with only 2 patients (0.02%) exceeding 7 days due to scheduling difficulties. All patients received EBRT with 65 (87.8%) receiving 30 Gy/10 fractions, and 9 (12.2%) receiving 20 Gy/5 fractions. The average field width was 8.5 cm (range: 7-17) and average field length was 14.6 cm (range: 9-27.5). Telecobalt was used in 71 (95.9%) and 15 MV photons in 3 patients (4.1%). The mean time interval between completion of ILRT and initiation of EBRT was 9.6 days (range: 2-50 days). Three patients had gaps during treatment ranging from 1-8 days (Table 5).
Table 5.
Treatment details of patients
| Length treated during ILBT | 7-18 cm, median 11 cm | |
| Nasogastric tube insertion prior to treatment | 30 (40.5%) | |
| EBRT dose | 30 Gy/10 fractions | 20 Gy/5 fractions |
| 65 (87.8%) | 9 (12.2%) | |
| Treatment gaps | 3 (4.05%), range 1-8 days | |
| Field width | Mean 8.4 cm (range 7-17) | |
| Field length | Mean 14.6 cm (range 9-27.5) | |
| Energy | Cobalt | Linac 15 MV |
| 71 (95.9%) | 3 (4.1%) | |
ILBT – intraluminal brachytherapy, EBRT – external beam radiotherapy
Follow-up and outcome
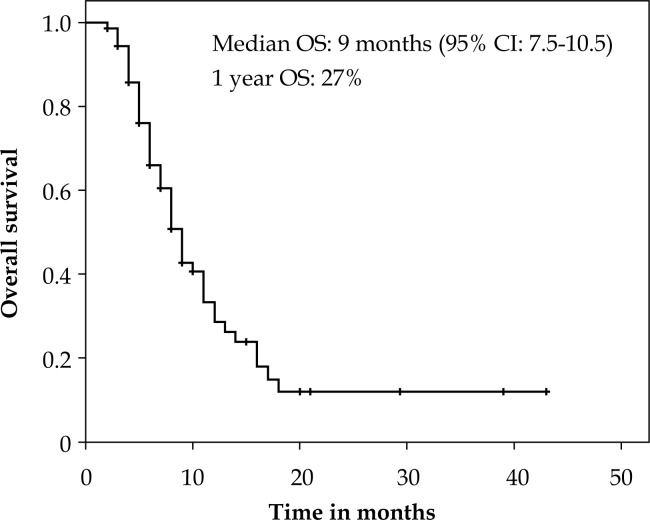
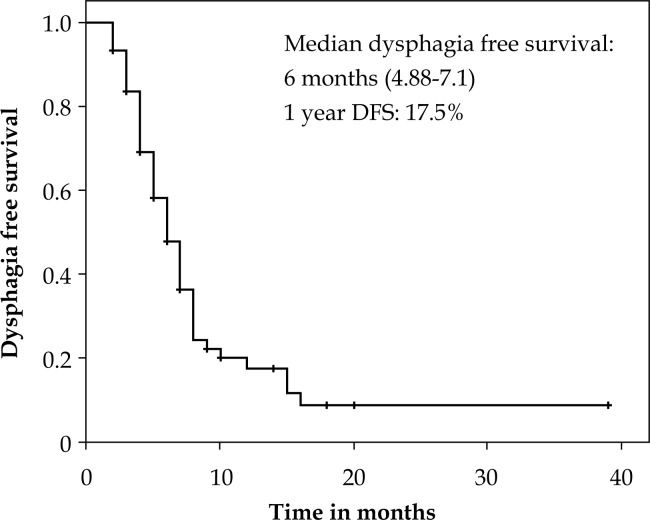
The median follow up was 6 months (range: 1-42 months). On Kaplan Meier analysis, the median OS was 9.5 months (95% CI: 7.5-10.5) with a 1 year OS of 27% (Figure 1). At last follow up, 28 patients were alive and there were 46 deaths. The causes of death were: esophageal cancer (41.4%), infections (2.7%), cardiovascular events (5.4%), and in 9 (12.2%) cases the cause was unknown. The duration of dysphagia relief was a median of 3 months (mean 4 months, range: 0-38). The median dysphagia free survival (DFS) was 6 months (95%CI: 4.8-7.1) with 1 year DFS of 17.5% (Figure 2).
Fig. 1.
Kaplan Meier plot showing overall survival
Fig. 2.
Kaplan Meier plot showing dysphagia free survival
A 2 tailed log rank p value analysis with stratification for age, sex, KPS, length of lesion, morphology, histology, and dose of EBRT was done. However, none of them were significant with respect to overall survival and dysphagia free survival.
Dysphagia scores
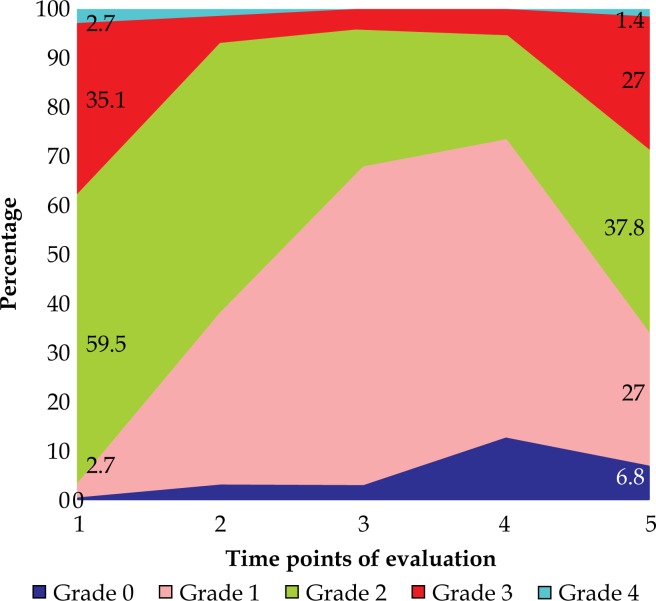
There was a significant improvement in dysphagia scores from baseline to completion of first fraction of ILBT (p = 0.000) with 50% experiencing 1 point, and 8.1% experiencing a 2 points improvement in scores. There was further significant improvement after completion of two ILBT's (p = 0.000) with 85.1% patients having 1 or 2 points improvement in score. There was further improvement in scores with addition of EBRT, which was significant compared to after completion of two ILBT (p = 0.02). The improvement in dysphagia scores were sustained even up to the last contact (p = 0.02) with about 35.1% having stable scores compared to 3 months after completion of EBRT (Table 6, Figure 3).
Table 6.
Change in dysphagia scores following treatment
| Change in score | One level improvement | Two or more level improvement | Remains same | Progressed | P value |
|---|---|---|---|---|---|
| Baseline to ILBT 1 | 37 (50%) | 6 (8.1%) | 30 (40.5%) | 1 (1.4%) | 0.000 |
| Baseline to ILBT 2 | 52 (70.2%) | 11 (14.9%) | 10 (13.5%) | 1 (1.4%) | 0.000 |
| Baseline to EBRT | 39 (52.7%) | 21 (28.4%) | 8 (10.8%) | 6 (8.1%) | 0.127 |
| ILBT 2 to EBRT | 21 (28.4%) | 1 (1.4%) | 41 (55.4%) | 11 (14.9%) | 0.02 |
| Baseline to last contact | 26 (35.1%) | 9 (12.2%) | 24 (32.4%) | 13 (17.6%) | 0.915 |
| EBRT to last contact | 4 (5.4%) | 1 (1.4%) | 26 (35.1%) | 43 (58.1%) | 0.02 |
ILBT – intraluminal brachytherapy, EBRT – external beam radiotherapy
Fig. 3.
Time trend for dysphagia scores
Odynophagia scores
There was improvement in odynophagia scores from baseline to completion of the first fraction of ILBT, which showed a significant trend (p = 0.05) with 32.4% having a 1 point, and 14.9% with a 2 point improvement in scores. There was further improvement in scores with completion of ILBT and EBRT; however, this was not statistically significant compared to baseline. There was worsening of scores in 13.5% patients after completion of 2 ILBT due to increase in pain scores. Compared to scores after 2 ILBTs, there were better scores after completion of EBRT with 63.5% having same scores and 20.5% having 1 level improvement (p = 0.03). At the point of last contact, compared to baseline 41.9% patients had 1 or 2 level improvement of scores, which was significant (p = 0.01) (Table 7).
Table 7.
Change in odynophagia scores following treatment
| Change in score | One level improvement | Two or more level improvement | Remains same | Progressed | P value |
|---|---|---|---|---|---|
| Baseline to ILBT 1 | 24 (32.4%) | 11 (14.9%) | 33 (44.6%) | 6 (8.1%) | 0.05 |
| Baseline to ILBT 2 | 18 (24.3%) | 13 (17.6%) | 33 (44.6%) | 10 (13.5%) | 0.465 |
| Baseline to EBRT | 22 (29.7%) | 9 (12.2%) | 35 (47.3%) | 8 (10.8%) | 0.165 |
| ILBT 2 to EBRT | 15 (20.5%) | – | 47 (63.5%) | 12 (16.2%) | 0.03 |
| Baseline to last contact | 24 (32.4%) | 7 (9.5%) | 29 (39.2%) | 13 (17.5%) | 0.016 |
| EBRT to last contact | 8 (10.8%) | 1 (1.4%) | 45 (66.8%) | 20 (27%) | 0.094 |
ILBT – intraluminal brachytherapy, EBRT – external beam radiotherapy
Regurgitation and pain scores
There were no significant changes in regurgitation scores at various time points with 35.1% patients having 1 point improvement, and 13.5% having deterioration of scores at point of last contact compared to baseline (Table 8). There were no significant changes in pain scores at various time points with majority of patients having a stable score 35% patients had worsening of pain scores at point of last contact (Table 9).
Table 8.
Change in regurgitation scores following treatment
| Change in score | One level improvement | Two or more level improvement | Remains same | Progressed | P value |
|---|---|---|---|---|---|
| Baseline to ILBT 1 | 24 (32.4%) | 1 (1.4%) | 34 (45.9%) | 15 (20.3%) | 0.55 |
| Baseline to ILBT 2 | 19 (25.7%) | 1 (1.4%) | 35 (47.3%) | 19 (25.7%) | 0.60 |
| Baseline to EBRT | 21 (28.4%) | 1 (1.4%) | 44 (59.5%) | 8 (10.8%) | 0.20 |
| ILBT 2 to EBRT | 24 (32.4%) | – | 39 (52.7%) | 11 (14.9%) | 0.85 |
| Baseline to last contact | 26 (35.1%) | 1 (1.4%) | 37 (50%) | 10 (13.5%) | 0.60 |
| EBRT to last contact | 14 (18.9%) | – | 49 (66.2%) | 11 (14.9%) | 0.0520 |
ILBT – intraluminal brachytherapy, EBRT – external beam radiotherapy
Table 9.
Change in pain scores following treatment
| Change in score | One level improvement | Two or more level improvement | Remains same | Progressed | P value |
|---|---|---|---|---|---|
| Baseline to ILBT 1 | 31 (41.9%) | 0 | 38 (51.4%) | 5 (6.8%) | 0.284 |
| Baseline to ILBT 2 | 32 (43.2%) | 0 | 34 (45.9%) | 8 (10.8%) | 0.71 |
| Baseline to EBRT | 32 (43.2%) | 0 | 28 (37.8%) | 14 (18.9%) | 0.20 |
| ILBT 2 to EBRT | 10 (13.5%) | 0 | 49 (66.2%) | 15 (20.3%) | 0.56 |
| Baseline to last contact | 23 (31.1%) | 0 | 25 (33.8%) | 26 (35.1%) | 0.24 |
| EBRT to last contact | 8 (10.8%) | 0 | 37 (50%) | 29 (39.2%) | 0.15 |
ILBT – intraluminal brachytherapy, EBRT – external beam radiotherapy
Weight
There was significant improvement in weight of patients after completion of 2 ILBT (p = 0.01) and after 3 months of completion of EBRT. The median improvement in weight was 1-2 kg. Thirty-nine percent has improvement in weight with 35% maintaining their weight, and 26% had deterioration of weight.
Complications/interventions
Forty-six (62.1%) patients had residual/stable disease at last follow-up while progressive disease was documented in 28 (37.8%), 6 of which were identified with distant metastasis during follow up. Thirty-one (41.9%) patients required nasogastric (NGT) tube insertion and 14 (18.9%) needed hospitalization for supportive care after completion of treatment. Two patients needed admission on two occasions. Twenty (27%) patients had complications in the form of stricture, fistula 4 (5.4%), and bleeding 3 (4%). Nine patients underwent NGT insertion, two had stent insertion, and seven required dilatation for management of the stricture. Two patients underwent stent placement for fistula. Six patients (8.1%) received palliative chemotherapy for progressive disease. In addition to the above interventions, 4 (5.4%) received radiation to metastatic sites; 2 to bony metastasis and 2 to progressive neck nodal disease (Table 10).
Table 10.
Complications/Interventions following treatment
| Complications/Interventions | Responders | Non-responders | Total |
|---|---|---|---|
| NGT insertions | 2 | 29 | 31 (41.9%) |
| Hospitalization | 2 | 12 | 14 (18.9%) |
| Stricture | 2 | 18 | 20 (27%) |
| Fistula | 0 | 4 | 4 (5.4%) |
| Bleeding | 0 | 3 | 3 (4%) |
| Palliative chemotherapy | 0 | 6 | 6 (8.1%) |
| Palliative radiation | 0 | 4 | 4 (5.4%) |
NGT – nasogastric tube insertion
Discussion
Dysphagia is a distressing symptom in advanced esophageal cancer. It is the presenting symptom in 80% of patients. Dysphagia may be due to intraluminal growth of tumor, extrinsic compression caused by lymphadenopathy, or maybe seen in the post treatment setting due to fibrosis at the anastomotic site as well as post radiation treatment or pseudo-achalasia due to infiltration of myenteric plexus by cancer [17]. It usually progresses rapidly leading to pain, nutritional compromise, and poor quality of life. About 60-70% patients are undernourished in advanced stage at presentation [18]. Hence, palliation of dysphagia is the major goal in the treatment of advanced esophageal cancer. However, there is no consensus on the best approach to this issue.
Intraluminal brachytherapy is one of the modalities for palliation of dysphagia. It can be used as the sole modality or in combination with EBRT. It improves dysphagia and swallowing status as it delivers very high dose to tumor leading to tumor regression while sparing the surrounding normal tissues [11]. Intraluminal brachytherapy is a very short treatment and gives rapid relief of symptoms.
Sur et al. conducted a randomized study of HDR brachytherapy as sole modality for palliation in 232 patients of advanced esophageal carcinoma. Patients were randomized to 18 Gy in 3 fractions (6 Gy per fractions) vs. 16 Gy in 2 fractions (8 Gy per fraction). Both fractionation schedules resulted in similar dysphagia free survival, overall survival, strictures, fistula, and were equally effective for palliation [11]. Sharma et al. evaluated the role of palliative brachytherapy in advanced/recurrent esophageal carcinoma. All patients received 2 fractions of 6 Gy each of ILBT. The median dysphagia free survival was 10 months and median overall survival was 7 months. Overall complication rates were 30%, with stricture seen in 9 patients (15%), ulceration in 6 (10%), and tracheo-esophageal fistula in 3 patients (5%) [19].
External beam radiotherapy treats the gross disease and not just the symptom. Although it takes at least 2 weeks to produce palliation, it produces a durable relief. Murray et al. performed a retrospective review of 148 patients of esophageal cancer, locally advanced or metastatic, not suitable for radical treatment. 89% patients received palliative EBRT to a dose of 20 Gy in 5 fractions. Seventy-five percent patients experienced an improvement in dysphagia and 25% gained weight. The median overall survival was 6.1 months [9]. The combination of ILBT and EBRT is likely to result in larger benefit. Randomized trials have been performed to study the benefit of additional EBRT to ILBT. Sur et al. evaluated the addition of EBRT to ILBT in a randomized trial of 60 patients. All patients received ILBT, 16 Gy in 2 fractions, and were then randomized to no further treatment or EBRT to a dose of 30 Gy in 10 fractions. There was no difference in dysphagia free survival or overall survival between the groups. Median overall survival was 7.5 months with EBRT and ILBT [14]. Rosenblatt et al. randomized 219 patients of advanced esophageal cancer after ILBT, 16 Gy in 2 fractions to EBRT or no further treatment. With a median follow-up of 197 days, the median overall survival was 188 days with 18% survival for 1 year. There was no difference between the arms in terms of overall survival. There was improvement in dysphagia with addition of EBRT, the absolute benefit being 18% at 200 days. The complications were also similar between the groups. Overall complications were perforations 2%, strictures 2%, dilatations 16%, fistula 7%, stent placement 4%, metastasis 10%, and second EBRT course in 11% [15].
Our study assessed this palliative schedule of ILBT and EBRT in patients with locally advanced esophageal carcinoma. The median OS was 9 months with 1 year OS of 27%. The median duration of dysphagia relief was 3 months. This is in concordance with the study by Rosenblatt et al.
In the study by Rosenblatt et al., the scores for dysphagia, odynophagia, regurgitation, and pain significantly improved with addition of EBRT. However, weight did not improve. In our study, the scores for dysphagia significantly improved after ILBT and EBRT. 47.3% patients had 1 or 2 point improvement in scores after EBRT, 32.4% patients having stable symptoms. There was a worsening of dysphagia immediately after EBRT likely due to esophagitis. 41.9% patients had 1 or 2 level improvement of odynophagia scores, which was significant. There was worsening of scores after second ILBT, likely due to increase in pain scores. There was no significant improvement in regurgitation and pain scores. Weight improved significantly after completion of 2 ILBTs and 3 months after EBRT. The scoring in the trial by Rosenblatt was done frequently, at monthly intervals. However, in a service setting, this may not feasible as majority patients have poor KPS and advanced disease, and in a tertiary cancer centre like ours, maybe from out of station. In our study, the time points for evaluation were more practical.
The complications were slightly higher with stricture in 20 patients (27%) and fistula in 4 (5.4%), 20 of whom required active intervention in the form of NGT insertion, dilatation, and stenting. The higher rate of stricture in the present study is inclusive of both local disease recurrence/progression (24.3%) and post RT fibrosis (2.7%) (Table 10). At the same time, we have also reported on the frequency/need for further interventions post radiotherapy.
An IAEA trial evaluating the dosage of EBRT used with ILBT, comparing 30 Gy/10 fractions vs. 20 Gy in 5 fractions has been concluded. The results are awaited. In our study, only 9 patients were treated with 20 Gy in 5 fractions. There was no difference in outcomes compared to 30 Gy in 10 fractions. However the number of patients is too small to draw definite conclusions.
There are other methods of palliation of dysphagia. Homs et al conducted a randomized trial of single dose brachytherapy (12 Gy) vs. stent placement. Stent was superior to brachytherapy (BT) with dysphagia improvement by at least 1 score 64% vs. 76% (p > 0.05) at the end of 30 days. However, in the longer term, brachytherapy was significantly better than stent with longer dysphagia relief (115 days vs. 82 days, difference of 33 days with p = 0.015). The quality of life was also significantly better with brachytherapy. Complications were significantly lesser with BT, 21% vs. 33% (p = 0.02). However, on further analysis what they discovered was that patients with poor prognostic factors such as lesion length > 10 cm, presence of metastatic disease and poor WHO performance were better palliated with stent placement [20].
In patients with incurable esophageal cancer, Amdal et al. compared self-expanding metal stent (SEMS) followed by brachytherapy (8 Gy × 3 fractions) with brachytherapy alone (8 Gy × 3 fractions). Combination of stent and brachytherapy had significant improvement in dysphagia at 3 weeks (0.02) providing immediate relief. Patients in both arms had less dysphagia at 7 weeks. There was no difference in pain [21]. Javed et al. conducted a randomized trial of stent alone vs. stent followed by EBRT (30 Gy in 10 fractions) in 84 patients with locally advanced disease. The scores for dysphagia improved significantly in both groups following stent insertion. However, dysphagia relief was more sustained in stent + EBRT arm than with stent alone (7 vs. 3 months, p = 0.002). Overall median survival was significantly higher in stent + EBRT (180 vs. 120 days, p = 0.009). Addition of radiotherapy following stenting prolonged mean dysphagia-free survival (118.6 ± 55.8 vs. 96.8 ± 43.0 days, p = 0.054). Overall complication rate was 35%. In comparison, our study showed higher dysphagia free survival and overall survival with lesser complications. However, a randomized study comparing stent + EBRT vs. ILBT + EBRT could give more insights about the superiority of one modality over the other [22].
Chemotherapy has provided dysphagia relief in majority of patients with advanced unresectable and metastatic cancer. Both monotherapy and combination chemotherapy has been used. The overall response rates are modest with similar survival rates (median 5-9 months). The overall dysphagia relief ranges from 64-90% with response rates of 15-50% [17]. The commonly used agents are platinum (cisplatin and carboplatin), 5-fluorouracil (5-FU), and the taxanes (paclitaxel, docetaxel). Cisplatin and 5-FU has response rates of 35-45% with few months of prolonged survival. Irinotecan and taxane based regimens have shown promising results. The benefits of chemotherapy are usually evident after 4-6 weeks. The use of chemotherapy for dysphagia relief must be balanced with toxicity considerations. Grade 3-4 toxicity, predominantly hematological, is seen in 20-50% of patients [23, 24, 25].
Conclusions
Intraluminal brachytherapy with EBRT is an effective option for palliation in advanced carcinoma esophagus, resulting in sustained symptomatic relief with acceptable adverse events and a need for additional interventions in only a small proportion of patients. The results of our study substantiate the results of the same regimen conducted in a controlled, trial setting.
Disclosure
Authors report no conflict of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods, and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Hanna WC, Sudarshan M, David M, et al. What is the optimal management of dysphagia in metastatic esophageal cancer? Curr Oncol. 2014;19:1–14. doi: 10.3747/co.19.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besharat S, Jabbari A, Semnani S, et al. Inoperable esophageal cancer and outcome of palliative care. World J Gastroenterol. 2008;14:3725–3728. doi: 10.3748/wjg.14.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki T, Osaka Y, Takagi Y, et al. Comparative study of self-expandable metallic stent and bypass surgery for inoperable esophageal cancer. Dis Esophagus. 2001;14:208–211. doi: 10.1046/j.1442-2050.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- 5.Konigsrainer A, Riedmann B, De Vries A, et al. Expandable metal stents versus laser combined with radiotherapy for palliation of unresectable esophageal cancer: a prospective randomized trial. Hepatogastroenterology. 2000;47:724–727. [PubMed] [Google Scholar]
- 6.Lindenmann J, Matzi V, Neuboeck N, et al. Individualized, multimodal palliative treatment of inoperable esophageal cancer: clinical impact of photodynamic therapy resulting in prolonged survival. Lasers Surg Med. 2012;44:189–198. doi: 10.1002/lsm.22006. [DOI] [PubMed] [Google Scholar]
- 7.Eroglu A, Turkyilmaz A, Subasi M, et al. The use of self-expandable metallic stents for palliative treatment of inoperable esophageal cancer. Dis Esophagus. 2010;23:64–70. doi: 10.1111/j.1442-2050.2009.00978.x. [DOI] [PubMed] [Google Scholar]
- 8.Grunberger B, Raderer M, Schmidinger M, et al. Palliative chemotherapy for Recurrent and Metastatic Esophageal Cancer. Anticancer Res. 2007;27:2705–2714. [PubMed] [Google Scholar]
- 9.Murray LJ, Din OS, Kumar VS, et al. Palliative radiotherapy in patients with esophageal carcinoma: A retrospective review. Pract Radiat Oncol. 2012;2:257–264. doi: 10.1016/j.prro.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Sur RK, Donde B, Levin V, et al. Fractionated high dose rate intraluminal brachytherapy in palliation of advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;40:447–453. doi: 10.1016/s0360-3016(97)00710-4. [DOI] [PubMed] [Google Scholar]
- 11.Sur RK, Levin CV, Donde B, et al. Prospective randomized trial of HDR brachytherapy as a sole modality in palliation of advanced esophageal carcinoma – an International Atomic Energy Agency study. Int J Radiat Oncol Biol Phys. 2002;53:127–133. doi: 10.1016/s0360-3016(02)02702-5. [DOI] [PubMed] [Google Scholar]
- 12.Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus. 2004;17:260–265. doi: 10.1111/j.1442-2050.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- 13.Amdal CD, Jacobsen AB, Tausjø JE, et al. Palliative interventions and prognosis in patients with advanced esophageal cancer. Dis Esophagus. 2011;24:502–509. doi: 10.1111/j.1442-2050.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 14.Sur RK, Donde B, Falkson C, et al. Randomized prospective study comparing high-dose-rate intraluminal brachytherapy (HDRILBT) alone with HDRILBT and external beam radiotherapy in the palliation of advanced esophageal cancer. Brachytherapy. 2004;3:191–195. doi: 10.1016/j.brachy.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Rosenblatt E, Jones G, Sur RK, et al. Adding external beam to intra-luminal brachytherapy improves palliation in obstructive squamous cell oesophageal cancer: a prospective multi-centre randomized trial of the International Atomic Energy Agency. Radiother Oncol. 2010;97:488–494. doi: 10.1016/j.radonc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Lettmaier S, Strnad V. Intraluminal brachytherapy in oesophageal cancer: defining its role and introducing the technique. J Contemp Brachytherapy. 2014;6:236–241. doi: 10.5114/jcb.2014.43780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javle M, Ailawadhi S, Yang GY, et al. Palliation of malignant dysphagia in oesophageal cancer: a literature based review. J Support Oncol. 2006;4:365–374. [PubMed] [Google Scholar]
- 18.Mehta S, Sharma SC, Kapoor R, et al. Quality of life assessment with different radiotherapy schedules in palliative management of advanced carcinoma esophagus: a prospective randomized study. Indian J Palliat Care. 2008;14:90–96. [Google Scholar]
- 19.Sharma V, Mahantshetty U, Dinshaw K, et al. Palliation of advanced/recurrent esophageal carcinoma with high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2002;52:310–315. doi: 10.1016/s0360-3016(01)01822-3. [DOI] [PubMed] [Google Scholar]
- 20.Homs M, Steyerberg E, Eijkenboom W, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomized trial. Lancet. 2004;364:1497–1504. doi: 10.1016/S0140-6736(04)17272-3. [DOI] [PubMed] [Google Scholar]
- 21.Amdal CD, Jacobsen A-B, Sandstad B, et al. Palliative brachytherapy with or without primary stent placement in patients with oesophageal cancer, a randomized phase III trial. Radiother Oncol. 2013;107:428–433. doi: 10.1016/j.radonc.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Javed A, Pal S, Dash NR, et al. Palliative stenting with or without radiotherapy for inoperable esophageal carcinoma: a randomized trial. J Gastrointest Cancer. 2012;43:63–69. doi: 10.1007/s12029-010-9206-4. [DOI] [PubMed] [Google Scholar]
- 23.Ilson DH. Esophageal Cancer Chemotherapy: Recent Advances. Gastrointest Cancer Res. 2008;2:85–92. [PMC free article] [PubMed] [Google Scholar]
- 24.El-Rayes BF, Shields A, Zalupski M, et al. A Phase II study of Carboplatin and paclitaxel in esophageal cancer. Ann Oncol. 2004;15:960–965. doi: 10.1093/annonc/mdh230. [DOI] [PubMed] [Google Scholar]
- 25.Rustgi AK, El-Serag HB. Esophageal Carcinoma. N Engl J Med. 2014;371:2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]