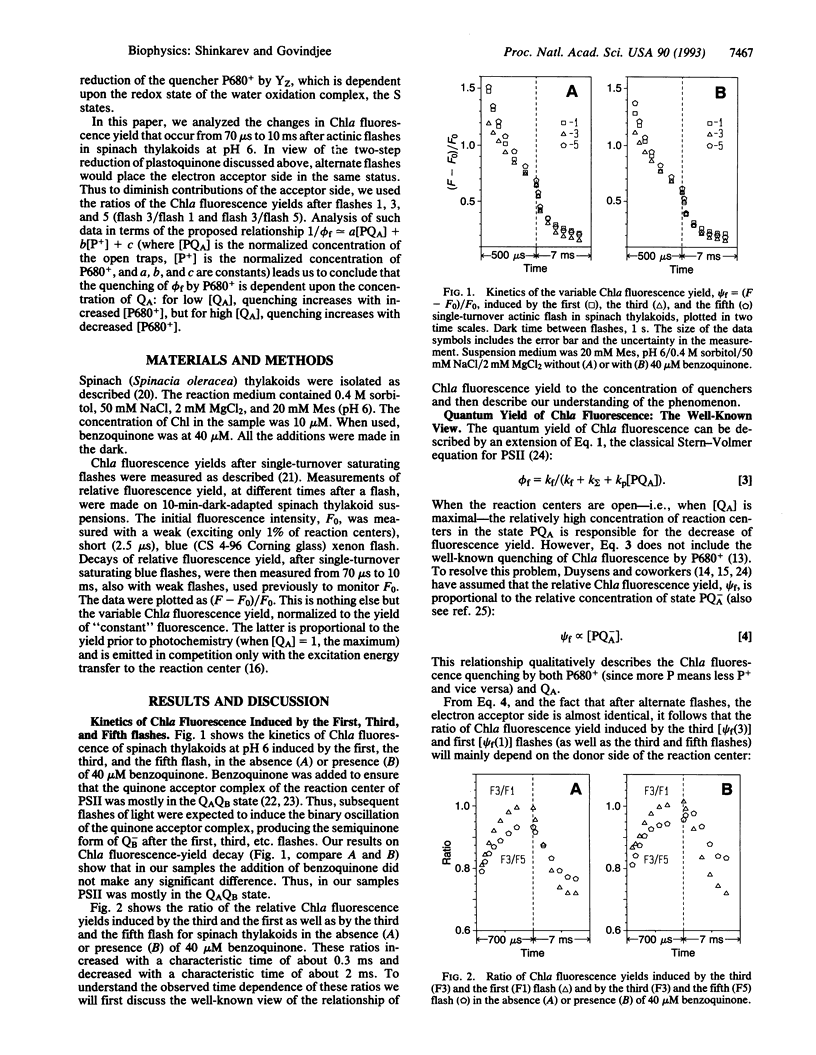
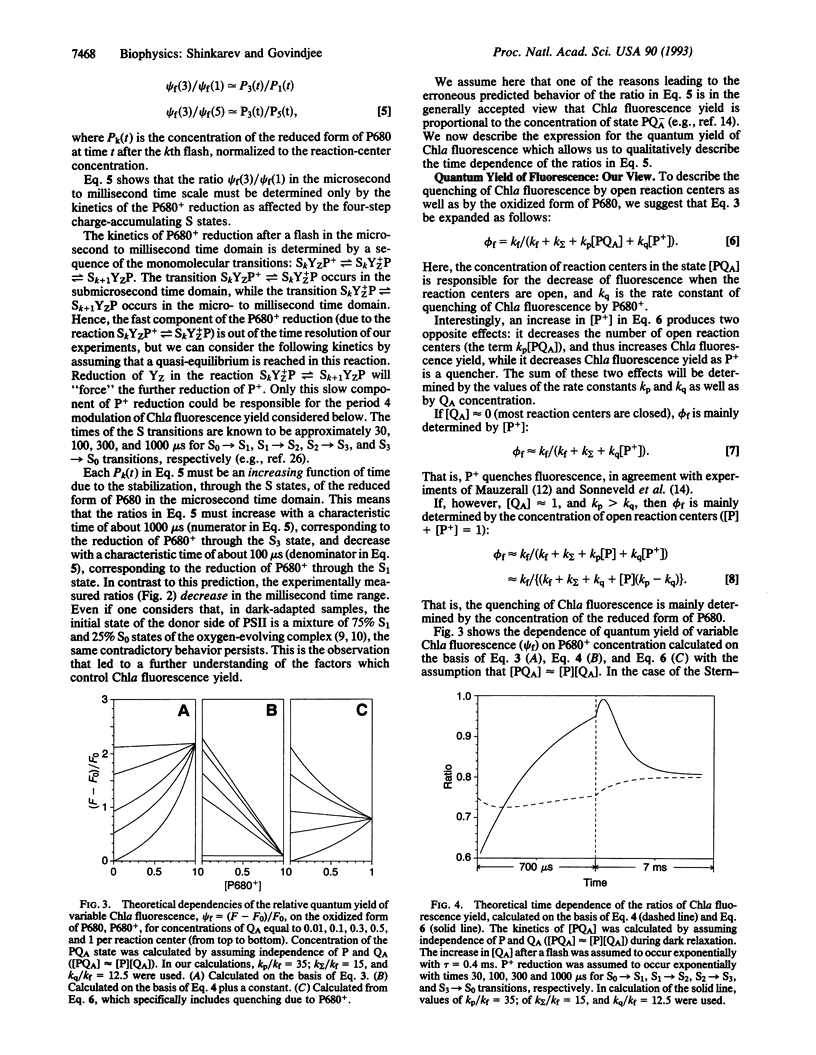
Abstract
Fluorescence of chlorophyll a (Chla) is a noninvasive and very sensitive intrinsic probe of photosynthesis. It monitors the composition and organization of the photosystems, the exciton energy transfer, the photochemistry, and the effects of various types of stress on plants. It is the most used as well as the most abused tool in photosynthesis. Thus, an understanding of its relationship to photosynthesis has been of paramount importance. Both the oxidized primary plastoquinone, QA, and the oxidized primary reaction-center Chla, P680+ (for short, P+), are known to be quenchers of Chla fluorescence yield (phi f) of photosystem II. Flash-number dependence of Chla fluorescence yield shows either a period 4, due to the four-step charge-accumulation process of water oxidation (donor side), or period 2 behavior, due to the two-step reduction of the plastoquinone QB (acceptor side) of photosystem II reaction centers. We provide here a further insight into the relationship of variable Chla fluorescence yield (phi f) to the concentration of the two quenchers. The observed time dependence of the ratio of psi f after flash 3 to that after flash 1 (or flash 5) in spinach thylakoids at pH 6 can be explained if we suggest that 1/psi f approximately equals a[PQA] + c, where a, b, and c are constants. From this it follows that the quenching of Chla fluorescence by P680+ after a flash is dependent on QA: for low [QA] (when most reaction centers are closed, [PQA] is low) the quenching of Chla fluorescence by P680+ predominates, while for high [QA] (when most reaction centers are open), the quenching of Chla fluorescence is due predominantly to the increased concentration of the reduced form of P680 ([P+] is low).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowes J. M., Crofts A. R. Binary oscillations in the rate of reoxidation of the primary acceptor of photosystem II. Biochim Biophys Acta. 1980 May 9;590(3):373–384. doi: 10.1016/0005-2728(80)90208-x. [DOI] [PubMed] [Google Scholar]
- Butler W. L. On the primary nature of fluorescence yield changes associated with photosynthesis. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3420–3422. doi: 10.1073/pnas.69.11.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Mauzerall D. Light-induced fluorescence changes in Chlorella, and the primary photoreactions for the production of oxygen. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1358–1362. doi: 10.1073/pnas.69.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld A., Rademaker H., Duysens L. N. Chlorophyll a fluorescence as a monitor of nanosecond reduction of the photooxidized primary donor P-680 Of photosystem II. Biochim Biophys Acta. 1979 Dec 6;548(3):536–551. doi: 10.1016/0005-2728(79)90063-x. [DOI] [PubMed] [Google Scholar]
- Sonneveld A., Rademaker H., Duysens L. N. Transfer and trapping of excitation energy in photosystem II as studied by chlorophyll alpha 2 fluorescence quenching by dinitrobenzene and carotenoid triplet. The matrix model. Biochim Biophys Acta. 1980 Dec 3;593(2):272–289. doi: 10.1016/0005-2728(80)90065-1. [DOI] [PubMed] [Google Scholar]
- Wasielewski M. R., Johnson D. G., Seibert M., Govindjee Determination of the primary charge separation rate in isolated photosystem II reaction centers with 500-fs time resolution. Proc Natl Acad Sci U S A. 1989 Jan;86(2):524–528. doi: 10.1073/pnas.86.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankel K. L. Rapid fluorescence changes observed in chloroplasts: their relationship to the O2 evolving system. Biochim Biophys Acta. 1973 Oct 19;325(1):138–148. doi: 10.1016/0005-2728(73)90159-x. [DOI] [PubMed] [Google Scholar]


