Abstract
Objectives
This study aimed to characterise the epidemiology of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections among men who have sex with men (MSM) and transgender women (TW) in Lima, Peru.
Setting
Cross-sectional study in Lima, Peru.
Participants
We recruited a group of 510 MSM and 208 TW for a subsequent community-based randomised controlled trial. The presence of CT and NG were evaluated using Aptima Combo2 in pharyngeal and anal swabs. We also explored correlates of these infections. Primary and secondary outcome measures: Study end points included overall prevalence of C. trachomatis and N. gonorrhoeae in anal and pharyngeal sites.
Results
Overall prevalence of CT was 19% (95% CI 16.1% to 22.1%) and 4.8% (95% CI 3.3% to 6.6%) in anal and pharyngeal sites, respectively, while prevalence of NG was 9.6% (95% CI 7.5% to 12.0%) and 6.5% (95% CI 4.8% to 8.5%) in anal and pharyngeal sites, respectively.
Conclusions
The prevalence of each infection declined significantly among participants older than 34 years (p<0.05). Efforts towards prevention and treatment of extraurogenital chlamydial and gonococcal infections in high-risk populations like MSM and TW in Lima, Peru, are warranted.
Trial registration number
NCT00670163; Results.
Keywords: PUBLIC HEALTH
Strengths and limitations of this study.
First study in Latin America to describe the prevalence of non-genital infections in high-risk men who have sex with men and transgender populations.
Comprehensive assessment of anal and pharyngeal Chlamydia trachomatis and Neisseria gonorrhoeae infections.
Ethnographic sampling might preclude generalisability of our findings to populations outside the geographic areas under study.
Oral sex was accessed in-depth; therefore, it is not possible to link specific oral sex practices and pharyngeal infections.
Introduction
The most common curable genital sexually transmitted infections (STI) worldwide are those caused by Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG).1 They remain a major public health challenge because of their high prevalence and incidence, especially among high-risk populations such as men who have sex with men (MSM) and transgender women (TW).2 3 Simultaneous testing for both bacteria is highly recommended and many current diagnostic approaches combine the detection of both agents since coinfection is common.4 5
With regard to CT and NG infections in extragenital sites, their transmission from the urogenital tract of one individual to the oropharynx of another and vice versa has been previously reported.6–8 Common sexual practices among MSM and TW such as fellatio, anilingus, and both receptive and insertive condomless anal intercourse can facilitate CT and NG infections in exposed anatomical sites. These facts highlight the importance and complexities of the transmission of these bacteria given the potential of the anorectum and oropharynx to be infected or colonised, and then to serve as silent reservoirs and new sources of infections. However, many public health programmes in low and middle income countries, such as Peru, still do not routinely screen for NG and CT in anorectal and pharyngeal sites.5 9 10
The importance and potential impact of screening for CT and NG in non-genital sites for both high-risk men and women has been shown in several previous studies.11 12 CT and NG infections in the oropharynx and/or the anorectum are much more frequent among MSM and TW than in heterosexual men.6 13–17 The inclusion of CT and NG screening in some STI programmes at public STI clinics worldwide has showed a significant increase in their detection, especially when testing is targeted and based on reported sexual practices and STI history in these high-risk populations.6 10 18
Our study aimed to characterise the epidemiology of CT and NG infections among MSM and TW in Lima, Peru.
Methods
Study design, study population and setting
We analysed the baseline data of the ‘Comunidades Positivas and Enhanced Partner Therapy Trial’ (ClinicalTrials.gov identifier: NCT00670163) collected during 2008–2009 for a larger clinical trial which was implemented in Lima, Peru, until 2012. This time frame included planning, implementation and data analysis. We recruited 718 participants who were 18–45-years-old, born biologically male, who lived and/or socialised in the study neighbourhoods, had anal or oral sex with other men in the previous 12 months, and expressed sexual preference for men. HIV status was not considered for enrolment purposes. Recruitment used a snowball-like, peer-based referral approach in sites previously identified by ethnographic methods. This sample size was defined a priori to meet the aims of the original trial. Among the trial participants, 701 agreed to provide self-collected anal swabs and 712 agreed to provide technician-assisted pharyngeal swabs for screening. We offered free treatment to all participants resulting with a positive test for CT and/or NG according to the Peruvian STI Management Guidelines (azithromycin, 1.0 g single dose orally for CT; cefixime, 400 mg single dose orally for NG).19
Data collection
Mobile study teams went to each study neighbourhood for 1–2 weeks to implement all study procedures using temporary offices. Once the participant volunteered and consented in written to participate in the study, they were asked to respond to a questionnaire using a Computer-Assisted Personal Interview (CAPI) system.20 The questionnaire collected information on demographic characteristics, general health and healthcare-seeking behaviour, exposure to HIV/STI prevention messages, HIV testing history, sexual risk behaviours and substance use, and took approximately 60 min to complete. After completing the questionnaire, all participants went through pretest counselling for STIs, including HIV infection, with a trained counsellor according to the Peruvian STI Management guidelines.19
Laboratory procedures
We trained all participants to self-collect anal swabs, using pictures and mirrors for guidance. Trained technicians collected pharyngeal swabs by swabbing the tonsils. We initially stored all collected samples in Aptima collection tubes and then transported them to our laboratory facilities in Lima where nucleic acid amplification testing (NAAT) was performed. Specifically, the detection of CT and/or NG infections was performed by means of a Transcription Mediated Amplification (TMA) with the Aptima Combo2 CT/NG test (Gen Probe Incorporated, San Diego, California, USA) following the manufacturer's directions. NAAT technology is well known for being highly sensitive and specific.15 18 21 22 The results were given to the participants within 2 weeks of sample collection, along with post-test counselling and treatment, as necessary, according to the Peruvian STI Management Guidelines.19 Laboratory quality assurance was performed on a randomly selected 10% of the samples and conducted by the Laboratory of the San Francisco Department of Public Health in California.
Variables and statistical analysis
Outcomes: The four outcome variables of interest for this analysis were anorectal chlamydiasis, anorectal gonorrhoea, oropharyngeal chlamydiasis and oropharyngeal gonorrhoea. We calculated their prevalences and 95% CIs overall and for each of the three self-reported sexual and gender identity subgroups (gay/homosexual, male-to-female transgender women and bisexual/heterosexual). Composite outcomes for any infection and concomitant coinfection at each anatomical site were also calculated. The concomitant coinfection outcome was generated in order to gain statistical power for later modelling analysis, especially for the oral infection outcomes given their very low absolute numbers.
Correlates: The following variables were used to describe the study population and analysed to explore their associations with the outcome variables: age, age at sexual debut and number of sexual partners over the past 6 months (given their distribution departure from normality, these were re-categorised in tertiles), educational attainment, self-reported sexual/gender identity, preferred role during anal sex, engagement in condomless anal intercourse, engagement in oral sex (without differentiation if giving or receiving) and ever engaging in compensated sex. Oral sex and condomless anal intercourse were summary binary variables constructed from responses provided by participants when asked for specific sexual behaviours with up to three most recent sexual partners in the past 6 months.
Statistical analysis: We tested associations between categorised variables and each of the four outcomes by using either χ2's test or Fisher's exact test when needed. Differences in the distribution of numeric variables between subjects with and without infection were assessed using the Wilcoxon's Rank Sum test. Within each sexual/gender identity group, we also assessed the linear trend of the prevalence of each infection across age groups using the χ2 test for linear trends. To model the outcomes as a function of the exposure (correlate) variables of a priori interest, we fit multivariable log-binomial models under the Generalised Linear Models framework using a logarithmic link and binomial family settings.23 This approach estimates prevalence ratios (PR), which are more appropriate for a cross-sectional study like the present.24 Moreover, in comparison to other alternatives, such as the Cox or Poisson regression models, the PR obtained are less prone to errors in interval estimation as well as less biased.25 26
The selection and inclusion of variables into the final multivariate models relied on a conceptual framework developed by the authors following guidelines previously described for epidemiological applications.27 Conceptually, we considered three hierarchical levels of variables related to the outcome: a distal level (age and education), an intermediate level (sexual identity, sexual role, number of partners and age at sexual debut) and a proximal level (compensated sex and either condomless receptive anal intercourse or oral sex—the latter according to the outcome under analysis). Each anal infection outcome was regressed on each variable at a time and only one variable from each level was selected according to its best fit measured by the lowest reported value of Akaike Information Criterion (AIC) for the distal and intermediate levels, or the Bayesian Information Criterion (BIC) for the proximal level. We adopted this approach given the specific properties of each information criterion: AIC tends to favour the best model among a set of candidate models while BIC tends to favour the ‘one true model’ among a set of candidate models.28 29 For the oral infections outcomes, we followed the same approach, but later further limited the number of correlates in the final model to avoid overfitting and stick to the proximal and distal levels only. In general, to avoid overfitting and instability in the final models, we limited the number of variables to include in the models in such a way that there are about 10 to 16 events in the outcome per dichotomous or dummy.30 We assessed potential collinearity among predictors by measuring uncentred variance inflation factors.
Results
Participants characteristics
During 2008 and 2009, we enrolled 718 participants in Lima and surrounding areas. Participants’ age range was 18–45-years. Most of them were born in the coastal region of Peru: 53.6% (385/718) in Lima and 32.2% (232/718) in other coastal cities. The remaining 14.1% (101/718) were born in non-coastal cities. Almost 80% of participants were concurrently working and/or studying (568/718). The mean age of sexual debut was 14 years. Most participants self-reported as gay or homosexual (64.8%), and having preference for a pasivo sexual role (67.5%). The median number of sexual partners in the past 6 months was 5. Further details are described in table 1.
Table 1.
Overall study population characteristics and their bivariate associations with anal and pharyngeal infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae among 718 MSM/TW participants in Lima, Peru, 2008–2009
| Total enrolled (N=718) n (%) |
Provided anal swabs (N=701) |
Provided throat swabs (N=712) |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Chlamydia trachomatis |
Neisseria gonorrhoeae |
Chlamydia trachomatis |
Neisseria gonorrhoeae |
||||||||||||||||||||
| Characteristics | Positive=133 n (%) |
Negative=568 n (%) |
Positive=67 n (%) |
Negative=634 n (%) |
Positive=34 n (%) |
Negative=678 n (%) |
Positive=46 n (%) |
Negative=666 n (%) |
|||||||||||||||
| Demographic characteristics | |||||||||||||||||||||||
| Age (years) | |||||||||||||||||||||||
| Median (IQR) | 29 | (23–35) | 26 | (22–30) | 29 | (24–36) | <0.05 | 24 | (21–29) | 29 | (23–36) | <0.05 | 25 | (22–29) | 29 | (23–36) | <0.05 | 25 | (23–29) | 29 | (23–36) | <0.05 | |
| Tertile 1: 18–25 | 261 | (36.4) | 61 | (24.1) | 192 | (75.9) | 37 | (14.6) | 216 | (85.4) | 18 | (7.0) | 241 | (93.0) | 24 | (9.3) | 235 | (90.7) | |||||
| Tertile 2: 26–33 | 242 | (33.7) | 50 | (21.2) | 186 | (78.8) | <0.05 | 25 | (10.6) | 211 | (89.4) | <0.05 | 12 | (5.0) | 227 | (95.0) | 0.05 | 16 | (6.7) | 223 | (93.3) | <0.05 | |
| Tertile 3: 34–45 | 215 | (29.9) | 22 | (10.4) | 190 | (89.6) | 5 | (2.4) | 207 | (97.6) | 4 | (1.9) | 210 | (98.1) | 6 | (2.8) | 208 | (97.2) | |||||
| Educational attainment | |||||||||||||||||||||||
| At least some beyond HS | 204 | (28.4) | 42 | (21.1) | 157 | (78.9) | 19 | (9.6) | 180 | (90.4) | 9 | (4.5) | 192 | (95.5) | 15 | (7.5) | 186 | (92.5) | |||||
| Full/partially attended HS | 448 | (62.4) | 83 | (19.0) | 353 | (81.0) | 0.27 | 38 | (8.7) | 398 | (91.3) | 0.25 | 22 | (4.9) | 423 | (95.1) | 0.96 | 24 | (5.4) | 421 | (94.6) | 0.22 | |
| Elementary or none | 66 | (9.2) | 8 | (12.1) | 58 | (87.9) | 10 | (15.2) | 56 | (84.8) | 3 | (4.6) | 63 | (95.4) | 7 | (10.6) | 59 | (89.4) | |||||
| Self-reported sexual identity | |||||||||||||||||||||||
| Gay/homosexual | 465 | (64.8) | 87 | (19.2) | 366 | (80.8) | 39 | (8.6) | 414 | (91.4) | 19 | (4.1) | 441 | (95.9) | 25 | (5.4) | 435 | (94.6) | |||||
| Transgender women | 208 | (29.0) | 41 | (20.1) | 163 | (79.9) | 0.40 | 25 | (12.3) | 179 | (87.7) | 0.28 | 14 | (6.7) | 194 | (93.3) | 0.25 | 20 | (9.6) | 188 | (90.4) | 0.06 | |
| Bisexual/heterosexual | 45 | (6.2) | 5 | (11.4) | 39 | (88.6) | 3 | (6.8) | 41 | (93.2) | 1 | (2.3) | 43 | (97.7) | 1 | (2.3) | 43 | (97.7) | |||||
| Sexual characteristics | |||||||||||||||||||||||
| Sexual role during anal sex | |||||||||||||||||||||||
| Pasivo | 485 | (67.5) | 89 | (18.8) | 384 | (81.2) | 45 | (9.5) | 428 | (90.5) | 23 | (4.8) | 458 | (95.2) | 30 | (6.2) | 451 | (93.8) | |||||
| Moderno | 188 | (26.2) | 41 | (22.2) | 144 | (77.8) | 0.07 | 20 | (10.8) | 165 | (89.2) | 0.46 | 9 | (4.8) | 177 | (95.2) | 0.99 | 13 | (7.0) | 173 | (93.0) | 0.94 | |
| Activo | 45 | (6.3) | 3 | (7.0) | 40 | (93.0) | 2 | (4.7) | 41 | (95.3) | 2 | (4.4) | 43 | (95.6) | 3 | (6.7) | 42 | (93.3) | |||||
| Sexual debut | |||||||||||||||||||||||
| Median (IQR) | 14 | (12–16) | 14 | (12–16) | 14 | (12–16) | 0.12 | 14 | (12–15) | 14 | (12–16) | 0.24 | 13 | (12–15) | 14 | (12–16) | 0.15 | 14 | (13–17) | 14 | (12–16) | 0.50 | |
| Tertile 1: 13 or younger | 294 | (41.0) | 61 | (21.0) | 229 | (79.0) | 28 | (9.7) | 262 | (90.3) | 18 | (6.2) | 274 | (93.8) | 15 | (5.1) | 277 | (94.9) | |||||
| Tertile 2: 14–15 | 188 | (26.2) | 38 | (20.8) | 145 | (79.2) | 0.16 | 24 | (13.1) | 159 | (86.9) | 0.08 | 9 | (4.8) | 179 | (95.2) | 0.25 | 14 | (7.5) | 174 | (92.5) | 0.49 | |
| Tertile 3: 16 or older | 236 | (32.8) | 34 | (14.9) | 194 | (85.1) | 15 | (6.6) | 213 | (93.4) | 7 | (3.0) | 225 | (97.0) | 17 | (7.3) | 215 | (92.7) | |||||
| Sexual partners over the past 6 months* | |||||||||||||||||||||||
| Median (IQR) | 5 | (2–15) | 5 | (2–15) | 4 | (2–15) | 0.67 | 7 | (4–30) | 4 | (2–15) | <0.05 | 7 | (3–30) | 4 | (2–15) | <0.05 | 6 | (2–18) | 4 | (2–15) | 0.56 | |
| 1 partner | 111 | (16.1) | 14 | (12.8) | 95 | (87.2) | 0.11 | 5 | (4.6) | 104 | (95.4) | <0.05 | 2 | (1.9) | 106 | (98.1) | 0.26 | 8 | (7.4) | 100 | (92.6) | 0.75 | |
| 2 partners | 117 | (16.9) | 29 | (25.7) | 84 | (74.3) | 4 | (3.5) | 109 | (96.5) | 5 | (4.3) | 112 | (95.7) | 5 | (4.3) | 112 | (95.7) | |||||
| 3–10 partners | 262 | (37.9) | 52 | (20.4) | 203 | (79.6) | 28 | (11.0) | 227 | (89.0) | 10 | (3.8) | 251 | (96.2) | 16 | (6.1) | 245 | (93.9) | |||||
| More than 10 partners | 201 | (29.1) | 37 | (18.7) | 161 | (81.3) | 26 | (13.1) | 172 | (86.9) | 13 | (6.5) | 187 | (93.5) | 14 | (7.0) | 186 | (93.0) | |||||
| Sexual behaviours over the past 6 months | |||||||||||||||||||||||
| C-IAI with men | Yes | 131 | (18.3) | 18 | (14.2) | 109 | (85.8) | 0.13 | 5 | (3.9) | 122 | (96.1) | <0.05 | 4 | (3.1) | 126 | (96.9) | 0.32 | 8 | (6.2) | 122 | (93.8) | 0.88 |
| No | 587 | (81.7) | 115 | (20.0) | 459 | (80.0) | 62 | (10.8) | 512 | (89.2) | 30 | (5.2) | 552 | (94.8) | 38 | (6.5) | 544 | (93.5) | |||||
| C-RAI sex with men | Yes | 469 | (65.3) | 97 | (21.2) | 360 | (78.8) | <0.05 | 49 | (10.7) | 408 | (89.3) | 0.15 | 23 | (5.0) | 441 | (95.0) | 0.76 | 30 | (6.5) | 434 | (93.5) | 0.99 |
| No | 249 | (34.7) | 36 | (14.8) | 208 | (85.2) | 18 | (7.4) | 226 | (92.6) | 11 | (4.4) | 237 | (95.6) | 16 | (6.5) | 232 | (93.5) | |||||
| Oral sex with men | Yes | 472 | (65.7) | 92 | (19.7) | 374 | (80.3) | 0.46 | 46 | (9.9) | 420 | (90.1) | 0.69 | 25 | (5.3) | 446 | (94.7) | 0.35 | 33 | (7.0) | 438 | (93.0) | 0.41 |
| No | 246 | (34.3) | 41 | (17.5) | 194 | (82.5) | 21 | (8.9) | 214 | (91.1) | 9 | (3.7) | 232 | (96.3) | 13 | (5.4) | 228 | (94.6) | |||||
| Compensated sex | Yes | 394 | (54.9) | 73 | (19.0) | 312 | (81.0) | 0.99 | 47 | (12.2) | 338 | (87.8) | <0.05 | 23 | (5.9) | 369 | (94.1) | 0.13 | 26 | (6.6) | 366 | (93.4) | 0.84 |
| No | 324 | (45.1) | 60 | (19.0) | 256 | (81.0) | 20 | (6.3) | 296 | (93.7) | 11 | (3.4) | 309 | (96.6) | 20 | (6.3) | 300 | (93.7) | |||||
p<0.05 for bold values; p<0.10 for underlined values.
Values between parentheses are percentages in row display except for the following variables: age, age at sexual debut and number of partners.
Bivariate associations between categorical variables tested either using χ2 or Fisher’s exact test according to expected values.
Differences in the distribution of age, age at sexual debut and number of partners between patients with and without each of the infections tested using Wilcoxon’s Rank Sum Test.
This table only includes data about positive cases for each infection under study. However, the statistical comparisons made reflect positive versus negative cases.
*n=691 for this variable.
C-IAI, condomless insertive anal intercourse; C-RAI, condomless receptive anal intercourse; CT+, positive result for Chlamydia trachomatis infection; HS, high school; MSM/TW, men who have sex with men transgender women; NG+, positive result for Neisseria gonorrhoeae.
Infections by anatomical site, infectious agent and sexual identity
Anal swabs were provided by 97.6% of participants (701/718), and 99.2% (712/718) provided pharyngeal swabs for CT and NG testing. Overall, anal infection by CT trachomatis was 19% (95% CI 16.1% to 22.1%), while pharyngeal infection was 4.8% (95% CI 3.3% to 6.6%). The prevalence of NT was 9.6% (95% CI 7.5% to 12.0%) for anal infection, and 6.5% (95% CI 4.8% to 8.5%) for pharyngeal infection. This pattern of higher CT infection in anorectum and higher NG infection in pharynx was evident within each sexual identity subgroup (gay/homosexual, TW and bisexual). Further details are described in table 2.
Table 2.
Prevalence of anal and pharyngeal infections caused by Chlamydia trachomatis and/or Neisseria gonorrhoeae among 718 MSM/TW participants and subgroups by self-reported sexual identity
| Type of infection | Total enrolled (N=718) |
Gay/homosexual (N=465) |
Transgender women (N=208) |
Bisexual/hetero (N=45) |
p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n/N | Per cent | n/N | Per cent | n/N | Per cent | n/N | Per cent | ||
| Anal | |||||||||
| Chlamydia trachomatis | 133/701 | (19.0) | 87/453 | (19.2) | 41/204 | (20.1) | 5/44 | (11.4) | 0.40 |
| Neisseria gonorrhoeae | 67/701 | (9.6) | 39/453 | (8.6) | 25/204 | (12.3) | 3/44 | (6.8) | 0.28 |
| Either | 169/701 | (24.1) | 105/453 | (23.2) | 57/204 | (27.9) | 7/44 | (15.9) | 0.18 |
| Both concurrently | 31/701 | (4.4) | 21/453 | (4.6) | 9/204 | (4.4) | 1/44 | (2.3) | 0.77 |
| Pharyngeal | |||||||||
| Chlamydia trachomatis | 34/712 | (4.8) | 19/460 | (4.1) | 14/208 | (6.7) | 1/44 | (2.3) | 0.25 |
| Neisseria gonorrhoeae | 46/712 | (6.5) | 25/460 | (5.4) | 20/208 | (9.6) | 1/44 | (2.3) | 0.06 |
| Either | 73/712 | (10.2) | 41/460 | (8.9) | 30/208 | (14.4) | 2/44 | (4.6) | 0.04 |
| Both concurrently | 7/712 | (1.0) | 3/460 | (0.7) | 4/208 | (1.9) | 0/44 | (0.0) | 0.24 |
p Value from χ2 or Fisher’s exact test to compare prevalence of infections between sex identity/gender groups.
Values between parentheses are percentages in column display.
Of 718 participants, 701 provided anal swabs and 712 pharyngeal swabs.
MSM/TW, men who have sex with men transgender women.
In the anorectum, the prevalence of CT infection was almost twice (19%) as common as for NG (9.6%); this was true for the overall study population and within each sexual identity group. In the oropharynx, the prevalence of NG was slightly higher than for CT in all cases, except for the bisexual subgroup. Comparing across sexual identities, the prevalence of each single and composite outcome infection was more common in TW participants, followed by gay/homosexual, and less common among bisexuals in most of the cases, though these differences were not statistically significant. The only statistically significant difference was for either pharyngeal infection, p value <0.05, see table 2.
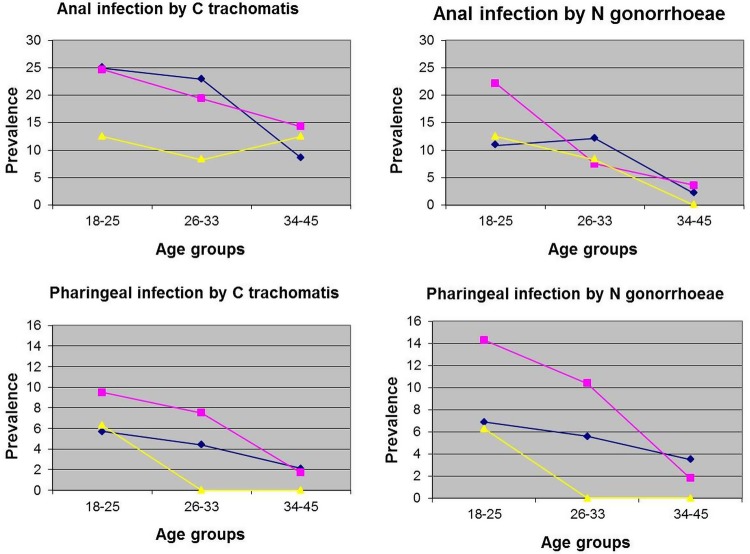
Overall, the prevalence of each of the four outcomes of the study decreases with increasing age. The test for trends over age group was significant (p<0.05) only in the following cases: within gay/homosexual for anal chlamydiasis, within gay/homosexual and TW for anal gonorrhoea, and within transgender women for pharyngeal gonorrhoea. For pharyngeal chlamydiasis, a borderline statistical significance for trend (p=0.08) was found within TW. The graphical description of trends is shown in figure 1.
Figure 1.
Trends of prevalence of anal and pharyngeal infections over age group, satisfied by agent and sexual identity.
Statistical models for outcomes and laboratory quality assurance
The six final multivariate models are presented in table 3. Briefly, the higher age group consistently remained inversely associated to all single and composite outcomes. For the oral infection outcomes, both oral sex and compensated sex were associated with higher proportions of infection though these were not statistically significant. For the anal infection outcomes, the number of sexual partners showed an erratic pattern of association and were not statistically significant in most of the cases, while condomless receptive anal intercourse remained directly and significantly associated with the infections in the models where it was included. The results of the laboratory quality assurance performed on 10% of the samples showed 100% of correlation (data not shown).
Table 3.
Multivariable models for each specific single or composite either infection by anatomic site among 718 MSM/TW participants in Lima, Peru 2008–2009
| Anatomic site | Variables included and their categories | Infection |
||
|---|---|---|---|---|
| Chlamydia trachomatis | Neisseria gonorrhoeae | Either bacteria | ||
| PR (95% CI) | PR (95% CI) | PR (95% CI) | ||
| Anal | C-RAI | |||
| Yes | 1.4 (1.0 to 2.0) | NA | 1.4 (1.0 to 1.9) | |
| No | Ref | NA | Ref | |
| Compensated sex | ||||
| Yes | NA | 1.4 (0.8 to 2.5) | NA | |
| No | NA | Ref | NA | |
| Partners | ||||
| >10 | 1.4 (0.8 to 2.5) | 2.0 (0.7 to 5.3) | 1.5 (1.0 to 2.5) | |
| 3–10 | 1.5 (0.9 to 2.7) | 2.0 (0.8 to 5.1) | 1.5 (0.9 to 2.3) | |
| 2 | 2.1 (1.2 to 3.7) | 0.7 (0.2 to 2.6) | 1.7 (1.0 to 2.8) | |
| 1 | Ref | Ref | Ref | |
| Age (years) | ||||
| 34–45 | 0.4 (0.3 to 0.7) | 0.2 (0.1 to 0.5) | 0.4 (0.2 to 0.6) | |
| 26–33 | 0.9 (0.6 to 1.2) | 0.8 (0.5 to 1.2) | 0.8 (0.6 to 1.1) | |
| 18–25 | Ref | Ref | Ref | |
| Pharyngeal | Oral sex | |||
| Yes | NA | 1.3 (0.7 to 2.4) | 1.3 (0.8 to 2.0) | |
| No | NA | Ref | Ref | |
| Compensated sex | ||||
| Yes | 1.7 (0.8 to –3.4) | NA | NA | |
| No | Ref | NA | NA | |
| Age (years) | ||||
| 34–45 | 0.3 (0.1 to –0.8) | 0.3 (0.1 to 0.7) | 0.3 (0.2 to 0.6) | |
| 26–33 | 0.7 (0.4 to –1.5) | 0.7 (0.4 to 1.3) | 0.7 (0.5 to 1.2) | |
| 18–25 | Ref | Ref | Ref | |
Partners: Number of sexual partners reported over the past 6 months.
Oral sex: No distinction in the specific role during oral sex, just engagement over the past 6 months.
Ref: Specific subcategory used as baseline reference for the analysis.
C-RAI, condomless receptive anal intercourse; MSM/TW, men who have sex with men transgender women; NA, variable not included in the model; PR, adjusted prevalence ratios along with 95% level CI.
Discussion
Using a snowball-like, peer-based referral approach, we were able to reach a group of MSM and TW at high risk as evidenced by their high prevalence of CT and NG infections. Using both technician-assisted pharyngeal swabs and self-collected anal swabs, we found that close to 25% of the study population had anal infection by either CT or NG, while close to 10% had oropharynx infection by the same bacteria. The prevalence of these infections tends to decrease with age. Therefore, these findings constitute a very important update in the Peruvian scenario and become one of the few available studies in Latin America.
Gonorrhoea prevalence in anorectal samples was higher than previously reported in HIV and STIs sentinel- surveillance studies conducted among MSM and TW by the Peruvian Minister of Health during 1996–2002; this report detailed a decrease in anal NG infection from 5.1% in 1996 to 0.2% in 2002,31 However, their method for gonorrhoea diagnostics used the regular culture of urethral secretion and it is very well known that culture only detects viable bacteria in people with active infection.32 Moreover, culturing NG from highly-contaminated samples can be very difficult. NAAT, especially TMA, are a more reasonable approach for the rapid and accurate diagnosis of anal and pharyngeal gonorrhoea without the limitations of culture.33 However, one limitation of the NAAT technology is that current non-active infections can result in a positive test since the method assesses the presence of RNA and/or DNA instead of live bacteria.34
Chlamydial infection prevalence also was quite high in this group of MSM and TW, especially in anorectal samples where we found the highest prevalence of infection. A previous report of Peruvian people living with HIV who were tested for pharyngeal chlamydia infection did not find any positive cases.35 Other studies among MSM and young men in Peru only detected chlamydia in urine samples.36 A previous country-level study conducted among young Peruvian adults from the general population found a prevalence of 4.2% for CT in urogenital samples.37 Consequently, our study adds important data to better understand the epidemiology of CT infection in Peru.
Prevalence of chlamydia and gonorrhoea decreases with age, and this can be explained by an increase in the awareness of STI infections and decreases in the number of sex partners with age.4 38 Even if not treated, chlamydia can be cleared in certain group of infected people.39 This inverse association between age and chlamydial infection has been also reported in other studies.40 41
This is the first study in Peru that uses TMA technology for testing CT and NG infection in extragenital sites, and our study highlight the importance of extragenital screening of CT and NG. Currently, the Peruvian STI Management Guidelines19 establish that all female and male sex workers are subject to periodic medical assessment, a monthly strategy which includes STI assessment based on a syndromic approach. If urethral discharge is found, gonorrhoea culture must be performed and treatment for chlamydia and gonorrhoea should be offered. There is no further testing for chlamydial infection; and asymptomatic cases for both CT and NG infections, especially in extragenital sites, can easily be missed and neglected. According to the current CDC recommendations, NAAT testing should be available for CT and NG diagnosis.33
There are some limitations in our analysis. The participants tested were not randomly selected and all of them belong to high-risk groups (since they originally were enrolled for a trial implementation) from low income neighbourhoods in the metropolitan area of Lima; thus, our results can show higher prevalences than random MSM or TW could have, and are not necessarily generalisable to all MSM and TW in Lima or Peru. However, given that this is a hidden population, the ethnographic techniques initially used to identify all of the MSM and TW within a neighbourhood represent a nearly saturated sample for each community included. At the moment, there are no Food and Drug Administration-cleared CT/NG tests available for non-urogenital sites, but the CT/NG Aptima Combo-2 test has shown good performance when used for pharyngeal and anal samples.15 42 43
In conclusion, infection by CT and NG in extragenital sites (anorectum and oropharynx) are very common among MSM and TW in Lima, Peru, and both infections need to be incorporated into the regular STI testing programmes in Peru. The overall prevalence of CT and NG tends to decrease over age, although this needs confirmation with further longitudinal analysis. Screening and treatment for extragenital CT and NG at non-healthcare facilities located in the actual venues where this populations are by means of temporary mobile teams is feasible through a combination of coordinated work with the community, use of NAAT technology and previous ethnographic work. These approaches should be used by the Ministry of Health in order to better control STIs in Lima and possibly in other cities of Peru.
Acknowledgments
The authors thank the mobile teams and the staff at the Laboratory of Sexual Health for their commitment to outstanding performance and collection of data. The authors also thank Hologic Inc. for the donation of the Aptima Combo2 CT/NG test kits used in the study. This study was sponsored by the US NIMH R01 MH078752.
Footnotes
Twitter: Follow Segundo Leon at @srleons
Contributors: SRL monitored data collection for the whole study, drafted and revised the paper. ERS and KAK wrote the statistical analysis plan, cleaned and analysed the data, drafted and revised the paper. JAF supervised the laboratory testing. AS-S monitored the data collection and revised the paper. JTG monitored data collection and supervised ethical issues of the study. JDK assessed the biological and treatment component. TJC and CFC designed the study and revised the paper.
Funding: This work was supported by the US National Institute of Mental Health grant number 5R01MH078752. SRL was also supported by NIH research training grant #r25 tw009345 funded by the Fogarty International Center, the National Institute of Mental Health, the NIH Office of the Director, the Office of Women’s Health and the Office of AIDS Research.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study was approved by the IRB of the University of California at Los Angeles, and by the Institutional Ethics Committee of the Universidad Peruana Cayetano Heredia.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.WHO. Prevalence and incidence in 2005 of selected sexually transmitted infections: methods and results. Geneva: WHO, 2011. [Google Scholar]
- 2.Organization W.H. Guidelines for the management of sexually transmitted infections. Geneva: World Health Organization, 2003. [PubMed] [Google Scholar]
- 3.Organization W.H. Sexually transmitted diseases: three hundred and thirty-three million new, curable cases in 1995 [press release]. Geneva: WHO, 1995. [Google Scholar]
- 4.Detels R, Green AM, Klausner JD et al. . The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex Transm Dis 2011;38:503–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Sweet RL. Pelvic inflammatory disease: current concepts of diagnosis and management. Curr Infect Dis Rep 2012:14:194–203. 10.1007/s11908-012-0243-y [DOI] [PubMed] [Google Scholar]
- 6.Hamasuna R, Takahashi S, Uehara S et al. . Should urologists care for the pharyngeal infection of Neisseria gonorrhoeae or Chlamydia trachomatis when we treat male urethritis? J Infect Chemother 2012;18:410–13. 10.1007/s10156-011-0355-6 [DOI] [PubMed] [Google Scholar]
- 7.Marcus JL, Kohn RP Barry PM et al. . Chlamydia trachomatis and Neisseria gonorrhoeae transmission from the female oropharynx to the male urethra. Sex Transm Dis 2011;38:372–3. 10.1097/OLQ.0b013e3182029008 [DOI] [PubMed] [Google Scholar]
- 8.Peters RP, Verweij SP, Nijsten N et al. . Evaluation of sexual history-based screening of anatomic sites for chlamydia trachomatis and neisseria gonorrhoeae infection in men having sex with men in routine practice. BMC Infect Dis 2011;11:203 10.1186/1471-2334-11-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards S, Carne C. Oral sex and transmission of non-viral STIs. Sex Transm Infect 1998;74:95–100. 10.1136/sti.74.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters RP, Nijsten N, Mutsaers J et al. . Screening of oropharynx and anorectum increases prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infection in female STD clinic visitors. Sex Transm Dis 2011;38:783–7. 10.1097/OLQ.0b013e31821890e9 [DOI] [PubMed] [Google Scholar]
- 11.Dudareva-Vizule S, Haar K, Sailer A et al. . Prevalence of pharyngeal and rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among men who have sex with men in Germany. Sex Transm Infect 2014;90:46–51. 10.1136/sextrans-2012-050929 [DOI] [PubMed] [Google Scholar]
- 12.Freeman AH, Bernstein KT, Kohn RP et al. . Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis 2011;38:1036–9. 10.1097/OLQ.0b013e318227713e [DOI] [PubMed] [Google Scholar]
- 13.Bachmann LH, Johnson RE, Cheng H et al. . Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010;48:1827–32. 10.1128/JCM.02398-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachmann LH, Johnson RE, Cheng H et al. . Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae oropharyngeal infections. J Clin Microbiol 2009;47:902–7. 10.1128/JCM.01581-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman AH, Bernstein Kyle T, Kohn Robert P et al. . Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis 2011;38:1036–9. 10.1097/OLQ.0b013e318227713e [DOI] [PubMed] [Google Scholar]
- 16.Hunte T, Alcaide M, Castro J. Rectal infections with chlamydia and gonorrhoea in women attending a multiethnic sexually transmitted diseases urban clinic. Int J STD AIDS 2010;21:819–22. 10.1258/ijsa.2010.009279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kent CK, Chaw JK, Wong W et al. . Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis 2005;41:67–74. 10.1086/430704 [DOI] [PubMed] [Google Scholar]
- 18.Schachter J, Philip SS. Testing men who have sex with men for urethral infection with Chlamydia trachomatis and Neisseria gonorrhoeae is only half the job, and we need the right tools. Sex Transm Dis 2011;38:925–7. 10.1097/OLQ.0b013e318230f3d6 [DOI] [PubMed] [Google Scholar]
- 19. Ministerio de Salud, Peru. Norma Técnica de salud para el manejo de infecciones de transmisión sexual en el Perú. Lima, Peru; 2009.
- 20.NIMH Collaborative HIV/STD Prevention Trial Group. The feasibility of audio computer-assisted self-interviewing in international settings. AIDS 2007;21(Suppl 2):S49–58. [DOI] [PubMed] [Google Scholar]
- 21.Chai SJ, Aumakhan B, Barnes M et al. . Internet-based screening for sexually transmitted infections to reach nonclinic populations in the community: risk factors for infection in men. Sex Transm Dis 2010;37:756–63. 10.1097/OLQ.0b013e3181e3d771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard EJ, Xu F, Taylor SN et al. . Screening methods for Chlamydia trachomatis and Neisseria gonorrhoeae infections in sexually transmitted infection clinics: what do patients prefer? Sex Transm Infect 2011;87:149–51. 10.1136/sti.2010.045807 [DOI] [PubMed] [Google Scholar]
- 23.Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol 1986;123:174–84. [DOI] [PubMed] [Google Scholar]
- 24.Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med 2008;65:481, 501–6 10.1136/oem.2007.034777 [DOI] [PubMed] [Google Scholar]
- 25.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003;3:21 10.1186/1471-2288-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen MR, Deddens JA. A comparison of two methods for estimating prevalence ratios. BMC Med Res Methodol 2008;8:9 10.1186/1471-2288-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Victora CG, Huttly SR, Fuchs SC et al. . The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol 1997;26:224–7. 10.1093/ije/26.1.224 [DOI] [PubMed] [Google Scholar]
- 28.Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Ecol Evol (Amst) 2004;19:101–8. 10.1016/j.tree.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 29.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods 2012;17:228–43. 10.1037/a0027127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710–18. 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 31.Sanchez J, Lama JR, Kusunoki L et al. . HIV-1, sexually transmitted infections, and sexual behavior trends among men who have sex with men in Lima, Peru. J Acquir Immune Defic Syndr 2007;44:578–85. 10.1097/QAI.0b013e318033ff82 [DOI] [PubMed] [Google Scholar]
- 32.Kapala J, Biers K, Cox M et al. . Aptima Combo 2 testing detected additional cases of Neisseria gonorrhoeae infection in men and women in community settings. J Clin Microbiol 2011;49:1970–1. 10.1128/JCM.02062-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prevention, C.f.D.C.a. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014;63(RR-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 34.Olshen E, Shrier L.A. Diagnostic tests for chlamydial and gonorrheal infections. Semin Pediatr Infect Dis 2005;16:192–8. 10.1053/j.spid.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 35.Press N, Chavez VM, Ticona E et al. . Screening for sexually transmitted diseases in human immunodeficiency virus-positive patients in Peru reveals an absence of Chlamydia trachomatis and identifies Trichomonas vaginalis in pharyngeal specimens. Clin Infect Dis 2001;32:808–14. 10.1086/319202 [DOI] [PubMed] [Google Scholar]
- 36.Group, N.C.H.S.P.T. Sexually transmitted disease and HIV prevalence and risk factors in concentrated and generalized HIV epidemic settings. AIDS 2007;21 Suppl 2:S81–90. [DOI] [PubMed] [Google Scholar]
- 37.Cárcamo CP, Campos PE, García PJ et al. . Prevalences of sexually transmitted infections in young adults and female sex workers in Peru: a national population-based survey. Lancet Infect Dis 2012;12:765–73. 10.1016/S1473-3099(12)70144-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.León SR, Konda KA, Klausner JD et al. . Chlamydia trachomatis infection and associated risk factors in a low-income marginalized urban population in coastal Peru. Rev Panam Salud Publica 2009;26:39–45. 10.1590/S1020-49892009000700006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renault CA, Israelski DM, Levy V et al. . Time to clearance of Chlamydia trachomatis ribosomal RNA in women treated for chlamydial infection. Sex Health 2011;8:69–73. 10.1071/SH10030 [DOI] [PubMed] [Google Scholar]
- 40.Miranda AE, Szwarcwald CL, Peres RL et al. . Prevalence and risk behaviors for chlamydial infection in a population-based study of female adolescents in Brazil. Sex Transm Dis 2004;31:542–6. 10.1097/01.olq.0000137899.25542.75 [DOI] [PubMed] [Google Scholar]
- 41.Acosta-Cázares B, Ruiz-Maya L, Escobedo de la Peña J. Prevalence and risk factors for Chlamydia trachomatis infection in low-income rural and suburban populations of Mexico. Sex Transm Dis 1996;23:283–8. 10.1097/00007435-199607000-00007 [DOI] [PubMed] [Google Scholar]
- 42.Chow JM, Bauer H.M., Bolan G. Repeating low-positive nucleic acid amplification test results for Chlamydia trachomatis and Neisseria gonorrhoeae: assessment of current practice in selected California public- and private-sector laboratories. J Clin Microbiol 2012;50:539 10.1128/JCM.06225-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moncada J, Schachter J, Liska S et al. . Evaluation of self-collected glans and rectal swabs from men who have sex with men for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of nucleic acid amplification tests. J Clin Microbiol 2009;47:1657–62. 10.1128/JCM.02269-08 [DOI] [PMC free article] [PubMed] [Google Scholar]