Abstract
Introduction
In clinical practice, it is difficult to distinguish between patients with refractory asthma from those with poorly controlled asthma, where symptoms persist due to poor adherence, inadequate inhaler technique or comorbid diseases. We designed an audio recording device which, when attached to an inhaler, objectively identifies the time and technique of inhaler use, thereby assessing both aspects of adherence. This study will test the hypothesis that feedback on these two aspects of adherence when passed on to patients improves adherence and helps clinicians distinguish refractory from difficult-to-control asthma.
Methods
This is a single, blind, prospective, randomised, clinical trial performed at 5 research centres. Patients with partially controlled or uncontrolled severe asthma who have also had at least one severe asthma exacerbation in the prior year are eligible to participate. The effect of two types of nurse-delivered education interventions to promote adherence and inhaler technique will be assessed. The active group will receive feedback on their inhaler technique and adherence from the new device over a 3-month period. The control group will also receive training in inhaler technique and strategies to promote adherence, but no feedback from the device. The primary outcome is the difference in actual adherence, a measure that incorporates time and technique of inhaler use between groups at the end of the third month. Secondary outcomes include the number of patients who remain refractory despite good adherence, and differences in the components of adherence after the intervention. Data will be analysed on an intention-to-treat and a per-protocol basis. The sample size is 220 subjects (110 in each group), and loss to follow-up is estimated at 10% which will allow results to show a 10% difference (0.8 power) in adherence between group means with a type I error probability of 0.05.
Trial registration number
NCT01529697; Pre-results.
Keywords: MEDICAL EDUCATION & TRAINING
Introduction
Approximately 10% of patients with asthma remain poorly controlled with persisting symptoms and severe exacerbations despite use of combination therapy with long-acting β agonists and inhaled corticosteroids.1 This poor control may be due to medication refractory asthma or due to difficult-to-manage asthma from issues such as poor inhaler technique, poor adherence or coexisting comorbid disease.1–3 In practice, distinguishing refractory from difficult-to-manage asthma is difficult. For example, adherence to medications, a particular problem in patients with severe asthma, is difficult to detect since self-report is unreliable,4 and pharmacy refill records only identify if the individual has collected a prescription. Some patients may demonstrate a reasonable inhaler technique when directly observed, but may be careless in their inhaler use on a day-to-day basis.5 Hence, without objective longitudinal information on inhaler adherence and technique it is challenging to distinguish a patient with refractory asthma from one who has difficult-to-manage asthma.6
We developed a device, INhaler Compliance Assessment (INCA), which makes a digital audio recording of an inhaler being used.5 7–13 Analysis of the audio recordings by automated signal processing techniques, provides an objective assessment of both the time and the technique of inhaler use. Validation of the device and the audio recordings have been previously presented.5 7–13 We hypothesised that this information could be used both as part of an educational consultation for patients and for clinicians to help distinguish refractory from difficult-to-manage asthma.
The objective of this study is to assess if inhaler use obtained from the INCA device on time and technique of use leads to better inhaler adherence and better clinical information than current best practice.
Methods
We describe the protocol of a randomised, single blind, nurse-delivered education study. The study will comprise patients with severe asthma attending specialist hospital asthma clinics, who remain uncontrolled and have experienced at least one recent severe asthma exacerbation. Basing on information obtained directly from the INCA acoustic recording device, one group (the active arm) will discuss patterns of adherence and training on technique of inhaler use. The second group (the control arm) will be given generalised strategies to improve adherence, while technique errors will be corrected using checklists.14 Adherence will be assessed objectively in all participants. Global outcomes will be quantified using the clinical, lung function, adherence and exacerbation data collected during the observation period.
Sponsorship
This is a researcher-initiated study, funded by the Health Research Board of Ireland (Pro/2011/57), and hosted within the Dublin Centre for Clinical Research clinical trials centres. The study sponsor, Royal College of Surgeons, is an independent medical university. The trial was approved by the Beaumont Hospital's Ethics committees. The trial is registered as NCT01529697 on Clinicaltrials.gov and a detailed statistical plan has been approved by an independent statistical team. The INCA device was manufactured and supplied by Vitalograph, Ennis, Ireland, and GlaxoSmithKline provided the salmeterol/fluticasone Diskus inhaler for this study.
Setting
This is a prospective, multicentre, single blind, randomised controlled trial of two nurse-delivered strategies to optimise inhaler technique and adherence of patients with stage 3 to 5 asthma. The study is being conducted at the Clinical Research Centres of five university hospitals within the republic of Ireland (4 in Dublin County, 1 in Cork County). At each centre between one and three nurses have been trained to provide either intervention. The lead clinical nurse was educated by the principal investigator and a respiratory nurse specialist. All other nurses were educated by our lead clinical nurse in a teach-to-goal method with demonstration. The study period is from 2011 with ongoing recruitment.
Participants (n=220)
Prior to study recruitment, an asthma diagnosis is made using a clinician diagnosis supported by one or more of the following: obstructive spirometry with a minimum of 10% reversibility, either spontaneously over time or with inhaled β agonist, or with a minimum 15% peak flow variability over time or through a positive bronchial provocation challenge.
Inclusion criteria
Patients already prescribed therapy equivalent to step 3 or higher on the Asthma Management Guidelines (1) for at least 3 months, and who had at least one exacerbation treated with systemic glucocorticoids in the prior year, and who are either uncontrolled or partially controlled by Global Initiative for Asthma (GINA) guidelines are eligible for inclusion.1 Patients must also be 18 years or older in age.
Exclusion criteria
Patients who are controlled, as defined by the GINA criteria1 on their current therapy are excluded. Additional exclusion criteria are those who are unwilling to participate in a clinical study, or prior hypersensitivity to salmeterol/fluticasone. There are no other exclusion criteria.
Study design
The study flow is indicated in table 1.
Table 1.
Details of study data collection
| Study procedure | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| Informed consent | X | |||
| Demographics | X | |||
| Medical history | X | |||
| Inclusion and exclusion criteria | X | |||
| Current medications | X | |||
| Physical examination | X | X | ||
| X | X | X | X | |
| AQLQ | X | X | X | X |
| ACT | X | X | X | X |
| Randomisation | X | |||
| Dispense adapted Seretide inhaler | X | X | X | |
| Dispense electronic PEFR monitor | X | X | X | |
| Download device readings active only | X | X | X | |
| Inhaler use education | X | X | X | X |
| Adverse events recorded | X | X | X | |
| Concomitant medications recorded | X | X | X |
The active group receive a copy of device readings and active feedback about adherence and inhaler technique, visit 1: screening visit: at time of enrolment (week 0); visit 2: at end of month 1 (week 4); visit 3: at end of month 2 (week 8); visit 4: final visit at the end of month 3 (week 12).
AQLQ, Asthma Quality of Life Questionnaire; ACT, Asthma Control Test; PEFR, peak expiratory flow rate.
Patients identified at specialist asthma clinics who meet the inclusion and not the exclusion criteria are invited to participate in the study. Once consented, each study visit is performed by a registered nurse.
The dose of inhaled corticosteroid and Long Acting Beta-Agonist (LABA) is not changed at recruitment and during the study procedure, as the main aim of the study is to improve adherence to current asthma treatment.
The audio recording technology has not yet been established for the turbohaler or the pressurised metered dose inhaler (pMDI), hence, for those participants who are currently prescribed formoterol/budesonide/beclomethasone at recruitment, their therapy is changed to an equivalent dose of salmeterol/fluticasone delivered via the Diskus device. This change is made by the physician looking after the patient in the outpatient clinic. Following this, the patient is then referred to the study. The patient may still refuse to enter the study. All other aspects of regular patient care are continued.
At the initial visit, the participant's age, sex, height, weight, duration of asthma, smoking history, number of courses of steroids in the prior year are recorded (self-reported by the participant). The dose of salmeterol/fluticasone and duration of taking this dose, use and dose of other inhaled therapy including short-acting β agonists, long acting muscarinic antagonist, nasal steroid and antihistamines are recorded. The nurse records the peak expiratory flow rate (PEFR) and an Inhaler Proficiency checklist Score (IPS; see online supplementary appendix 1), a 10-point checklist score. The Asthma Control Test (ACT) score and Asthma Quality of Life Questionnaire (AQLQ)15 are completed by the participant. Serum total and specific immunoglobulin E levels and peripheral blood eosinophil levels, prior spirometry and bronchial provocation test are recorded from the clinical notes.
The participants receive a salmeterol/fluticasone Diskus inhaler with an INCA device attached, and they are asked to use the inhaler twice per day and to take reliever salbutamol, as required, for breakthrough symptoms. All participants are informed at recruitment that the device would provide information on how and when they use their inhaler. Participants are asked to record their peak expiratory flow with an electronic monitor (ASMA-1, Vitalograph, Ennis, Ireland) twice daily.
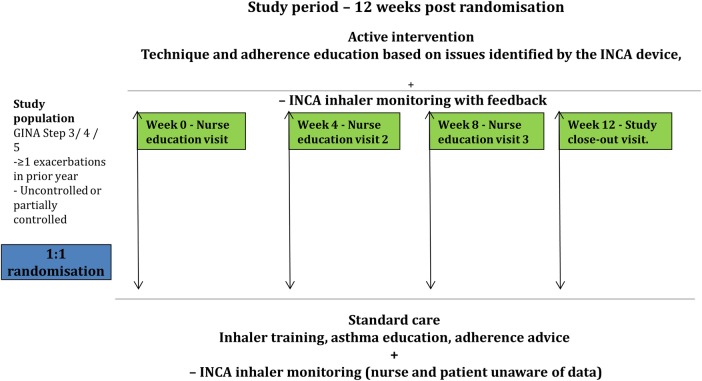
Visits are scheduled 4, 8 and 12 weeks later, summarised in figure 1. At these visits, the participants return their inhaler and electronic peak flow monitor. Additionally, at each of these visits the ACT, AQLQ, PEFR as well as any exacerbations and changes in medications, including new medications are recorded. Owing to an omission in the original study protocol, where ACT was not a measured variable, it was not recorded on the first 60 participants. The training, as per allocation, is then given. Details of the clinical visits are included in the clinical training manual, online supplementary appendix 2.
Figure 1.
The study participants are patients with a diagnosis of asthma attending a severe asthma clinic who remain uncontrolled or partially controlled and have experienced at least one severe exacerbation of asthma in the prior year. With no medication change, adherence and inhaler technique are re-enforced over the 12-week monitoring period (INCA, INhaler Compliance Assessment).
Interventions
Control group: behavioural intervention and inhaler training
The key points of each of the visit consultations includes: participant-identified goals for outcomes, exploration of barriers to achieving goals, explanation of the purpose of asthma treatment and provision of an asthma management plan for exacerbations. A checklist is used to review and correct errors in inhaler technique (IPS). To promote adherence during the education, emphasis is given on the individual developing a habit in time of use of the inhaler. Four, 8 and 12 weeks later the participants return their inhaler and receive an identical structured consultation as at the first meeting. Participants and nursing staff are unaware of data from the INCA device in the control arm. A video and manual describing the exact steps of usual care is shown as https://www.youtube.com/watch?v=PlTkhVuogaI.
Active group: feedback using recordings from the INCA device
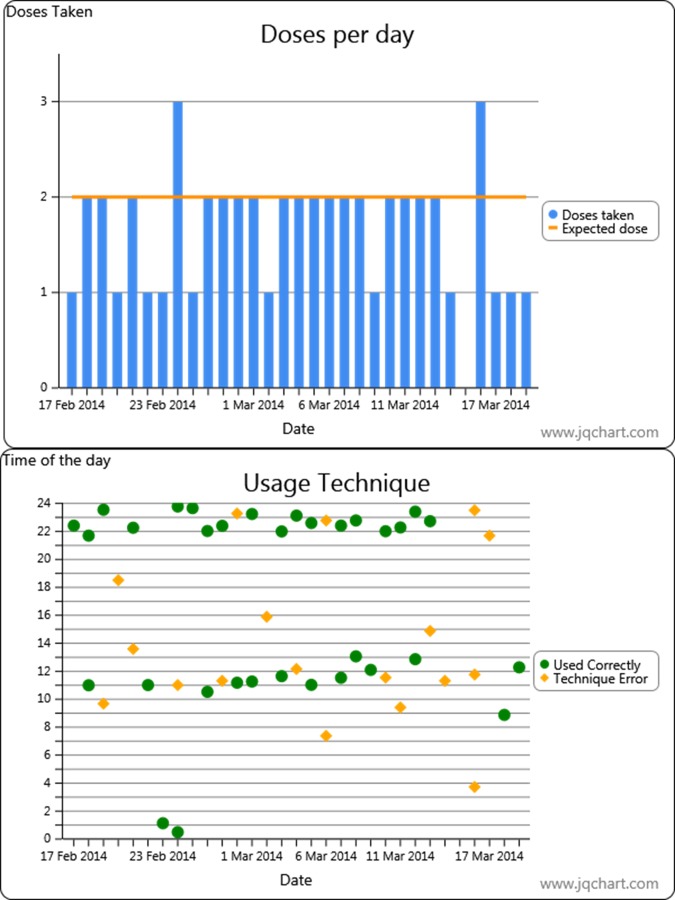
The content of the first visit was the same as for the control group. At 4, 8 and 12 weeks later, the participants together with the nurse review the information recorded on the INCA device and electronic PEFR (ePEFR), in the form of a graph, see figure 2 for an example of graphs produced by the INCA device. This graph leads to a consultation that focuses on the time of use, patterns of inhaler use, attempting to identify barriers to good adherence, development of habit of use as well as remediation of errors in inhaler use, as identified by the analysis of the data.
Figure 2.
A screen shot of the data presented to the patient for discussion of their adherence to the salmeterol/fluticasone Diskus inhaler over the prior month. In this example, the patient has good time of use, in particular in the evening, suggesting they are developing a regular habit of use. However, they show intermittent errors in inhaler technique. In this example, they used the inhaler incorrectly on almost half of all occasions in which the inhaler was used.
Data collection
INCA device
The original INCA device was designed at the Department of Bioengineering, Trinity College Dublin, Ireland and Conformité Européenne (CE) marked and manufactured by Vitalograph, Ennis, Ireland.
Analysis of the audio data
Analysis of the digital recordings is performed as previously described.5 The files are uploaded to a server and analysed using signal processing methods. For patients in the active arm, these audio recordings are uploaded during the visit by the nurse and feedback is given to the patient based on an automated analysis of the audio files. The sensitivity and specificity details have been published.7
At a later date, two independent raters over-read all files from all patients. Their agreed, combined analysis will be used in the calculation of the actual adherence. These raters are unaware of either the patient allocation or any of the patient clinical outcomes and are not involved in any aspect of the patient care during the trial. Critical inhaler errors including whether the drug was primed, whether the patient exhaled after priming but before inhalation, whether an adequate flow rate was achieved, the exact flow rate, whether there were multiple inhalations indicating inadequate breath-holds and correct sequence of events/timing of events were performed. Non-critical errors such as not holding the device vertically (as described by the manufacturer) were not recorded. The sensitivity and agreement within the two raters and between the raters and the algorithm have been published.9 Any disagreements within raters were reviewed by a third rater who made the final decision on the audio file.
Electronic peak expiratory flow rate
Participants receive an ePEFR device at each visit, which are then collected at the subsequent visit. For participants in the active arm, data from these devices are downloaded during the visit and information on PEFR, in conjunction with adherence data from the INCA device, are feedback to the participant.
Objectives
The objective of this study is to assess if feedback obtained from the INCA device on adherence and technique errors yields better adherence and better clinical information than best practice.
Primary outcome
The actual inhaler adherence, expressed as cumulative drug exposure, is calculated by combining the time of use along with the interval between doses (correct time is twice a day, in a period not <6 h between the last dose and the subsequent doses, or at a time >18 h apart from the previous dose) and incorporating, by audio analysis, if the inhaler was used correctly (ie, no evidence of critical technique errors aforementioned). The rate of actual adherence for the last month of the intervention will be compared between the active and control patients.
Secondary outcome analysis will include
Clinical outcomes, PEFR, ACT, AQLQ, reliever use and exacerbations between active and control arms at the end of the study will be compared. A composite score of these values, the global clinical outcome profile, see table 2 comprising the observed adherence, peak flow data, asthma control, quality of life and reliever use, as well as exacerbations over the study period will be calculated. Exacerbations is defined as an increase in symptoms (ie, shortness of breath, wheeze, cough) requiring a course of systemic glucocorticoids. Healthcare utilisation (ie, unscheduled general practitioner visits, hospitalisation and emergency department visits) will also be compared between active and control arms.
Table 2.
Clinical decision tool
| Non-adherence | Refractory asthma | Controlled asthma | Comorbidity | |
|---|---|---|---|---|
| Actual adherence >80% | No | Yes | Yes or no | Yes |
| PEFR >80 of area under the curve | Yes or no | No | Yes | Yes |
| AQLQ >5 and ACT >19 | Yes or no | No | Yes | No |
| Exacerbations | Yes or no | Yes or no | No | Yes or no |
The outcome decision tool, at the end of the study the cumulative information on actual adherence. PEFR rate, calculated as the AUC within 80% of normal predicted. ACT and AQLQ considered to be optimal, and exacerbations will be used to describe one of four possible outcomes. Sufficiently non-adherent as the likely reason for failure to progress, asthma that is refractory because despite optimal adherence, both symptoms and lung function and exacerbations occur. Controlled asthma, patients who are no longer impaired nor have exacerbations, and a group of patients who have good adherence and lung function but who continue to have symptoms, therefore suggesting that a significant comorbidity is the likely driver for the ongoing symptoms.
ACT, Asthma Control Test; AQLQ, Asthma Quality of Life Questionnaire; PEFR, peak expiratory flow rate.
Comparison of the proportion of patients in each group who achieved full adherence at the end of the study (≥80%), as well as changes in patterns of adherence in the two groups, the number progressing to good actual technique will be assessed. A comparison of the morning and evening habits of inhaler use, error rates, overdose rates, interval and attempted rates of adherence in the two groups at the end of the study will be compared. The factors associated with improving adherence will be described.
The relationship between adherence and asthma control, asthma quality of life and PEFR will be assessed by comparing the proportion of patients who are GINA 2011, ACT controlled and no longer require regular β agonist. The ACT, PEFR rate, AQLQ and reliever use between the two groups will also be compared.
The κ score between raters and a sensitivity analysis of the algorithm will be calculated.
Sample size calculation
The usual rate of adherence to inhalers is reported to be calculated from the dose counter, and is expressed as an average adherence. Most studies in trials of patients with inhalers report adherence of >0.8. Therefore, we anticipate that there is going to be high adherence in the setting of a clinical trial. However, we also expect that there will be a number of patients with poor inhaler technique which will lead to a lower actual adherence. We shall assume that when this is accounted for then the actual adherence is 0.15 lower, that is, 0.65 adherence at the end of the first month. Our preliminary data in primary care and on the wards indicates an SD of adherence is 0.25.5 The primary end point is the rate of adherence at the end of the study period, that is, during the last visit at month 3 between active and control groups. We expect the adherence to improve over the study period in the control group, as they are repeatedly educated in inhaler use by 0.05, and we expect the active group to get closer to the physician-reported ideal rate of 0.8, that is, a 0.15 improvement in actual adherence. Hence, with a power of 0.8 at the 0.05 significance level, with a 0.1 difference in the actual adherence rate, then a sample size of 100 in each limb is required. We expect a 10% dropout; hence the target recruitment sample size is 220 in total.
Randomisation and allocation
Randomisation will use a stratified-by-site random block design, with blocks varying in size of 8–12. Allocation ratio is 1:1 with a central computer-generated randomisation. This is a single blind study, the nurse may deliver either intervention and is not blinded to the allocation. The participants are aware which group they are allocated to and aware that data on adherence is being collected for analysis. Patients in the control arm will be blinded to their adherence data from the INCA device.
Statistical methods
Data analysis
Data will be analysed on an intention-to-treat and a per-protocol basis. Data will be presented as means with SDs, and Student t test will be used to compare differences in mean adherence rates between the groups. Significance will be set at the 5% level. Stratification of patients by new versus previous use with respect to use of the Diskus device (ie, if patient's are using the Diskus device for the first time, or if they have previously used the device), stratification based on severity of disease according to GINA guidelines.
Discussion
Most management guidelines suggest that for poorly controlled asthma patients that before changing therapy, issues with adherence and inhaler technique need to be addressed.1–3 However, this is difficult to achieve in clinical practice. The aim of this study is to see if a nurse-delivered educational intervention with repeated education and monitored adherence can improve both aspects of adherence, the time and the technique of inhaler use. We will record when and how well an individual has used their inhaler over time with a device that makes a digital audio recording of an individual using their inhaler. Analysis of the recorded time of use, the interval between use and technique of use provides a measure termed actual adherence. The study's primary outcome will be a comparison of the actual adherence between the two groups at the end of the third month of participation in the study.
It is expected that over the study period some patients will become fully controlled while others will remain poorly controlled despite being fully adherent over the study period, and others may remain uncontrolled and also poorly adherent. This information may help with clinical decision-making for individual patients, for example, by helping decide who should have their therapy increased, their inhaler device changed or further interventions to promote adherence such as motivational interviewing. Hence, by combining clinical outcomes with the longitudinally collected adherence, asthma control and PEFR data, the composite outcome may assist a clinician in identifying the cause of difficult-to-manage asthma and increase the clinical confidence that the patient has refractory asthma.
This study has several novel features; this is the first study to use a technology that objectively assesses adherence to inhalers both in terms of technique of use as well as time of use. This technology involves not simply the device but also the automated algorithms, the feedback tools and the content of the feedback delivered by the nurse during the consultation.
It could be argued that the results of this clinical research study, performed in a research setting, will lead to greatly improved adherence, which is not reflective of clinical practice. Additionally, both control and active patients will be reviewed on a monthly basis for 3 months. This approach itself will more than likely also lead to an increase in adherence in the active and control group and may not fully reflect ‘usual care’. To add to this, patients in both the active and control arms are aware that their inhaler use is being ‘monitored’, and this may lead to increased inhaler adherence. The authors see no alternative way of performing the study, as practical challenges in a more real-world setting may lead to a significant loss of patient follow-up, and hence, less precise information. The benefits of regular visits and PEFR measurements outweigh the disadvantages. Another limitation of the study is that it has a limited follow-up time frame, hence, the long-term effect on clinical outcomes and persistence of the observed benefits will not be established.
In regard to safety, patients enrolled in this study have to be uncontrolled or partially controlled by GINA guidelines. However, for the duration of the study their regular medical treatment of their asthma will not be changed (ie, their ICS/LABA dose will remain unchanged). The rationale behind this is to see the effect 3 months of adherence training would have on asthma control, potentially reducing the need to increase patient medication (step-up), and possibly allowing physicians to reduce asthma treatment (step-down). Patients can withdraw at any time during the study without any impact on their clinical care. Additionally, if the clinician feels there is a clinical indication, patients can be removed from the study in the patient's best interest.
In summary, this study proposes to assess the impact of a series of consecutive educational visits on adherence and inhaler use by patients with severe asthma. In addition, by combining objective measurement of lung function, clinical outcomes and objectively assessed adherence, this may also provide clinicians greater precision in decision-making for the future care of this group of patients.
Acknowledgments
The authors would like to thank Deidre Hyland, John McCourt and D Kenny, RCSI Centre for Clinical Research; Professor D Kelleher and Professor P Murray from the Dublin Centre for Clinical Research; Professor Eavan Daly and Professor Cheryl Marron from GSK; Professor Frank Keane and Professor Enda Kelly from Vitalograph Ireland; the research staff of the Centres for Clinical Research at St Vincent's Hospital, Adelaide, Meath and National Children's Hospital (AMNCH), St James's Hospital and Cork University Hospital.
Footnotes
Collaborators: Professor D Kelleher, Professor P Murray, Professor Eavan Daly, Professor Cheryl Marron, Professor Frank Keane, Professor Enda Kelly, Professor Deirdre Flynn.
Contributors: RBR, CH and RWC conceived the INCA device. RWC conceived and designed the study. EMacH, IS, GD, SDS and MF made substantial contributions to study design; JS, MH, TT, IS, SDS, IK and VR have all been involved in defining the characteristics of the INCA device and the associated acoustic and other analysis. GC provided the statistical support and contributed to the drafting of the manuscript. All authors were involved in the writing of the manuscript and revising it critically for intellectual content; and have given final approval of the version to be published. All authors read and approved the final manuscript.
Funding: This study is funded by the Health Research Board of Ireland.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Beaumont Hospital Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All the available data will be published in this paper.
Contributor Information
Collaborators: D Kelleher, P Murray, Eavan Daly, Cheryl Marron, Frank Keane, Enda Kelly, and Deirdre Flynn
References
- 1.GINA Report, Global strategy for asthma management and prevention- 2010. http://www.ginasthma.org
- 2.British Thoracic Society, Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax 2014;69(Suppl 1):1–192. [PubMed] [Google Scholar]
- 3.National Heart Lung and Blood Institute. National Asthma Education and Prevention Program. Expert PanelReport 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health, 2007. [Google Scholar]
- 4.Tommelein E, Mehuys E, Van Tongelen I et al. Accuracy of the Medication Adherence Report Scale (MARS-5) as a quantitative measure of adherence to inhalation medication in patients with COPD. Ann Pharmacother 2014;48:589–95. 10.1177/1060028014522982 [DOI] [PubMed] [Google Scholar]
- 5.D'Arcy S, MacHale E, Seheult J et al. A method to assess adherence in inhaler use through analysis of acoustic recordings of inhaler events. PLoS ONE 2014;9:e98701 10.1371/journal.pone.0098701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Boven JFM, Trappenburg JCA, Van de Molen T et al. Towards tailored and trageted adherence assessment to optomise asthma management. NPJ Prim Care Respir Med 2015;25:15046 10.1038/npjpcrm.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes MS, Le Menn M, D'Arcy S et al. Automatic identification and accurate temporal detection of inhalations in asthma inhaler recordings. Conf Proc IEEE Eng Med Biol Soc 2012;2012:2595–8. 10.1109/EMBC.2012.6346495 [DOI] [PubMed] [Google Scholar]
- 8.Holmes MS, Seheult J, Geraghty C et al. Using acoustics to estimate inspiratory flow rate and drug removed from a dry powder inhaler. Conf Proc IEEE Eng Med Biol Soc 2013;2013:6866–9. 10.1109/EMBC.2013.6611135 [DOI] [PubMed] [Google Scholar]
- 9.Holmes MS, D'Arcy S, Costello RW et al. An acoustic method of automatically evaluating patient inhaler technique. Conf Proc IEEE Eng Med Biol Soc 2013;2013:1322–5. 10.1109/EMBC.2013.6609752 [DOI] [PubMed] [Google Scholar]
- 10.Holmes MS, Seheult JN, Geraghty C et al. A method of estimating inspiratory flow rate and volume from an inhaler using acoustic measurements. Physiol Meas 2013;34:903–14. 10.1088/0967-3334/34/8/903 [DOI] [PubMed] [Google Scholar]
- 11.Taylor TE, Holmes MS, Sulaiman I et al. An acoustic method to automatically detect pressurized metered dose inhaler actuations. Conf Proc IEEE Eng Med Biol Soc 2014;2014:4611–14. 10.1109/EMBC.2014.6944651 [DOI] [PubMed] [Google Scholar]
- 12.Holmes MS, Seheult JN, O'Connell P et al. An acoustic-based method to detect and quantify the effect of exhalation into a dry powder inhaler. J Aerosol Med Pulm Drug Deliv 2015;28: 247–53. 10.1089/jamp.2014.1169 [DOI] [PubMed] [Google Scholar]
- 13.Seheult JN, O'Connell P, Tee KC et al. The acoustic features of inhalation can be used to quantify aerosol delivery from a Diskus dry powder inhaler. Pharm Res 2014;31:2735–47. 10.1007/s11095-014-1371-x [DOI] [PubMed] [Google Scholar]
- 14.Basheti IA, Bosnic-Anticevich SZ, Armour CL et al. Checklists for powder inhaler technique: a review and recommendations. Respir Care 2014;59:1140–54. 10.4187/respcare.02342 [DOI] [PubMed] [Google Scholar]
- 15.Juniper EF, Guyatt GH, Epstein RS et al. Evaluation of impairment of health-related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax 1992;47:76–83. 10.1136/thx.47.2.76 [DOI] [PMC free article] [PubMed] [Google Scholar]