Abstract
An 18-year-old man suffered a sudden cardiac arrest with ventricular fibrillation and was successfully resuscitated. He had neither a medical nor family history of cardiac disease/sudden death, but was known to have Graves’ disease, for which he was treated with radioactive iodine. Recently, block-and-replacement therapy had been discontinued to evaluate thyroid functioning. On admission, thyroid hormone levels were markedly elevated, suggesting thyroid storm due to residual Graves’ disease. The patient was treated with propylthiouracil, hydrocortisone and Lugol solution. ECG showed repolarisation patterns suggestive of an underlying type 1 Brugada syndrome (BS). These findings were confirmed by an additional ajmaline test. An implantable cardioverter defibrillator was implanted to prevent future arrhythmias. The patient underwent total thyroidectomy 9 months later and recovered completely. To the best of our knowledge, this is the first reported case of a sudden cardiac arrest as a presentation of BS unmasked by thyroid storm.
Background
Thyroid storm is a rare life-threatening endocrine emergency needing prompt diagnosis and aggressive therapy. It predominantly occurs in women, and in patients with underlying Graves’ disease (GD).1 Diagnosis is based on clinical signs and symptoms, including unconsciousness, fever, heart failure, diarrhoea and jaundice. Recent data from Japan show an incidence of 0.22% in hospitalised patients and a mortality rate of more than 10%.2 The main causes of death are multiple organ failure, congestive heart failure, respiratory failure and arrhythmia mainly of supraventricular origin.2 Ventricular tachycardia and ventricular fibrillation (VF) are rare, and usually only occur in those with underlying cardiac disease.3 4 Brugada syndrome (BS) is a rare autosomal dominant disease that predisposes to the development of ventricular arrhythmias. It is diagnosed most commonly in men aged 40–45 years. Most patients with BS are asymptomatic but presenting symptoms include syncope and sudden cardiac death mainly during sleep or at rest.5
Case presentation
An 18-year-old man with a history of GD presented to the emergency room after treatment for sudden cardiac arrest (SCA). Family members started basic life support after he was found unconscious at night in bed. On arrival at emergency medical services, primary rhythm revealed VF. He was defibrillated 15 times and return of spontaneous circulation was achieved after 45 min without the patient regaining consciousness. He was transferred to the intensive care unit (ICU) for haemodynamic and ventilatory support, and for immediate induction of therapeutic hypothermia for cerebral protection (target temperature 32.5°C).
The patient's medical history included residual GD, for which he was treated with radioactive iodine 4 months prior to the present admission. Block-and-replacement therapy (strumazol 30 mg and levothyroxine 100 µg), resulting in euthyroidism, was given until 2 weeks before this admission, after which he was advised to discontinue the medication in order to evaluate thyroid function. During this period, the patient did not report any symptoms. He had no family history of sudden cardiac death.
The patient presented as a comatose (E1M4V1) young man with unstable vital signs (100% saturation on pressure controlled ventilation with level 12, PEEP 8 and FiO2 0.41, blood pressure 160/95 mm Hg, pulse rate 120 bpm). Pupils were isochoric with symmetrical light reflexes. Body temperature on admission was normal. The thyroid gland was not enlarged and examination of the heart, lungs and abdomen was unremarkable.
Investigations
Laboratory analysis on admission showed normal blood cell counts; electrolytes, kidney function and hepatic enzymes were normal. Cardiac markers were elevated (creatine kinase (CK) 1363 IU/L and troponin-I 3.92 µg/L). The postresuscitation ECG revealed sinus tachycardia, with intermediate axis in the frontal plane, normal P-wave and PQ-interval, with prolonged QRS duration of 110 ms and ST-depression in the inferolateral leads. These findings are neither diagnostic nor suspicious for the presence of BS. The initial transthoracic echocardiogram (TTE) showed no wall motion abnormalities and ventricular dimensions were unremarkable.
Two hours after admission, haemodynamic instability occurred with development of a right bundle branch block and recurrent episodes of VF. The patient was resuscitated multiple times according to the advanced cardiac life support (ACLS) shock protocol, with return of spontaneous circulation. Further diagnostic work up revealed markedly elevated free thyroxine levels (62 pmol/L, normal 10–24 pmol/L), while thyroid-stimulating hormone was suppressed (<0.01 mIU/L, normal 0.3–4.5 mIU/L). This hyperthyroidism in the presence of tachycardia and coma strongly suggested the diagnosis of thyroid storm.
Treatment
Two and a half hours after admission, treatment with propylthiouracil (PTU) 200 mg every 4 h, hydrocortisone 100 mg intravenously every 6 h and acetaminophen 500 mg every 6 h was initiated. After PTU was given, 20 drops of Lugol solution were administered every 8 h. The use of propranolol was contraindicated due to haemodynamic instability.
Despite the aforementioned treatment, pulseless electric activity developed 3 h after admission and repeated TTE revealed distended hypokinetic ventricles. Enoximone 6.4 µg/kg/min and epinephrine 2 mg/h continuously did not improve haemodynamics. Therapeutic hypothermia was reached but discontinued because of cardiogenic shock and the patient was resuscitated according to the ACLS non-shock protocol. Circulatory support with implantation of a left ventricular (LV) assist device was considered, for which the patient was transferred to an academic hospital. On arrival, the patient underwent coronary angiography, which showed no abnormalities.
Outcome and follow-up
Circulatory parameters improved gradually and after 2 days the patient was weaned from inotropic support. Sedation was discontinued and the patient awoke with no neurological deficits. Mechanical ventilation could be discontinued on the fourth day after his cardiac arrest. Toxicology screening tests from admission (cannabis, amphetamines, cocaine, opiates, benzodiazepines, GHB and XTC) came back negative.
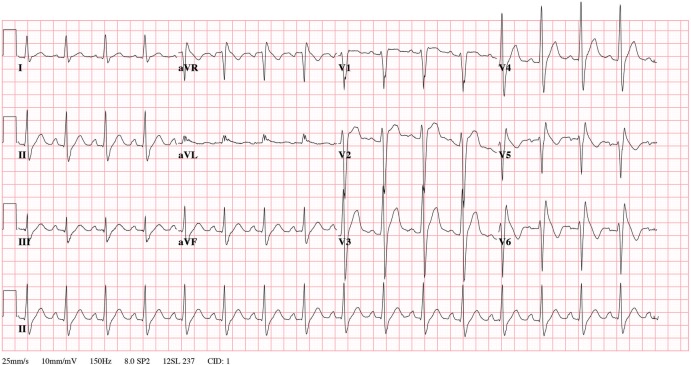
One week after starting treatment, the free thyroxine levels normalised and the patient was transferred to the cardiology ward for further analysis. Cardiac MRI suggested left ventricular (LV) hypertrophy, normal right ventricular (RV) dimensions and no signs of fibrosis, excluding an underlying structural heart disease. Post critical care ECG showed an incomplete right bundle branch block and upsloping ST-segment in V2 and V3. With ajmaline (an intravenously administered sodium channel blocking agent), the right precordial leads (V5 and V6 were placed cranial to V1 and V2) changed with J-point elevation, a coved type ST-segment elevation and an inverted T-wave (figure 1). Based on clinical presentation and ECG findings on ajmaline, an underlying BS type 1 was diagnosed.
Figure 1.
ECG with ajmaline: the right precordial leads (V5 and V6 were placed cranial to V1 and V2) changed with J point elevation, a coved type ST-segment elevation and an inverted T-wave.
Cardiac function normalised completely. An implantable cardioverter defibrillator (ICD) was implanted before discharge to prevent further cardiac arrhythmias. Nine months later, an uncomplicated total thyroidectomy was performed. Genetic linkage analysis revealed no presence of the SCN5A gene mutation. At writing, one year after the arrest, the patient is fully recovered and experienced no further ICD shocks during the past year. He continued his studies in ICT and remembers little of his experience in the ICU.
Discussion
We describe a case of an 18-year-old man presenting with an SCA due to primary VF in the presence of thyroid storm. Ventricular arrhythmias are an important cause of SCA, usually in combination with structural heart disease, predominantly coronary disease with myocardial infarction.6 Less commonly, VF may be associated with metabolic disorders, drug toxicity or prolonged or short QT syndrome. Rhythm disturbances in thyroid storm are, in general, supraventricular, including atrial premature conduction and atrial fibrillation.7 Ventricular tachycardia and VF are rare, and usually only occur in those with underlying cardiac disease.3 4
In our case, the diagnosis of thyroid storm was based on the findings of coma and tachycardia in a state of hyperthyroidism, with a score of 45 in the scoring system by Burch and Wartofsky8 being highly indicative of thyroid storm. On admission, our patient was not known to have cardiac disease, but given the rarity of his presentation, we instantly suspected an underlying condition. After initial resuscitation and postresuscitation care, BS type 1 was diagnosed based on the ECG and ajmaline test. BS is characterised by a typical ST segment elevation in the right precordial leads often accompanied by an apparent right bundle branch block without underlying structural heart defects.5 It is diagnosed most commonly in men aged 40–45 years. Most patients with BS are asymptomatic but presenting symptoms include syncope and SCA, mainly during sleep or at rest.5
In most cases describing SCA in thyroid storm, the underlying thyroid pathology is GD, either as a first presentation9–11 or due to discontinuation of medication,12 13 such as in our case. However, thyroid storm with subsequent SCA may also occur in other thyroid conditions, including iatrogenic thyrotoxicosis (amiodarone13 or iodine contrast-induced).14 Underlying structural heart disease plays a role in some of these,13 but not in all.9–12 A Brugada pattern has never been reported in association with thyroid storm, but did occur in a case of hypokalaemic periodic paralysis as a complication of thyrotoxicosis.15
The pathophysiological mechanism in which arrhythmias occur in thyroid storm is unknown. In general, excess thyroid hormone is known to cause alterations in cardiovascular haemodynamics, including increased heart rate, ejection fraction and cardiac output.16 The associated increase in myocardial oxygen consumption and cardiac workload may give rise to a high output cardiac failure and can cause reversible dilated cardiomyopathy and cardiac ischaemia. The postulated underlying pathophysiological mechanism in BS is an ion channel defect leading to heterogeneous loss of the action potential in the right ventricular epicardium and dispersion of repolarisation.17 We hypothesise that the dilated cardiomyopathy (as shown on TTE) and possible subsequent cardiac ischaemia, in combination with the altered repolarisation in BS, led to ventricular arrhythmia in our patient. To the best of our knowledge, this is the first reported case of SCA as a presentation of BS unmasked by thyroid storm.
Conclusion
We described an unusual case of an 18-year-old man presenting with an SCA due to primary VF in the presence of thyroid storm. During work up, an underlying BS type 1, a rare autosomal dominant disease that predisposes to the development ventricular arrhythmias, was diagnosed. After medical treatment in the ICU, an ICD was implanted and total thyroidectomy performed. A year later, the patient did not experience any shocks and is able to continue his studies in ICT.
Learning points.
Cessation of antithyroid drugs to evaluate thyroid function testing, even after treatment with radioactive iodine, is not without risk.
Determination of thyroid function should be considered in patients who develop idiopathic ventricular, and not just supraventricular, arrhythmias.
Underlying cardiac disease predisposes to the development of potentially fatal ventricular arrhythmias in thyroid storm.
Specifically in Brugada syndrome type 1, dispersion of repolarisation predisposes the heart to the development of malignant ventricular arrhythmias.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am 2006;35:663–86, vii 10.1016/j.ecl.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 2.Akamizu T, Satoh T, Isozaki O et al. . Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid 2012;22:661–79. 10.1089/thy.2011.0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biondi B, Kahaly GJ. Cardiovascular involvement in patients with different causes of hyperthyroidism. Nat Rev Endocrinol 2010;6:431–43. 10.1038/nrendo.2010.105 [DOI] [PubMed] [Google Scholar]
- 4.Ladénson PW. Thyrotoxicosis and the heart: something old and something new. J Clin Endocrinol Metab 1993;77:332–3. 10.1210/jcem.77.2.8345036 [DOI] [PubMed] [Google Scholar]
- 5.Berne P, Brugada J. Brugada syndrome 2012. Circ J 2012;76:1563–71. 10.1253/circj.CJ-12-0717 [DOI] [PubMed] [Google Scholar]
- 6.John RM, Tedrow UB, Koplan BA et al. . Ventricular arrhythmias and sudden cardiac death. Lancet 2012;380:1520–9. 10.1016/S0140-6736(12)61413-5 [DOI] [PubMed] [Google Scholar]
- 7.Osman F, Gammage MD, Franklyn JA. Hyperthyroidism and cardiovascular morbidity and mortality. Thyroid 2002;12:483–7. 10.1089/105072502760143854 [DOI] [PubMed] [Google Scholar]
- 8.Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am 1993;22:263–77. [PubMed] [Google Scholar]
- 9.Ando T, Henmi T, Haruta D et al. . Graves’ disease complicated by ventricular fibrillation in three men who were smokers. Thyroid 2011;21:1021–5. 10.1089/thy.2010.0368 [DOI] [PubMed] [Google Scholar]
- 10.Anjo D, Maia J, Carvalho AC et al. . Thyroid storm and arrhythmic storm: a potentially fatal combination. Am J Emerg Med 2013;31:1418.e3–5. 10.1016/j.ajem.2013.04.026 [DOI] [PubMed] [Google Scholar]
- 11.Munoz-Camacho JF, Sagrista-Sauleda J. Malignant ventricular arrhythmias as the initial manifestation of hyperthyroidism. Rev Esp Cardiol 2007;60:449–50. 10.1157/13101650 [DOI] [PubMed] [Google Scholar]
- 12.Jao YT, Chen Y, Lee WH et al. . Thyroid storm and ventricular tachycardia. South Med J 2004;97:604–7. 10.1097/00007611-200406000-00020 [DOI] [PubMed] [Google Scholar]
- 13.Nadkarni PJ, Sharma M, Zinsmeister B et al. . Thyrotoxicosis-induced ventricular arrthythmias. Thyroid 2008;18:1111–14. 10.1089/thy.2007.0307 [DOI] [PubMed] [Google Scholar]
- 14.Alkhuja S, Pyram R, Odeyemi O. In the eye of the storm: iodinated contrast induced thyroid storm presenting as cardiopulmonary arrest. Heart Lung 2013;42:267–9. 10.1016/j.hrtlng.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 15.Tsai CF, Wu DJ, Lin MC et al. . A Brugada-pattern electrocardiogram and thyrotoxic periodic paralysis. Ann Intern Med 2010;153:848–9. 10.7326/0003-4819-157-1-201207030-01001 [DOI] [PubMed] [Google Scholar]
- 16.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501–9. 10.1056/NEJM200102153440707 [DOI] [PubMed] [Google Scholar]
- 17.Gussak I, Antzelevitch C, Bjerregaard P et al. . The Brugada syndrome: clinical, electrophysiologic and genetic aspects. J Am Coll Cardiol 1999;33:5–15. 10.1016/S0735-1097(98)00528-2 [DOI] [PubMed] [Google Scholar]