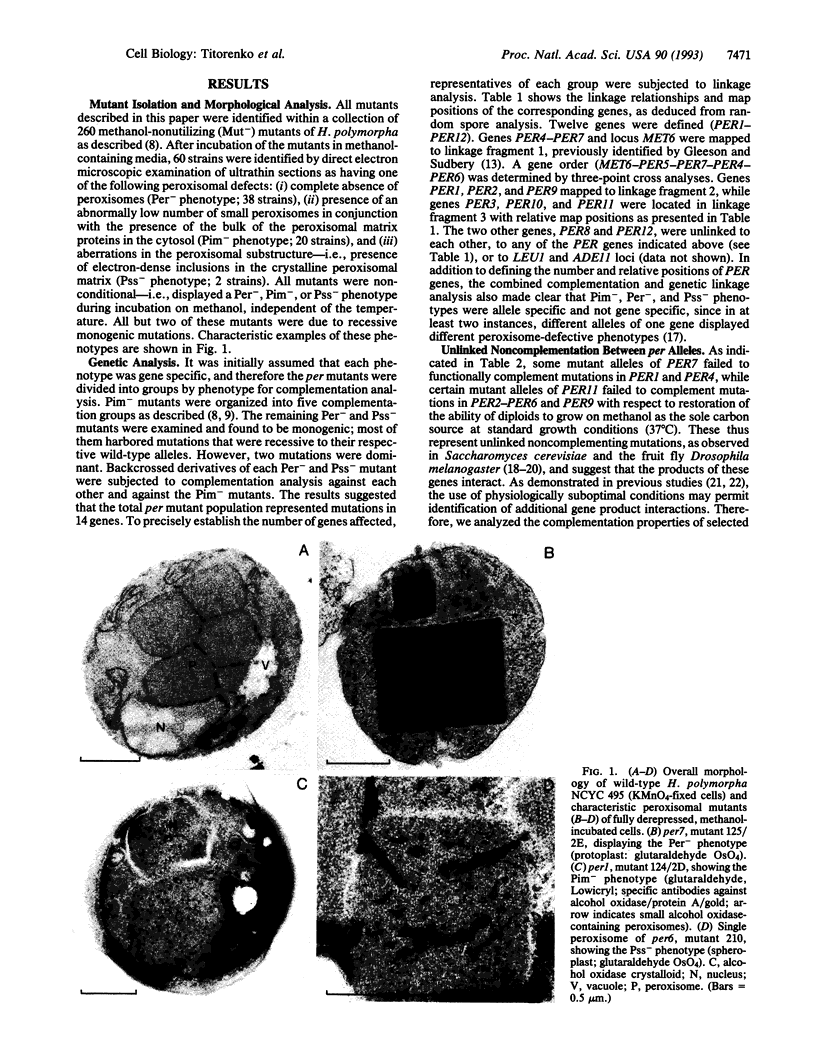
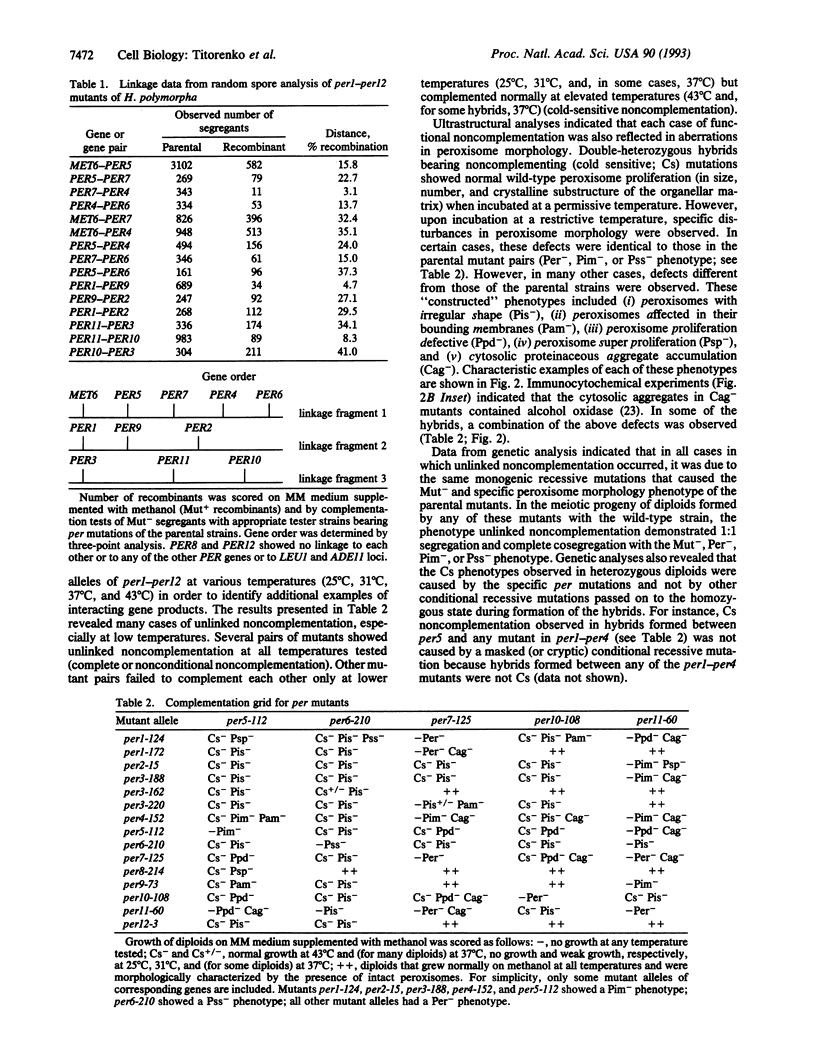
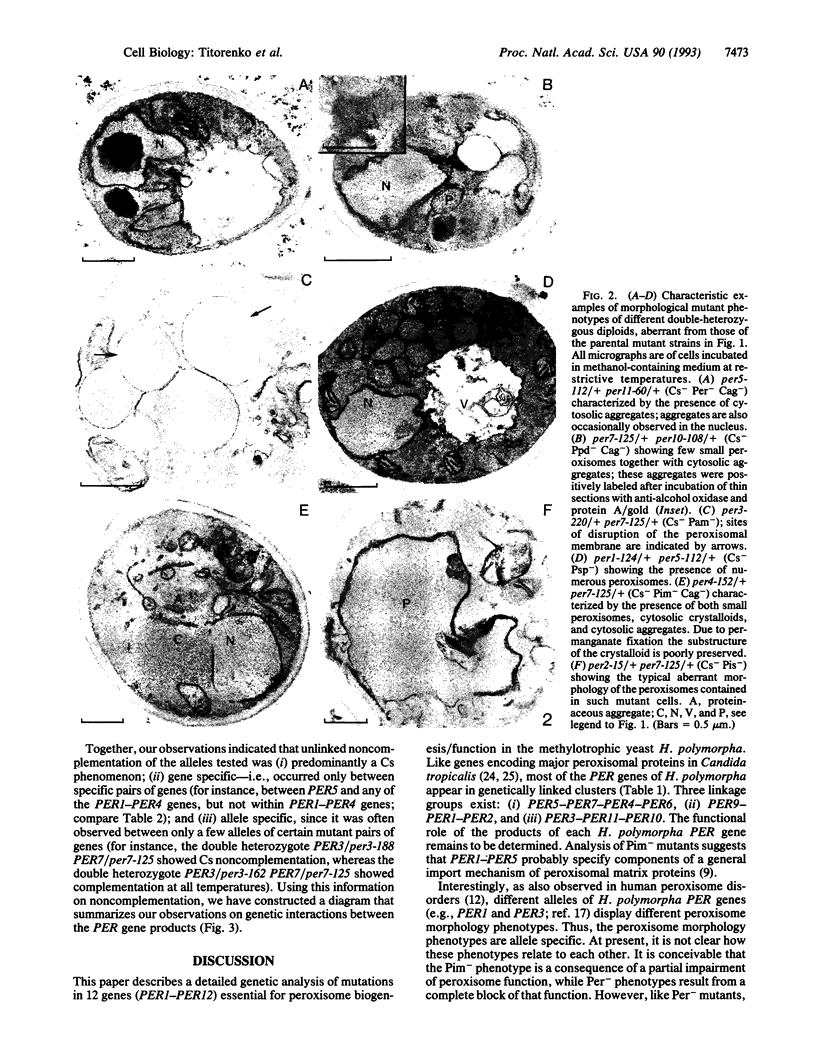
Abstract
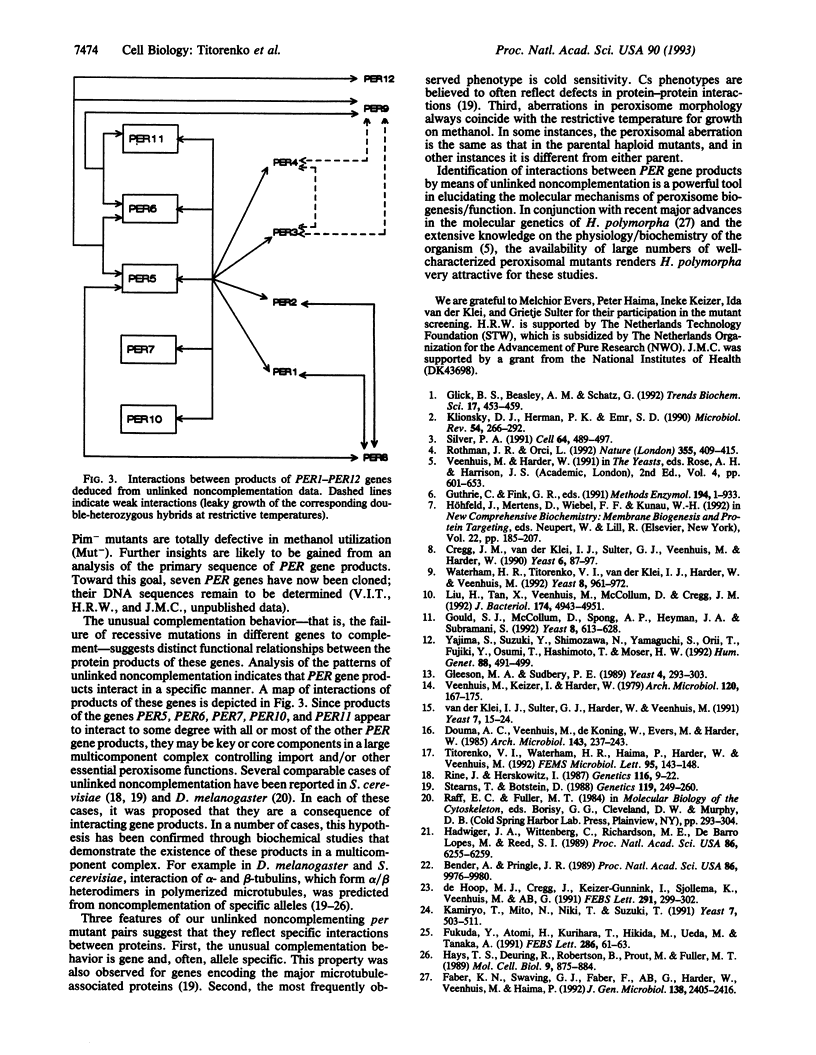
We have studied the genetic interactions between mutant alleles in 12 genes, designated PER1-PER12, which are essential for peroxisome biogenesis in the yeast Hansenula polymorpha. Recessive mutations in any of these genes determined three different morphological phenotypes: (i) complete absence of peroxisomes (Per-); (ii) presence of small peroxisomes in conjunction with a major fraction of peroxisomal matrix proteins in the cytosol (Pim-); and (iii) presence of peroxisomes with aberrant crystalline matrix substructure (Pss-). Extensive complementation analysis showed many cases of noncomplementation--that is, diploids that contained both wild-type and mutant alleles of two different PER genes were unable to grow on methanol and showed peroxisomal defects. The observed cases of unlinked noncomplementation appeared to be gene and allele specific and were predominantly observed at lower temperatures (cold sensitive). The genetic results obtained were used to formulate a model of PER gene product interactions. In this model, five PER gene products are key or core components of the complex. Other PER gene products appear to play a more peripheral role.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender A., Pringle J. R. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9976–9980. doi: 10.1073/pnas.86.24.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber K. N., Swaving G. J., Faber F., Ab G., Harder W., Veenhuis M., Haima P. Chromosomal targeting of replicating plasmids in the yeast Hansenula polymorpha. J Gen Microbiol. 1992 Nov;138(11):2405–2416. doi: 10.1099/00221287-138-11-2405. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Atomi H., Kurihara T., Hikida M., Ueda M., Tanaka A. Genes encoding peroxisomal enzymes are not necessarily assigned on the same chromosome of an n-alkane-utilizable yeast Candida tropicalis. FEBS Lett. 1991 Jul 29;286(1-2):61–63. doi: 10.1016/0014-5793(91)80940-5. [DOI] [PubMed] [Google Scholar]
- Glick B. S., Beasley E. M., Schatz G. Protein sorting in mitochondria. Trends Biochem Sci. 1992 Nov;17(11):453–459. doi: 10.1016/0968-0004(92)90487-t. [DOI] [PubMed] [Google Scholar]
- Gould S. J., McCollum D., Spong A. P., Heyman J. A., Subramani S. Development of the yeast Pichia pastoris as a model organism for a genetic and molecular analysis of peroxisome assembly. Yeast. 1992 Aug;8(8):613–628. doi: 10.1002/yea.320080805. [DOI] [PubMed] [Google Scholar]
- Hadwiger J. A., Wittenberg C., Richardson H. E., de Barros Lopes M., Reed S. I. A family of cyclin homologs that control the G1 phase in yeast. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6255–6259. doi: 10.1073/pnas.86.16.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays T. S., Deuring R., Robertson B., Prout M., Fuller M. T. Interacting proteins identified by genetic interactions: a missense mutation in alpha-tubulin fails to complement alleles of the testis-specific beta-tubulin gene of Drosophila melanogaster. Mol Cell Biol. 1989 Mar;9(3):875–884. doi: 10.1128/mcb.9.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Mito N., Niki T., Suzuki T. Assignment of most genes encoding major peroxisomal polypeptides to chromosomal band V of the asporogenic yeast Candida tropicalis. Yeast. 1991 Jul;7(5):503–511. doi: 10.1002/yea.320070510. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990 Sep;54(3):266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Tan X., Veenhuis M., McCollum D., Cregg J. M. An efficient screen for peroxisome-deficient mutants of Pichia pastoris. J Bacteriol. 1992 Aug;174(15):4943–4951. doi: 10.1128/jb.174.15.4943-4951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J., Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987 May;116(1):9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Stearns T., Botstein D. Unlinked noncomplementation: isolation of new conditional-lethal mutations in each of the tubulin genes of Saccharomyces cerevisiae. Genetics. 1988 Jun;119(2):249–260. doi: 10.1093/genetics/119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titorenko V. I., Waterham H. R., Haima P., Harder W., Veenhuis M. Peroxisome biogenesis in Hansenula polymorpha: different mutations in genes, essential for peroxisome biogenesis, cause different peroxisomal mutant phenotypes. FEMS Microbiol Lett. 1992 Aug 15;74(2-3):143–148. doi: 10.1016/0378-1097(92)90420-s. [DOI] [PubMed] [Google Scholar]
- Yajima S., Suzuki Y., Shimozawa N., Yamaguchi S., Orii T., Fujiki Y., Osumi T., Hashimoto T., Moser H. W. Complementation study of peroxisome-deficient disorders by immunofluorescence staining and characterization of fused cells. Hum Genet. 1992 Mar;88(5):491–499. doi: 10.1007/BF00219334. [DOI] [PubMed] [Google Scholar]
- de Hoop M. J., Cregg J., Keizer-Gunnink I., Sjollema K., Veenhuis M., Ab G. Overexpression of alcohol oxidase in Pichia pastoris. FEBS Lett. 1991 Oct 21;291(2):299–302. doi: 10.1016/0014-5793(91)81306-s. [DOI] [PubMed] [Google Scholar]