Abstract
Acute flaccid paralysis (AFP) has a changing epidemiology with ongoing polio outbreaks and emerging causes such as nonpolio enteroviruses and West Nile virus (WNV). We report a case of AFP from the Horn of Africa that was initially classified as probable polio but subsequently found to be due to WNV.
Keywords: enterovirus, paralysis, polio, West Nile virus
Acute flaccid paralysis (AFP) is an infrequent clinical syndrome with a multitude of infectious and noninfectious causes, but recent global outbreaks of disease have been reported. In May 2014, the World Health Organization declared an international health emergency due to ongoing polio epidemics in the Horn of Africa, Afghanistan, Iraq, Pakistan, and Syria [1]. Wild polio virus (WPV) has also been detected in sewer collection samples in Israel and Egypt, indicating international spread to previously polio-free countries [2]. Acute flaccid paralysis due to emerging nonpolio enteroviruses has been concurrently reported in the United States, Europe, and China [3–5]. These events continue to make the recognition of AFP a priority. West Nile virus (WNV) continues to be an important cause of AFP in Europe and North America [6].
We report a case of AFP that was initially classified as probable polio by Australian public health authorities but was subsequently found to be due to WNV. This is the first case of AFP due to WNV in Australia. Despite being an imported case, it also highlights the need for recognition of other pathogens such as enterovirus D68 (EV D68) that are emerging causes of AFP [3, 4].
CASE REPORT
A 49-year-old resident of Djibouti who recently arrived in Australia presented to our hospital 1 week after onset of AFP of the left arm and left facial weakness. His illness began 4 weeks earlier with headaches, neck pain, fever, altered conscious state, and nausea and vomiting while traveling in Somalia for work. There was no history of tick bite. The patient was born in Somalia and his history of polio vaccination was unknown. A household member had received the oral polio vaccine as part of a campaign in Djibouti in response to a polio outbreak in the Horn of Africa approximately 3 months before onset of the patient's illness. The patient had been hospitalized in Djibouti initially and treated for presumptive meningitis with intravenous ceftriaxone and dexamethasone. He elected to return to Australia when an evolving flaccid paralysis of his left arm was noted after resolution of his other symptoms. During travel to Australia, the patient continued to feel unwell and was admitted to a hospital in Dubai (United Arab Emirates), where he was treated with intravenous vancomycin and meropenem and oral prednisolone 40 mg daily.
After return to Australia, the patient presented directly to our hospital and was noted to have lower motor neuron pattern deficit of the left arm, with complete paralysis of shoulder abduction and adduction, severe weakness of other proximal arm muscles, but preserved distal hand function. Biceps and brachioradialis reflexes were absent. In the first 2 days of admission, the patient developed asymmetric bilateral facial weakness in a lower motor neuron pattern. Lumbar puncture was performed, with cerebrospinal fluid (CSF) demonstrating elevated protein 0.88 g/L (normal range 0.15–0.4 g/L), 54 mononuclear cells, 0 neutrophils, and normal glucose. No bacteria were seen on Gram or Ziehl-Neeson stains. Cerebrospinal fluid cryptococcal antigen was negative (Immuno Mycologics, Inc., Norman, OK), as was polymerase chain reaction (PCR) for enteroviruses (real-time TaqMan PCR for enterovirus detection, primers and probe targeting 50 untranslated regions; [7]), flaviviruses (pan-flavivirus real-time PCR using heminested primers that amplify a conserved sequence in the NS5 gene; modified from Scaramozzino et al [8]), herpes viruses (modified from Druce et al [9]), Mycobacterium tuberculosis (GeneXpert MTB/RIF; Cepheid, Sunnyvale, CA), and Toxoplasma (reverse transcription-PCR [10]). Enterovirus PCR of throat and stool samples, human immunodeficiency virus serology (COBAS Core HIV Combi; Roche Diagnostics, Mannheim, Germany), and rickettsial serology (indirect micro-immunofluorescence assay [11]) were all negative. Serology for Borrelia afzelii suggested past infection with 5 immunoglobulin (Ig)G immunoblot bands being positive.
Magnetic resonance imaging (MRI) of the brain was unremarkable (using a standard protocol on 1.5T scanner [General Electric Signa Excite; GE Healthcare, Milwaukee, WI]) [12], but imaging of the cervical and thoracic spine revealed high T2 signal in the central cervical cord gray matter from C3 to C6, particularly in the anterior horn cells (Figure 1). Electromyography/nerve conduction studies were consistent with a proximal motor axonopathy or anterior horn cell disease affecting both upper limbs and facial muscles. Empiric antituberculous therapy (rifampicin, isoniazid, pyrazinamide, ethambutol, and moxifloxacin), doxycycline, prednisolone, and intravenous Ig (31 g daily for 5 days) were commenced pending further results.
Figure 1.
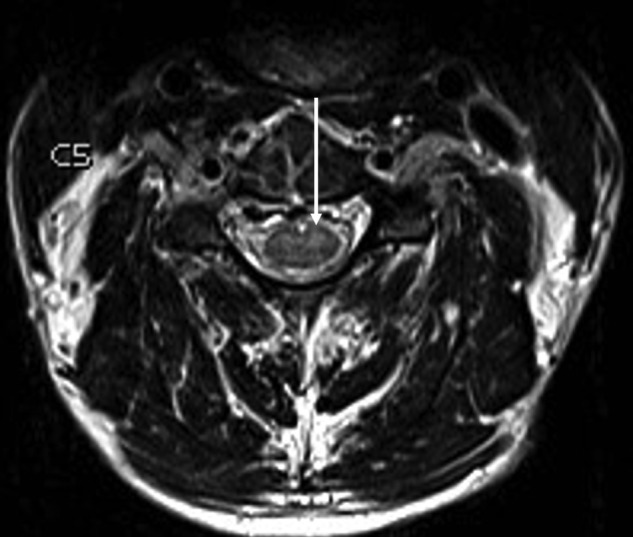
Axial T2-weighted magnetic resonance image of the cervical spine at C5 level demonstrates high T2 signal corresponding to central cervical cord gray matter greater on the left than the right, particularly involving the anterior horn cells (arrow).
Clinically, acute poliomyelitis was suspected and prompted isolation of the patient and notification to health authorities in Australia, Djibouti, and Dubai. After assessment by the Australian National Polio Expert Committee, the patient was classified as a polio-compatible case [13]. An initial stool sample had been insufficient for polio culture. Polio PCR and viral culture performed on 3 subsequent fecal samples and CSF were negative. Serology for polio subsequently demonstrated antibodies to all 3 polio types (WPV-1, 2.1 international units [IU], WPV-2 3.5IU, WPV-3 0.3IU [14]), consistent with previous vaccination. Serum WNV enzyme-linked immunosorbent assay (ELISA) IgM (Focus Diagnostics, Cypress, CA) taken day 1 of admission (day 25 of illness) was positive at 1:1280, indicating recent infection; this positive serology was subsequently confirmed by plaque reduction neutralization tests with a result of 1:2560 [15]. Cerebrospinal fluid (taken day 38 of illness) analyses for WNV (ELISA IgM and PCR; Procleix WNV Assay, Gen-Probe, and Chiron) were negative. Serial serum sampling over 2 months showed a 4-fold decrease in titer, consistent with the history of infection 4 weeks before presentation. After 3 months, the patient had complete resolution of facial weakness but ongoing weakness in his left shoulder (grade 0) and proximal arm muscles (grade 0 elbow flexion and extension).
DISCUSSION
Acute flaccid paralysis continues to be a public health priority due to disease caused by emerging pathogens and remains the primary means of polio surveillance. Although Guillain-Barré Syndrome remains the most common cause of AFP worldwide, our case highlights the threat of polio importation and the need to consider nonendemic pathogens such as WNV. Clinicians need to consider a broad differential when investigating patients with AFP (see Table 1) and take into account that there will be a shift in the epidemiology of AFP in previously polio-endemic countries, as demonstrated by the diagnosis of WNV in our case.
Table 1.
Infectious Causes of AFP: Clinical and Diagnostic Features
| Causes | Endemic Regions | Epidemiological and Clinical Features | Diagnostic Aspects | References |
|---|---|---|---|---|
| Viral | ||||
| Polio virus | ||||
| Wild polio virus | Africa, Middle East, Pakistan | Ongoing endemic transmission in Pakistan, Afghanistan, Nigeria, central Africa. No new cases in Horn of Africa since August 2014. Viral culture is gold standard |
Viral culture is gold standard but can take 1–3 weeks. Polio PCR on CSF and feces Serology (acute and convalescent titers) |
[16] |
| Vaccine-derived polio virus | Similar epidemiology to wild polio virus | |||
| Nonpolio enteroviruses | Worldwide | Clinical syndromes similar to wild polio virus | PCR on CSF, feces, respiratory secretions, blood | |
| Enterovirus D68 | North America, Europe | Recent outbreak of “acute flaccid myelitis” in United States and Europe. Most reports in pediatric population. | [3] | |
| Enterovirus 71 | Asia, Australia | Outbreaks described in Asia-Pacific region. Recent vaccine trials. | [17] | |
| Arthropod-borne viruses | PCR on CSF and blood Serological testing (virus-specific information below) |
[18, 19] | ||
| West Nile virus | North America, Europe, Africa | Approximately 5%–10% of patients with neuroinvasive disease develop AFP with case fatality rate of 10%–50%. | Viremia transient, therefore serological diagnosis key. Plaque reduction neutralization assays are done for confirmation of serological testing. |
[20] |
| Kunjin virus | Australia | Substrain of West Nile virus endemic to Australia. Similar clinical presentation. | [21] | |
| Japanese encephalitis | Asia | Mosquito-borne flavivirus. Classically presents with encephalitic illness but case series of AFP described. | [22] | |
| Chikungunya | Africa, Asia, Central and South America | Case reports of AFP described. Recent epidemics in Western Hemisphere. Frequently causes arthralgia. | [23] | |
| Dengue | Africa, Asia, Central and South America | Case reports of AFP described. Recent epidemics in Western Hemisphere. Frequently associated with rash. | NS1 antigen testing on plasma | [24] |
| Murray Valley encephalitis virus | Australia | Mosquito-borne flavivirus, presentation similar to Japanese encephalitis. AFP presentation described, particularly in children. One-third mortality rate. | [25] | |
| La Crosse virus | North America | Major cause of pediatric encephalitis in United States, particularly in central and eastern United States. Most cases in patients <15 years old. | [6, 26] | |
| Tickborne encephalitis virus | Europe | Tickborne flavivirus, endemic in northern Europe. Usually biphasic illness with initial systemic symptoms followed by neurological symptoms. |
[27] | |
| Toscana virus | Europe and Africa | Transmitted by sandflies. Typically found in Mediterranean countries. Coinfection with West Nile described. | [19, 28] | |
| Rabies | Africa, Asia, South Asia | AFP described in rare cases. | PCR testing of skin and saliva Serological testing on CSF and serum |
[29] |
| Cosavirus | Worldwide | Noted in fecal samples of nonpolio AFP cases. Causative role uncertain. | PCR on fecal samples | [30] |
| Bacterial | ||||
| Diphtheria | Africa, Central and South America, Asia, Europe | Neurological toxicity from absorption and dissemination of diphtheria toxin | Culture from throat and nose Need confirmation of toxin production | [31] |
| Lyme disease | North America, Europe | AFP is rare presentation | Serological testing PCR on CSF |
[32] |
| Botulism | Worldwide | Isolation of Clostridium botulinum from wound site Serum assay for botulinum toxin Electromyography |
[33] | |
| Rickettsia conorii | Europe, Africa | Cause of Mediterranean spotted fever. Rash very common. Case reports of AFP. | Serological testing | [34] |
Abbreviations: AFP, acute flaccid paralysis; CSF, cerebrospinal fluid; PCR, polymerase chain reaction.
Although polio was ultimately excluded in our case, in 2007 there was an importation of polio to Australia by a Pakistani student reflecting the ongoing possibility of transmission while WPV continues to circulate [35]. Despite declining polio incidence, outbreaks secondary to importation seem to disproportionately affect adults and have high mortality [36]. To complicate matters further, there has been “silent” circulation of WPV in sewage in Israel despite high vaccine coverage [37]. Furthermore, there are multiple reports of vaccine-derived polio transmission, and this was considered in our case due to a family member receiving oral polio vaccine but was thought unlikely because oral poliovirus is typically cleared within 6 weeks after vaccination [38–41]. Clinicians need to offer polio vaccination to individuals traveling to regions affected by the recent epidemics and also to migrant populations with undocumented vaccination histories, as was the case in our patient.
Currently, there is an increasing recognition of nonpolio enteroviruses taking the place of WPV as a global cause of AFP. The Centers for Disease Control and Prevention reported a cluster of acute limb weakness in children in Colorado that was subsequently associated with EV D68 [3, 4]. The clinical features have been grouped in a syndrome now termed “acute flaccid myelitis” characterized by flaccid limb weakness, cranial nerve dysfunction, bulbar weakness, and an MRI imaging showing gray matter lesions with anterior horn cell involvement. This is highly similar to our case and is seen in other viruses with a tropism for motor nerve cells such as polio and enterovirus 71 (EV71). Enterovirus 71 has caused outbreaks of AFP in the Asia-Pacific region [17], leading to the development of an EV71 vaccine due to concerns regarding neuroinvasive disease [42].
CONCLUSIONS
Acute flaccid paralysis remains an important clinical syndrome with a changing epidemiology. Although classically associated with polio, efforts at polio eradication are likely to cause a shift to pathogens such as WNV and nonpolio enteroviruses. Our case underscores the need for infectious diseases clinicians to remain vigilant about emerging “new” causes of AFP such as WNV and EV D68, re-emerging “old” pathogens such as WPV, and the need to prevent possibly devastating disease through vaccination.
Supplementary Material
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Porter KA, Diop OM, Burns CC et al. Tracking progress toward polio eradication - worldwide, 2013–2014. MMWR Morb Mortal Wkly Rep 2015; 64:415–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Delpeyroux F, Colbère-Garapin F. Editorial commentary: emerging problems impeding the elimination of the last polioviruses: silent circulation of wild strains in a well-immunized population. Clin Infect Dis 2015; 60:1065–7. [DOI] [PubMed] [Google Scholar]
- 3.Greninger AL, Naccache SN, Messacar K et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): a retrospective cohort study. Lancet Infect Dis 2015; 15:671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messacar K, Schreiner TL, Maloney JA et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet 2015; 385:1662–71. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Jiang L, Peng HL. Clinical analysis of 134 children with nervous system damage caused by enterovirus 71 infection. Pediatr Infect Dis J 2015; 34:718–23. [DOI] [PubMed] [Google Scholar]
- 6.Lindsey NP, Lehman JA, Staples JE et al. West Nile virus and other arboviral diseases - United States, 2013. MMWR Morb Mortal Wkly Rep 2014; 63:521–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Papadakis G, Chibo D, Druce J et al. Detection and genotyping of enteroviruses in cerebrospinal fluid in patients in Victoria, Australia, 2007–2013. J Med Virol 2014; 86:1609–13. [DOI] [PubMed] [Google Scholar]
- 8.Scaramozzino N, Crance JM, Jouan A et al. Comparison of flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J Clin Microbiol 2001; 39:1922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Druce J, Catton M, Chibo D et al. Utility of a multiplex PCR assay for detecting herpesvirus DNA in clinical samples. J Clin Microbiol 2002; 40:1728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalifa KK, Roth A, Roth B et al. Value of PCR for evaluating occurrence of parasitemia in immunocompromised patients with cerebral and extracerebral toxoplasmosis. J Clin Microbiol Infect Dis 1994; 32:2813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graves SR, Dwyer BW, McColl D, McDade JE. Flinders Island spotted fever: a newly recognised endemic focus of tick typhus in Bass Strait. Part 2. Serological investigations. Med J Aust 1991; 154:99–104. [DOI] [PubMed] [Google Scholar]
- 12.Arakawa S, Wright PM, Koga M et al. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke 2006; 37:1211–6. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organisation. WHO recommended surveillance standard of poliomyelitis. Available at: http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/poliomyelitis_standards/en/. Accessed 19 September 2015.
- 14.Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. J Infect Dis 1997; 175:S215–27. [DOI] [PubMed] [Google Scholar]
- 15.Oceguera LF, Patiris PJ, Chiles RE et al. Flavivirus serology by Western blot analysis. Am J Trop Med Hyg 2007; 77:159–63. [PubMed] [Google Scholar]
- 16.Immunology and Vaccine-Preventable Diseases – Pink Book - Polio - polio.pdf. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/polio.pdf Accessed 21 September 2015.
- 17.Ooi MH, Wong SC, Lewthwaite P et al. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 2010; 9:1097–105. [DOI] [PubMed] [Google Scholar]
- 18.Lindsey NP, Lehman JA, Staples JE, Fischer M. West Nile virus and other nationally notifiable arboviral diseases - United States, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:929–34. [DOI] [PubMed] [Google Scholar]
- 19.Tyler KL. Emerging viral infections of the central nervous system: part 1. Arch Neurol 2009; 66:939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sejvar JJ. Clinical manifestations and outcomes of West Nile virus infection. Viruses 2014; 6:606–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prow N. The changing epidemiology of Kunjin virus in Australia. Int J Environ Res Public Health 2013; 10:6255–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen KM, Tsai HC, Sy CL et al. Clinical manifestations of Japanese encephalitis in southern Taiwan. J Microbiol Immunol Infect 2009; 42:296–302. [PubMed] [Google Scholar]
- 23.Singh SS, Manimunda SP, Sugunan AP et al. Four cases of acute flaccid paralysis associated with chikungunya virus infection. Epidemiol Infect 2008; 136:1277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seet RC, Lim EC, Wilder-Smith EP. Acute transverse myelitis following dengue virus infection. J Clin Virol 2006; 35:310–2. [DOI] [PubMed] [Google Scholar]
- 25.Douglas MW, Stephens DP, Burrow JN et al. Murray Valley encephalitis in an adult traveller complicated by long-term flaccid paralysis: case report and review of the literature. Trans R Soc Trop Med Hyg 2007; 101:284–8. [DOI] [PubMed] [Google Scholar]
- 26.Gaensbauer JT, Lindsey NP, Messacar K et al. Neuroinvasive arboviral disease in the United States: 2003 to 2012. Pediatrics 2014; 134:e642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumpis U, Crook D, Oksi J. Tick-borne encephalitis. Clin Infect Dis 1999; 28:882–90. [DOI] [PubMed] [Google Scholar]
- 28.Dionisio D, Esperti F, Vivarelli A, Valassina M. Epidemiological, clinical and laboratory aspects of sandfly fever. Curr Opin Infect Dis 2003; 16:383–8. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC). Recovery of a patient from clinical rabies--California, 2011. MMWR Morb Mortal Wkly Rep 2012; 61:61–5. [PubMed] [Google Scholar]
- 30.Maan HS, Chowdhary R, Shakya AK, Dhole TN. Genetic diversity of cosaviruses in nonpolio acute flaccid paralysis cases of undefined etiology, Northern India, 2010–2011. J Clin Virol 2013; 58:183–7. [DOI] [PubMed] [Google Scholar]
- 31.Mateen FJ, Bahl S, Khera A, Sutter RW. Detection of diphtheritic polyneuropathy by acute flaccid paralysis surveillance, India. Emerg Infect Dis 2013; 19:1368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salonen R, Rinne JO, Halonen P et al. Lyme borreliosis associated with complete flaccid paraplegia. J Infect 1994; 28:181–4. [DOI] [PubMed] [Google Scholar]
- 33.Sobel J. Botulism. Clin Infect Dis 2005; 41:1167–73. [DOI] [PubMed] [Google Scholar]
- 34.Caroleo S, Longo C, Pirritano D et al. A case of acute quadriplegia complicating Mediterranean spotted fever. Clin Neurol Neurosurg 2007; 109:463–5. [DOI] [PubMed] [Google Scholar]
- 35.Stewardson AJ, Roberts JA, Beckett CL et al. Imported case of poliomyelitis, Melbourne, Australia, 2007. Emerg Infect Dis 2009; 15:63–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mach O, Tangermann RH, Wassilak SG et al. Outbreaks of paralytic poliomyelitis during 1996–2012: the changing epidemiology of a disease in the final stages of eradication. J Infect Dis 2014; 210 (Suppl 1):S275–82. [DOI] [PubMed] [Google Scholar]
- 37.Shulman LM, Martin J, Sofer D et al. Genetic analysis and characterization of wild poliovirus type 1 during sustained transmission in a population with >95% vaccine coverage, Israel 2013. Clin Infect Dis 2015; 60:1057–64. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins HE, Aylward RB, Gasasira A et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N Engl J Med 2010; 362:2360–9. [DOI] [PubMed] [Google Scholar]
- 39.Avellon A, Cabrerizo M, de Miguel T et al. Paralysis case and contact spread of recombinant vaccine-derived poliovirus, Spain. Emerg Infect Dis 2008; 14:1807–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander JP, Ehresmann K, Seward J et al. Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J Infect Dis 2009; 199:391–7. [DOI] [PubMed] [Google Scholar]
- 41.DeVries AS, Harper J, Murray A et al. Vaccine-derived poliomyelitis 12 years after infection in Minnesota. N Engl J Med 2011; 364:2316–23. [DOI] [PubMed] [Google Scholar]
- 42.Li R, Liu L, Mo Z et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 2014; 370:829–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


