We found that ICU setting is associated with higher Clostridium difficile infection (CDI) prevalence than general hospital population and 25% among CDI cases in ICU develop pseudomembranous colitis. CDI also affects adversely overall hospital mortality, ICU and overall hospital stay.
Keywords: C difficile infection, ICU, length of stay, meta-analysis, mortality
Abstract
Background. Intensive care unit (ICU) patients are at higher risk for Clostridium difficile infection (CDI).
Methods. We performed a systematic review and meta-analysis of published studies from 1983 to 2015 using the PubMed, EMBASE, and Google Scholar databases to study the prevalence and outcomes of CDI in this patient population. Among the 9146 articles retrieved from the studies, 22 articles, which included a total of 80 835 ICU patients, were included in our final analysis.
Results. The prevalence of CDI among ICU patients was 2% (95% confidence interval [CI], 1%–2%), and among diarrheic ICU patients the prevalence was 11% (95% CI, 6%–17%). Among CDI patients, 25% (95% CI, 5%–51%) were diagnosed with pseudomembranous colitis, and the estimated length of ICU stay before CDI acquisition was 10.74 days (95% CI, 5%–51%). The overall hospital mortality among ICU patients with CDI was 32% (95% CI, 26%–39%), compared with 24% (95% CI, 14%–36%) among those without CDI presenting a statistically significant difference in mortality risk (P = .030). It is worth noting that the length of ICU and hospital stay among CDI patients was significantly longer, compared with non-CDI patients (standardized mean of difference [SMD] = 0.49, 95% CI, .39%–.6%, P = .00 and SMD = 1.15, 95% CI, .44%–1.91%, P = .003, respectively). It is noteworthy that the morbidity score at ICU admission (Acute Physiology and Chronic Health Evaluation II [APACHE II]) was not statistically different between the 2 groups (P = .911), implying that the differences in outcomes can be attributed to CDI.
Conclusions. The ICU setting is associated with higher prevalence of CDI. In this setting, CDI is associated with increased hospital mortality and prolonged ICU and overall hospital stay. These findings highlight the need for additional prevention and treatment studies in this setting.
In the United States, Clostridium difficile infection (CDI) is associated with 250 000 hospitalizations and over 14 000 deaths per year. These rates result in more than $1 billion in excess medical cost per year, and the Centers for Disease Control and Prevention (CDC) assigned C difficile as an urgent hazard that requires aggressive action (http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf). Patients in intensive care units (ICUs) are at particularly high risk for CDI due to the presence of multiple risk factors [1], and CDI is the most common infectious cause of diarrhea in this setting [2]. Although it has been estimated that 0.9% of the general hospital population suffers from CDI [3], aggregate data on CDI rates among ICU patients are scarce. The purpose of this systematic review and meta-analysis is to assess the prevalence and clinical outcomes of CDI among ICU patients.
MATERIALS AND METHODS
A systematic search of PubMed, EMBASE, and Google Scholar databases was performed for pertinent studies up to May 31, 2015. We used terms (ICU AND [clostrid* OR difficile OR diarrhea OR infect* OR [clostridium difficile] OR pseudomembranous colitis]) to identify all published studies reporting cases of CDI in ICU patients among the total ICU population. The terms infect* and diarrhea were included in the search term in an effort to retrieve all articles that reported episodes of CDI along with other infections, as well as episodes of CDI along with other causes of diarrhea in ICU patients. Reference lists of the retrieved studies, systematic reviews, and meta-analyses relevant to our study were also reviewed. Our analysis included published literature and abstracts from conference proceedings published in EMBASE. The study was performed in line with the PRISMA recommendations [4].
Inclusion Criteria
All studies that reported the prevalence of CDI among patients after they were admitted in ICU were included in our meta-analysis. Studies that did not explicitly report the presence of symptoms or signs related to CDI (such as diarrhea, pain, fever, abdominal pain or tenderness) along with the subsequent laboratory confirmation were excluded because they circumvent the current CDI definition defined by the CDC and overestimate the relevant rates including C difficile colonization (http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_faqs_HCP.html#changed). In an effort to include studies that had appropriately attributed the CDI cases to ICUs, we included only those that explicitly reported so or those that described symptoms and screening after 24 hours after the ICU admission. Patients who had diarrhea or CDI before they were admitted to the ICU were excluded from the analysis. In case of randomized trials, we set the criterion to include both arms if the applied intervention did not result in a significant outcome. Language restriction was imposed with the exclusion of articles published in languages other than English. Only studies that met the quality assessment criteria were included (discussed below under Quality Assessment).
Data Extraction
Studies that were considered for inclusion in the meta-analysis were independently evaluated by 2 reviewers (S.P. and S.K.), and discrepancies were discussed and resolved by consensus. For each study apart from prevalence, we extracted data on study population, patient demographics, study duration, study design, location, laboratory method for C difficile detection, time after ICU admission when they are deemed as ICU patients, lower limit of age of included patients, type and size of ICU along with type of hospital for which they serve. The primary outcome of interest was the prevalence of CDI among ICU patients and among ICU patients with diarrhea. Prevalence was calculated by dividing the number of patients diagnosed with CDI while in the ICU among the total ICU patients or among ICU patients suffering from diarrhea, respectively. Laboratory confirmation of CDI among diarrheic patients included polymerase chain reaction, enzyme-linked immunosorbent assay, cytotoxin test, other enzyme immune-absorbent technique, or stool culture. As secondary outcomes, we estimated the difference in ICU and overall (all-cause) hospital mortality risk among CDI (associated to CDI) and non-CDI patients, the difference in ICU length of stay (LoS) and overall (all-cause) hospital length among CDI and non-CDI patients, the prevalence of pseudomembranous colitis among CDI patients, and the required time for CDI manifestation upon admission to ICU. We also extracted information on patient age and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores to evaluate patient characteristics and health status at ICU admission and to identify any potential difference between CDI and non-CDI patients. It is worth noting that we were unable to compare the APACHE II score between dead versus alive patients because there were no extractable data. We used studies that provided concurrently secondary outcomes in both CDI and non-CDI groups, assuring objective comparison.
Quality Assessment
The methodological quality of eligible studies was assessed by 2 reviewers (S.P. and S.K.) using the Newcastle-Ottawa Scale (NOS), which is a “star-based” rating system [5]. The 3 parameters used to evaluate the quality of individual studies were as follows: selection, comparability, and exposure/outcome assessments. The NOS assigns a maximum of 4 points for selection, 2 points for comparability, and 3 points for exposure/outcome. We considered the study population representative of the exposed cohort if data on CDI were provided for all available ICU patients and not among a specific subpopulation. The outcome was based on cases with symptoms and laboratory diagnosis of CDI. Studies that received 5 stars were considered of adequate quality for extraction of relevant information. If the study was an abstract, 4 stars were considered enough to indicate adequate quality. Any discrepancies regarding quality assessment were resolved by joint re-evaluation of the original article or abstract.
Data Synthesis and Analysis
We performed the meta-analysis using a random-effects model to estimate the pooled prevalence and the 95% confidence intervals (CIs) using DerSimonian and Laird [6] weights. The variance of the raw proportions was stabilized using the Freeman-Tukey arcsine methodology [7]. Metaprop command was used so that no studies with 0% or 100% proportions were excluded from the meta-analysis [8, 9]. To check for publication bias, we used the Egger's test [10]. Between-study variance τ2 estimation was used to assess statistical heterogeneity [10]. The effect of CDI on mortality compared with non-CDI cases was evaluated using random effects meta-analysis and reported as unadjusted risk difference (RD) estimates and 95% CIs. The effect of CDI on LoS compared with non-CDI cases was evaluated using random or fixed effects meta-analysis and reported as standardized mean of difference (SMD) estimates and 95% CIs. Median values and their interquartile ranges or range extracted from included studies were transformed to means and standard deviations according to Wan et al [11]. To model the time trends for CDI, an index year of each eligible study was determined, and then the model coefficients were transformed to rates and plotted against this year along with the observed prevalence rates [12]. The year that the study was conducted was used as the index year, and for studies whose study period extended for more than 1 calendar year the mid-year was calculated. For studies not reporting the time frame of the study conduction, we assumed that the study period was 2 years before the publication year or 1 year for the abstracts. Stata version 13 software package (Stata Corporation, College Station, TX) and Excel Microsoft Office 2010 were used to perform the statistical analysis. The statistical significance threshold was set at 0.05.
RESULTS
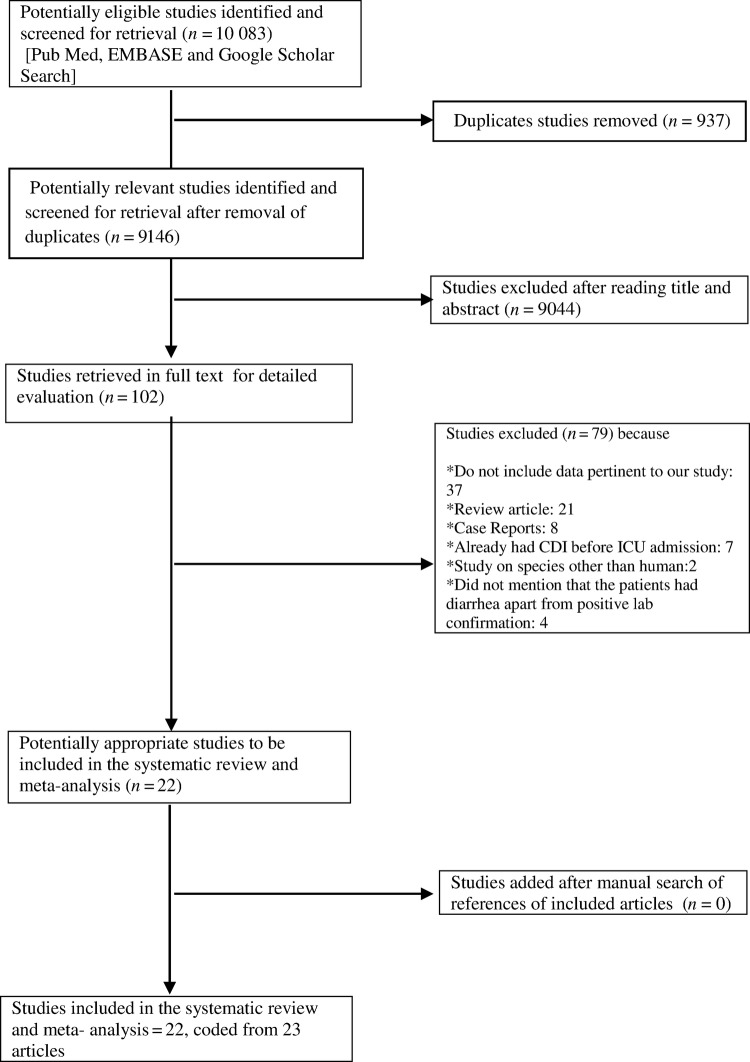
The initial databases search retrieved 10 083 potentially relevant citations. After screening the titles and abstracts and removing 937 duplicates, 102 studies were identified as potentially eligible for review and analysis. The articles were reviewed, and 75 articles were excluded from the final analysis. Among them, 37 studies did not provide data relevant to our study including those that did not report extractable data for our primary outcomes, 21 were review articles, 8 were case reports, 7 studies included data among patients who already had CDI before admission in ICU, 4 did not mention that the patients had diarrhea apart from positive laboratory confirmation, and 2 studies reported data in species other than human. The review of the reference lists of the full-texted articles did not add any additional studies. Finally, 22 studies were included in our meta- analysis [13–34], and they were coded from 23 papers [13–34] (2 studies included overlapped data [17, 35]). The main characteristics of the studies included in our meta-analysis are represented in Table 1, and the detailed selection process is illustrated in a flow chart (Figure1).
Table 1.
Individual Studiesa
| Author | Study Period | Mid-Year | Continent | Study Design | N | N Diarrhea (%) | n (%) | n Diarrhea (%) | Method | Time | Definition | Age | ICU type (beds) Hospital |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ang et al [13] | April 2004–April 2007 | 2005 | Europe | R Chart review | 1852 | NR | 62 (3.34%) | NR | Stool cytotoxin assay | >48 h | CDI: diarrhea and lab confirmation Diarrhea: ≥3 unformed stools/day for >2 d | >18 | General (8) Multiple injuries (8) |
| – | |||||||||||||
| Balassiano et al [14] | January 2006–July 2009 | 2007 | Europe | R Chart review | 458 | 218 | 43 (9.3%) | 43 (19.72%) | ELISA, culture, PCR targeting tpi gene and detecting cdtA cdtB cdtC | NR | Samples were tested at the discretion of the attending physician | NR | Medical/ Surgical (30) Tertiary |
| Buendgens et al [15] | February 1999–June 2010 | 2005 | Europe | R Chart review | 3286 | NR | 110 (3.35%) | NR | ELISA ± A/B DNA toxin detection through PCR | >48 h | CDI: a new onset diarrhea with typical endoscopy image ± microbiological confirmation | >14 | Medical (NR) University |
| Custovic et al [16] | January 2010–December 2010 | 2010 | Europe | P Cohort study | 834 | NR | 9 (1.1%) | NR | Reference to CDC | NR | Reference to CDC | NR | Surgical (NR) University |
| Dodek et al [17] | April 2006–December 2011 | 2008 | North America | R Chart review | 15 314 | NR | 236 (1.54%) | NR | EIA for toxins and common antigen, cytotoxin assay (tissue culture), or PCR | >24 h (after day 2) | CDI: ≥3 loose stools/day without other etiology and lab confirmation, typical pseudomembrane on endoscopy or toxic megacolon | >18 | Medical-Surgical University and community |
| Kelly et al [19] | March 1980–March 1981 | 1980 | Europe | P Cohort | 88 | 33 | 0 (0.00%) | 0 (0.00%) | Cytotoxin from fecal supernatants inoculated onto human embryonic lung fibroblast cells | >48 h | CDI: diarrhea and lab confirmation Diarrhea: >3 liquid stools a day as diarrhea | >9 | NR |
| Lawrence et al [20] | July 1997–December 1999 | 1998 | North America | R Cohort | 1872 | NR | 40 (2.14%) | NR | Cytotoxic assay | >24 h | CDI: positive for toxin A or B in an ordered specimen by the treating ICU physician | >16 | Medical (19) Tertiary Hospital |
| Lumpkins et al [21] | July 2004–October 2006 | 2005 | North America | P Cohort | 581 | NR | 19 (3.2%) | NR | Immunoassay for A and B toxins | NR | Samples were tested per signs and symptoms | NR | Trauma (NR) |
| Micek et al [22] | January 2009–December 2010 | 2009–2010 | North America | R Cohort | 5852 | NR | 267 (4.6%) | NR | Rapid membrane-filter immunoassay for both toxins A and B or real-time PCR to detect toxins or tcdC gene, Positive if toxin A, B, or both were detected. | >48 h | CDI: diarrhea or pseudomembranous colitis and lab confirmation | >18 | Medical and surgical (NR) University |
| Musa et al [23] | February 2003–January 2008 | 2005 | Europe | R Cohort | 5199 | NR | 27 (0.52%) | NR | EIA | >48 h | CDI: diarrhea officially defined and lab confirmation | >18 | Cardiothoracic – |
| Noto et al [24] | July 2012–July 2013 | 2013 | North America | R Cohort | 8068 | NR | 23 (0.29%) | NR | Reference to CDC | NR | Reference to CDC | >18 | Cardio (27), Medical (34), Neuro (34), Surgical (34) Tertiary |
| Rotimi et al [26] | July 1999–June 2000 | 2000 | Asia | P Cohort | 212 | 25 | 8 (3.77%) | 8 (32%) | ELISA | >96 h | CDI: diarrhea and lab confirmation ± endoscopic evidence and no other explanation for diarrhea Diarrhea: ≥6 loose stools/36 h |
>3 mo | General ICU (NR) Cancer ICU (NR) – |
| Salva et al [27] | January 2010–December 2011 | 2010 | Europe | R Chart review | 1936 | 177 | 7 (0.36%) | 7 (3.95%) | Immunochromatography or enzyme immunoassay | >48 h | CDI: diarrhea and lab confirmation Diarrhea: >3 watery stools/day or >200 g/day stool amount | >18 | Medical/Surgical (34) Tertiary |
| Shaughnessy et al [28] | January 2005–June 2006 | 2005 | North America | R Cohort | 1770 | NR | 87 (4.91%) | NR | ELISA for toxins A and B | >48 h | Sample was sent on the basis of physicians' clinical discretion | >15 | Medical (20) Tertiary |
| Silva et al [29] | June 2005–December 2009 | 2007 | Europe | R Cohort | 10 754 | 1080 | 15 (0.14%) | 15 (1.39%) | ELISA for either A and/or B toxin | >72 h | CDI: diarrhea or pseudomembranous colitis or toxic megacolon and lab confirmation | >18 | Open model (38) Tertiary |
| Thibault et al [30] | For 2 months | 2011b | Europe | P Cohort | 278 | 42 | 2 (0.78%) | 2 (4.76%) | NR | >24 h (up to 14 d) | CDI: diarrhea and lab confirmation Diarrhea: ≥3 liquid stools/day |
NR | Mixed medical/surgical (NR) Tertiary |
| Tripathy et al [32] | April 2008–August 2010 | 2009 | Europe | R Chart review | 2212 | NR | 9 (0.41%) | NR | EIA followed by GDH test and a positive toxin test | >48 h | CDI: diarrhea, toxic megacolon, or ileostomy with lab confirmation, pseudomembranous colitis (endoscopy or CT), positive biopsy result, positive fecal post-mortem sample with pseudomembranous colitis | NR | Neuro (14–20) Tertiary |
| Wang et al [33] | May 2012–January 2013 | 2012 | Asia | P cohort | 1277 | 124 | 31 (2.43%) | 31 (25%) | Stool DNA PCR kit for either toxin A or B detection. If positive followed by culture and toxinogenicity confirmation with multiplex PCR | >24 h | Sample collected if 3 or more loose stools/day | NR | Medical (50) University hospital |
| Zahar et al [34] | January 1999–January 2009 | 2004 | Europe | P Cohort | 5260 | 512 | 47 (0.9%) | 47 (9.18%) | EIA. Positive result if either toxin A or B is detected | ≥72 h | CDI: watery or unformed stools/day and lab confirmation of a stool sample positive | >18 | Medical-Surgical (NR) – |
| Hasham et al [18] | August 2009–October 2009 | 2009 | North America | P Chart review | 307 | 16 | 2 (0.65%) | 2 (12.5%) | NR | >72 h | CDI: diarrhea and lab confirmation Diarrhea: >3 episodes of loose stools/day | NR | NR |
| Pinto et al [25] | 2011 | 2011c | North America | R Chart Review | 2131 | 116 | 32 (1.5%) | 32 (27.59%) | PCR | NR | NR | 1 mo–21 y | Pediatric (NR) |
| Tirlapur et al [31] | January 2010– December 2010 | 2010 | Europe | R Cohort | 11 294 | 1278 | 119 (1%) | 119 (9.31%) | Antigen or toxin test | NR | CDI: diarrhea and lab confirmation | NR | NR (35) Tertiary |
Abbreviations: CDC, Centers for Disease Control and Prevention; CDI, Clostridium difficile infection; CT, computed tomography; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; GDH, glutamate dehydrogenase; ICU, intensive care unit; lab, laboratory; NR, not reported; PCR, polymerase chain reaction.
a Characteristics of 22 studies: study period, mid-year, continent, number of patients evaluated and screened, number of patients with diarrhea, number of patients with CDI, method of C difficile detection, the age lower limit, information about the initial, time after ICU admission, definitions used per author, size and type of ICU and hospital.
b Two years before publication of the study.
c One year before the presentation/publication of the abstract.
Figure 1.
PRISMA flow diagram of meta-analysis. Abbreviations: CDI, Clostridium difficile infection; ICU, intensive care unit.
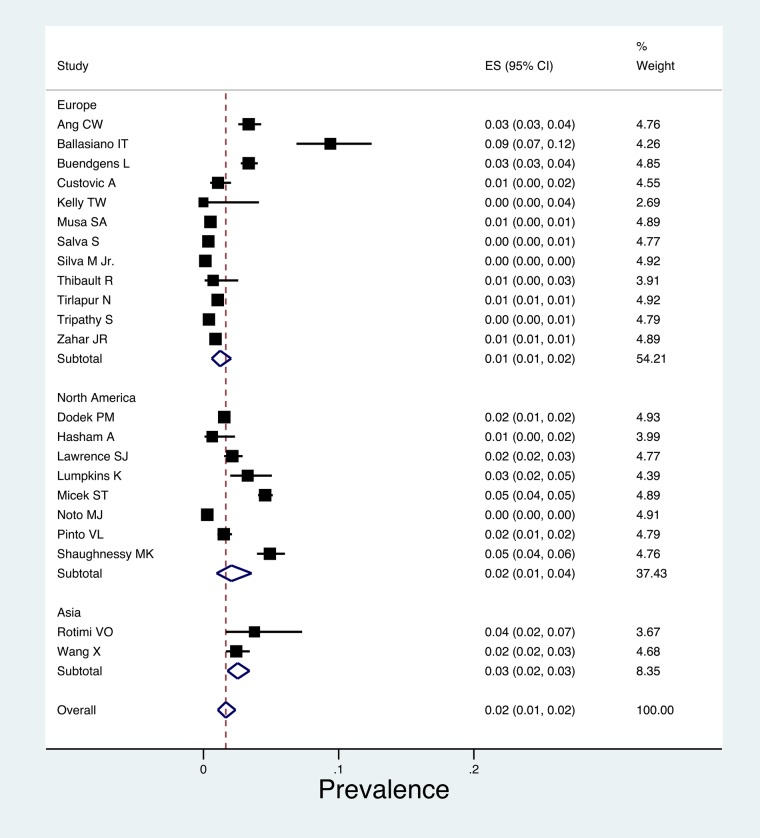
Among the 80 835 ICU patients in our analysis, the pooled prevalence of CDI was 2% (95% CI, 1%–2%; τ2 = 0.01) (Figure 2), with no evidence of small study effects across studies (Egger's bias 1.53, P = .142). The prevalence of CDI across North America studies was 2% [95% CI (1%–4%), τ2 = 0.02] [17, 18, 20–22, 24, 25, 28], whereas the estimated prevalence across Europe was 1% (95% CI, 1%–2%; τ2 = 0.01) [13–16, 19, 23, 27, 29–32, 34] and the estimated prevalence across Asia was 3% (95% CI, 1%–2%; τ2 = 0.01) [26, 33]. Among 3621 diarrheic ICU patients, the pooled prevalence of CDI was 11% (95% CI, 6%–17%; τ2 = 0.08), with no small study effect (Egger's bias 1.01, P = .156) [14, 18, 19, 25–27, 29–31, 33, 34] (Supplementary Figure 3). Time trend plot with index year of each eligible study revealed an increasing trend in CDI among diarrheic patients (P = .33).
Figure 2.
Forest plot of included studies stratified by continent. Individuals and combined prevalence estimates of Clostridium difficile infection among intensive care unit patients.
Based on 4 studies [26, 27, 32, 34], we found that 25% (95% CI, 5%–51%; τ2 = 0.17) of ICU patients with confirmed CDI had pseudomembranous colitis determined either with imaging or endoscopic findings [26, 27, 32, 34] (Supplementary Figure 4). It is worth noting that we excluded from this calculation the study by Buendgens et al [15], where only patients with pseudomembranous colitis were deemed to have CDI, in an effort to avoid overestimating the result. From 6 studies that included 391 CDI patients, the estimated pooled LoS in ICU (mean-days) before the CDI manifestation was 10.74 days (95% CI, 8.04%–13.44%; τ2 = 0.17) without small-study effects (Egger's bias 2.28, P = .1) [17, 20, 21, 23, 33, 34] (Supplementary Figure 5).
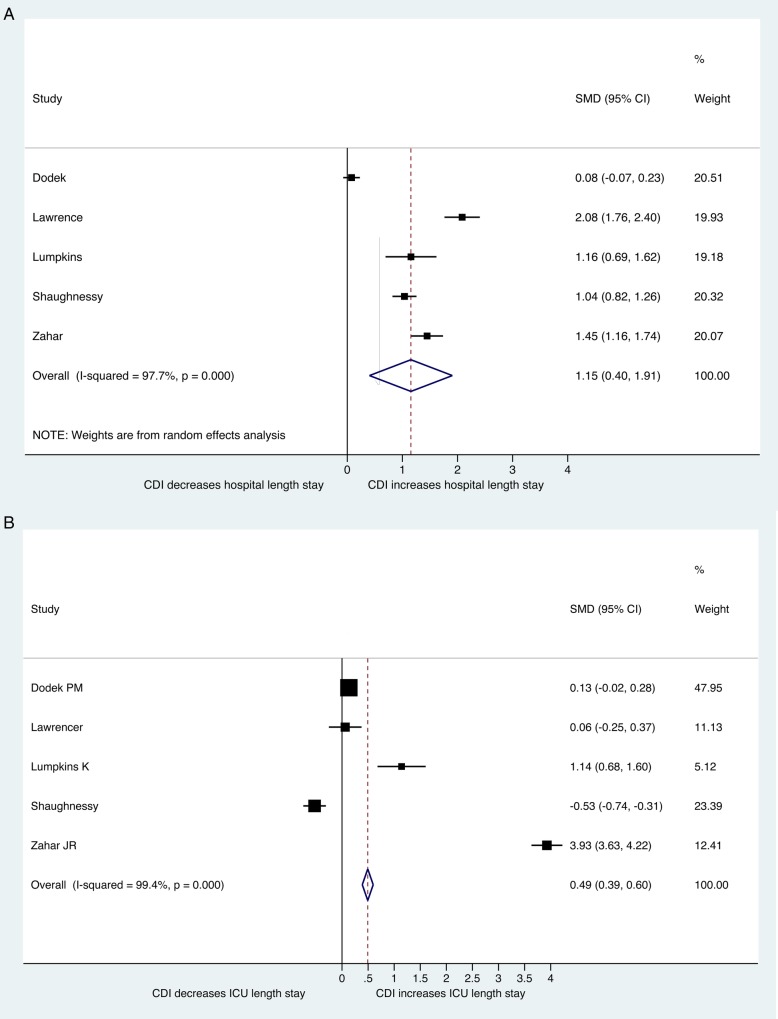
Regarding LoS in the ICU, based on 5 studies and 10 327 patients, CDI patients had an average ICU stay of 23.54 days (95% CI, 19.19%–27.90%), compared with 19.16 days (95% CI, 6.06%–31.46%) for the non-CDI group. The pooled mean difference in ICU LoS was statistically significant (SMD = 0.49; 95% CI, .39%–.6%; P = .00) [17, 20, 21, 28, 34] (Figure 3, Supplementary Figure 6). In terms of overall hospital LoS, based on 5 studies with 10 327 patients, CDI patients were hospitalized for 49.58 days (95% CI, 40.99%–58.16%), and non-CDI patients were hospitalized for 29.90 days (95% CI, 22.82%–36.99%) [17, 20, 21, 28, 34]. The pooled mean difference in hospital overall was again statistically significant (SMD = 1.15; 95% CI, .4%–1.91%; P = .003) (Figure 3, Supplementary Figure 7).
Figure 3.
Forest plot of included studies. Standard mean difference (SMD) in length of stay between Clostridium difficile infection (CDI) and non-CDI group (A) in intensive care unit (ICU) (B) in hospital. Abbreviation: CI, confidence interval.
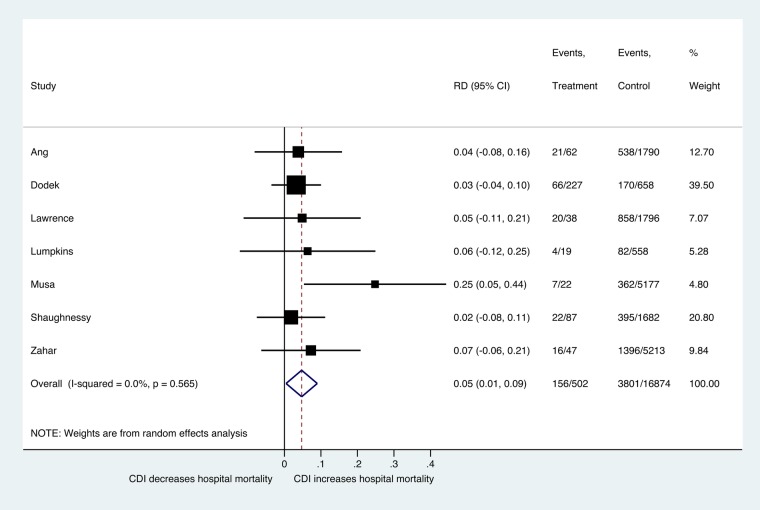
Based on 5 studies with 14 855 patients, the pooled mortality rate among CDI patients in the ICU was 20% (95% CI, 15%–26%; τ2 = 0.02), and the rate among non-CDI patients was 19% (95% CI, 17%–21%; τ2 = 0.00). The difference in mortality was not statistically significant (P = .989) (pooled RD = 0.2%; 95% CI, −27.9% to 28.3%; τ2 = 0.12) [15, 17, 20, 28, 33, 34] (Supplementary Figure 8). When we assessed the overall hospital mortality based on 7 studies with 12 165 patients, the pooled mortality rate among CDI patients was 32% (95% CI, 26%–39%; τ2 = 0.02), and among non-CDI patients the pooled mortality rate was 24% (95% CI, 14%–36%; τ2 = 0.13). The difference in overall hospital mortality risk was statistically significant (P = .030) (pooled RD = 4.7%; 95% CI, 1%–9%; τ2 = 0.00) [13, 17, 20, 21, 23, 28, 34] (Figure 4, Supplementary Figure 9).
Figure 4.
Forest plot of included studies. Risk difference (RD) estimate in hospital mortality between Clostridium difficile infection (CDI) and non-CDI group. Abbreviation: CI, confidence interval.
Evaluating the patient characteristics and morbidity between CDI and non-CDI group hospitalized in ICU, there was no statistically significant difference either in terms of age (SMD = 0.06; 95% CI, −.05% to .16%; P = .282 based on 5 studies with 14 343 patients) [17, 21, 22, 28, 34] or the APACHE II score (SMD = 0.023; 95% CI, −.37% to .42%; P = .911 based on 3 studies with 7922 patients) [17, 20, 22].
DISCUSSION
Intensive care unit patients represent an important risk group for developing CDI [36], and our analysis demonstrated that 2% of ICU patients manifest CDI (with lower incidence in Europe and higher incidence in the United States and Asia) and 11% of ICU patients with diarrhea are diagnosed with CDI. More importantly, regarding clinical outcomes, 1 of 4 of these patients will develop pseudomembranous colitis, and CDI in this population is associated with longer ICU and hospital stay and increased overall mortality.
The prevalence of CDI among ICU patients was significantly higher compared with what has been reported for general hospital population (0.9%) [3]. Diarrhea is common among ICU patients with a reported prevalence of 15%–38% [37]. It is notable that the estimated prevalence of CDI among diarrheic ICU patients was higher than the relevant estimation for general hospital diarrheic population (9.7%) [38]. These findings help us quantify the risk in this patient population and they are not surprising, because ICU patients are exposed concurrently to numerous risk factors for C difficile, such as treatment with multiple and broad-spectrum antibiotics, corticosteroids, proton pumps inhibitors, and enteral feeding, and they suffer from comorbidities, such as renal insufficiency, diabetes, gastrointestinal surgeries, immunodeficiency, malnutrition, and low serum albumin level that have been associated with CDI [13, 39, 40].
Cumulative data on ICU-acquired CDI and subsequent hospital mortality are scarce, and our analysis presents the first estimation on overall hospital mortality in this specific patient population with a rate of 32%. Of note, estimating the comorbidity scale between both groups, we confirmed that the general health status is similar, and it does not account for the difference in overall hospital mortality rates. Taken in their totality, our analysis indicates that among patients admitted in the ICU, the mortality rate is significantly higher in CDI group. It is worth noting that this higher mortality extends to the overall hospital stay, and patients with CDI and ICU admission should be closely monitored throughout their hospital stay. However, it should also be noted that we were unable to evaluate the hospital mortality attributable to CDI, and we reported all-cause mortality associated to CDI. In the general hospital population, previous reports estimated the all-cause mortality associated to CDI was between 13% and 18.6% [44, 45], whereas the mortality due to CDI (attributable mortality) has been reported to be approximately 7%, but this percentage referred to the era before the new aggressive virulent strains [46].
Of note is that prediction score tools specifically for mortality among CDI patients using variables, such as white blood count, renal function tests, levels of C-reactive protein or albumin, age, APACHE II score, presence of clinically severe disease, or cancer, can significantly contribute to the accuracy of the decision making and cost effectiveness. Although those tools have been recently developed with promising diagnostic capacity, none of them has gained widespread acceptance [44].
Our analysis demonstrated that the mean duration of ICU hospitalization before CDI is approximately 10 days. This finding seems to be an additional reason for physicians to minimize ICU stay by discharging or at least stepping-down ICU patients. The increased LoS of ICU and hospital stay found in our study is more difficult to analyze, because the LoS is both a risk factor as well as a consequence of CDI [34]. Previous studies have reported that diarrhea among critically ill patients is associated with increased LoS in the ICU [46, 47], whereas others argue that the increase in LoS among patients with diarrhea in the ICU is due to the severity of illness in this patient population [48]. Because there was no difference among CDI and non-CDI patients in terms of ICU morbidity and initial severity of illness (based on the APACHE II score), it is reasonable to assume that CDI has a direct effect in the LoS. However, it should be noted that our analysis does not allow us to quantify the direct effects of CDI in the LoS, and our estimations are unadjusted for potential confounders apart from the APACHE II score. Based on the high failure rates [49] and the increasing severity [50] and recurrence [51] associated with CDI, the Infectious Diseases Society of America and the Society for Healthcare and Epidemiology of America have updated the guidelines for the management of this infection [45, 52]. We were unable to evaluate the effect of the new guidelines on the clinical outcomes in ICU, because none of the studies conducted after 2010 provided data on mortality or LoS. In addition, regarding alternative therapies, fecal microbiota transplantation has been studied as part of the management of recurrence cases [53]. However, no studies evaluated this treatment in the context of severe CDI in the ICU.
CONCLUSIONS
In conclusion, ICU patients are at high risk for CDI, and the prevalence of CDI among critically ill patients was almost double that reported among the general hospital population. More than 1 of 10 cases with diarrhea suffer from CDI, and, subsequently, CDI affects adversely multiple clinical outcomes, such as the hospital mortality rate and LoS. These findings highlight the need for strict implementation of preventive policies in the critical care setting. Studies are needed to identify interventions, such as infection control measures and antibiotic stewardship programs, which may reduce the incidence and severity of CDI in this patient population.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Riddle DJ, Dubberke ER. Clostridium difficile infection in the intensive care unit. Infect Dis Clin North Am 2009; 23:727–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liolios A, Oropello JM, Benjamin E. Gastrointestinal complications in the intensive care unit. Clin Chest Med 1999; 20:329–45, viii. [DOI] [PubMed] [Google Scholar]
- 3.Lucado J, Gould C, Elixhauser A. Clostridium difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #124. Rockville, MD: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs, 2006. [PubMed] [Google Scholar]
- 4.Rowland SP, Dharmarajah B, Moore HM et al. Inferior vena cava filters for prevention of venous thromboembolism in obese patients undergoing bariatric surgery: a systematic review. Ann Surg 2015; 261:35–45. [DOI] [PubMed] [Google Scholar]
- 5.Wells G, Shea B, O'Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 December 2015.
- 6.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 7.Puli SR, Graumlich JF, Pamulaparthy SR, Kalva N. Endoscopic transmural necrosectomy for walled-off pancreatic necrosis: a systematic review and meta-analysis. Can J Gastroenterol Hepatol 2014; 28:50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndiaye C, Mena M, Alemany L et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 2014; 15:1319–31. [DOI] [PubMed] [Google Scholar]
- 10.Karanika S, Zervou FN, Zacharioudakis IM et al. Risk factors for meticillin-resistant Staphylococcus aureus colonization in dialysis patients: a meta-analysis. J Hosp Infect 2015; 91:257–63. [DOI] [PubMed] [Google Scholar]
- 11.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zacharioudakis IM, Zervou FN, Pliakos EE et al. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol 2015; 110:381–90; quiz 91. [DOI] [PubMed] [Google Scholar]
- 13.Ang CW, Heyes G, Morrison P, Carr B. The acquisition and outcome of ICU-acquired Clostridium difficile infection in a single centre in the UK. J Infect 2008; 57:435–40. [DOI] [PubMed] [Google Scholar]
- 14.Balassiano IT, Dos Santos-Filho J, de Oliveira MP et al. An outbreak case of Clostridium difficile-associated diarrhea among elderly inpatients of an intensive care unit of a tertiary hospital in Rio de Janeiro, Brazil. Diagn Microbiol Infect Dis 2010; 68:449–55. [DOI] [PubMed] [Google Scholar]
- 15.Buendgens L, Bruensing J, Matthes M et al. Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile-associated diarrhea. J Crit Care 2014; 29:696e11–5. [DOI] [PubMed] [Google Scholar]
- 16.Custovic A, Smajlovic J, Hadzic S et al. Epidemiological surveillance of bacterial nosocomial infections in the surgical intensive care unit. Mater Sociomed 2014; 26:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodek PM, Norena M, Ayas NT et al. Length of stay and mortality due to Clostridium difficile infection acquired in the intensive care unit. J Crit Care 2013; 28:335–40. [DOI] [PubMed] [Google Scholar]
- 18.Hasham A, Fless KG, Rezai F et al. Noninfectious diarrhea: Impact on resource utilization in the intensive care unit. Chest 2010; 138(4_MeetingAbstracts):273A. doi:10.1378/chest.10702. [Google Scholar]
- 19.Kelly TW, Patrick MR, Hillman KM. Study of diarrhea in critically ill patients. Crit Care Med 1983; 11:7–9. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence SJ, Puzniak LA, Shadel BN et al. Clostridium difficile in the intensive care unit: epidemiology, costs, and colonization pressure. Infect Control Hosp Epidemiol 2007; 28:123–30. [DOI] [PubMed] [Google Scholar]
- 21.Lumpkins K, Bochicchio GV, Joshi M et al. Clostridium difficile infection in critically injured trauma patients. Surg Infect (Larchmt) 2008; 9:497–501. [DOI] [PubMed] [Google Scholar]
- 22.Micek ST, Schramm G, Morrow L et al. Clostridium difficile infection: a multicenter study of epidemiology and outcomes in mechanically ventilated patients. Crit Care Med 2013; 41:1968–75. [DOI] [PubMed] [Google Scholar]
- 23.Musa SA, Moran C, Thomson SJ et al. Clostridium difficile-associated disease acquired in the cardiothoracic intensive care unit. J Cardiothorac Vasc Anesth 2011; 25:263–7. [DOI] [PubMed] [Google Scholar]
- 24.Noto MJ, Domenico HJ, Byrne DW et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA 2015; 313:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinto VL, Ambati S, Totapally B. Clostridium difficile associated disease among children in a pediatric intensive care unit. Pediatr Crit Care Med 2012; 13:708. [Google Scholar]
- 26.Rotimi VO, Mokaddas EM, Jamal WY et al. Hospital-acquired Clostridium difficile infection amongst ICU and burn patients in Kuwait. Med Princ Pract 2002; 11:23–8. [DOI] [PubMed] [Google Scholar]
- 27.Salva S, Duran N, Rodriguez V et al. Clostridium difficile in the ICU: study of the incidence, recurrence, clinical characteristics and complications in a university hospital. Med Intensiva 2014; 38:140–5. [DOI] [PubMed] [Google Scholar]
- 28.Shaughnessy MK, Micielli RL, DePestel DD et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol 2011; 32:201–6. [DOI] [PubMed] [Google Scholar]
- 29.Silva M Jr, Marra AR, Camargo TZ et al. Secular trends in the epidemiology of Clostridium difficile infection (CDI): relationship with alcohol gel and antimicrobial usage in a hospital. Int J Infect Dis 2013; 17:e418–21. [DOI] [PubMed] [Google Scholar]
- 30.Thibault R, Graf S, Clerc A et al. Diarrhoea in the ICU: respective contribution of feeding and antibiotics. Crit Care 2013; 17:R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tirlapur N, Matejowsky C, Coen P et al. Diarrhoea is associated with adverse outcomes in critical care. Intensive Care Med 2012; 38:S106. [Google Scholar]
- 32.Tripathy S, Nair P, Rothburn M. Clostridium difficile associated disease in a neurointensive care unit. Front Neurol 2013; 4:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Cai L, Yu R et al. ICU-onset Clostridium difficile infection in a university hospital in China: a prospective cohort study. PLoS One 2014; 9:e111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zahar JR, Schwebel C, Adrie C et al. Outcome of ICU patients with Clostridium difficile infection. Crit Care 2012; 16:R215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norena M, Wong H, Ayas N et al. ICU length of stay and mortality attributed to ICU-acquired Clostridium difficile associated diarrhea. Am J Respir Crit Care Med 2011; 183:2011–05. [Google Scholar]
- 36.Sabau L, Meybeck A, Gois J et al. Clostridium difficile colitis acquired in the intensive care unit: outcome and prognostic factors. Infection 2014; 42:23–30. [DOI] [PubMed] [Google Scholar]
- 37.Wiesen P, Van Gossum A, Preiser JC. Diarrhoea in the critically ill. Curr Opin Crit Care 2006; 12:149–54. [DOI] [PubMed] [Google Scholar]
- 38.Barbut F, Rame L, Petit A et al. [Prevalence of Clostridium difficile infection in hospitalized patients with diarrhea: results of a French prospective multicenter bi-annual point prevalence study]. Presse Med 2015; 44:e75–83. [DOI] [PubMed] [Google Scholar]
- 39.Morrison RH, Hall NS, Said M et al. Risk factors associated with complications and mortality in patients with Clostridium difficile infection. Clin Infect Dis 2011; 53:1173–8. [DOI] [PubMed] [Google Scholar]
- 40.Kwok CS, Arthur AK, Anibueze CI et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol 2012; 107:1011–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.