Abstract
The World Health Organization recommends artemisinin-based combination therapies (ACTs) for the treatment of uncomplicated falciparum malaria in the second and third trimesters of pregnancy. We conducted a meta-analysis to compare efficacy, safety and tolerability of ACTs versus quinine and other non-ACT antimalarials. The median PCR-adjusted failure rate by days 28 to 63 in the non-ACT group was 6 (range 0–37) per 100 women, lower in the ACT group overall (pooled risk ratio [PRR] random effects, 0.41; 95% confidence interval [CI], 0.16–1.05; 6 trials), and significantly lower compared with oral quinine (PRR, 0.20; 95% CI, 0.08–0.49; 4 trials). There were no differences in fetal deaths and congenital abnormalities. Compared with quinine, artemisinin-based combinations therapies were associated with less tinnitus (PRR, 0.19; 95% CI, 0.03–1.11; 4 studies), dizziness (PRR, 0.64; 95% CI, 0.44–0.93; 3 trials), and vomiting (PRR, 0.33; 95% CI, 0.15–0.73; 3 trials). Artemisinin-based combination therapies are better than quinine in the second and third trimesters; their use should be encouraged among health workers.
Keywords: treatment, artemisinins, malaria, pregnancy, quinine
Malaria is a potentially dangerous infection during pregnancy and can lead to maternal anemia, miscarriage, stillbirth, or infant low birth weight [1]. Since 2006, the World Health Organization has recommended the use of an artemisinin-based combination therapy (ACT) for the treatment of uncomplicated falciparum malaria in the second and third trimester of pregnancy [2, 3]. By 2013, ACTs had been adopted as national policy for first-line treatment in 79 of 88 countries where Plasmodium falciparum is endemic [4]; however, it has not always been clear whether this policy is also applicable to treatment of pregnant women in the second and third trimester. In countries where the policy has been adopted, many health workers continue to provide oral quinine, the drug recommended for treatment in the first trimester [5]. Quinine is associated with low adherence because it has to be taken 3 times per day for 7 days and is associated with cinchonism, characterized by tinnitus, hearing impairment, postural hypotension, and dizziness [6]. By contrast, ACTs are administered once or twice daily for only 3 days, and they are better tolerated than quinine. Over the past 2 decades, several studies have compared the efficacy and safety of ACTs to quinine and other non-ACTs in the second and third trimester [7–15], but individual studies often lack the power to draw definitive conclusions. A Cochrane review examining maternal treatment response, fetal outcomes, and drug safety of drug regimens for uncomplicated falciparum malaria considered each drug combination compared with the control drug as reported in studies, but it did not evaluate ACTs as a group compared with non-ACTs [16]. We conducted a meta-analysis of randomized controlled trials (RCTs) to compare the efficacy, safety, and tolerance of ACTs to quinine and other non-ACT antimalarials for the case management of uncomplicated falciparum malaria in the second and third trimester of pregnancy to provide policymakers with the evidence needed to improve adherence among health providers to the current policy on treatment with ACTs.
METHODS
Search Strategy
We searched the Malaria in Pregnancy Library up to May 2015 [17, 18] to identify all trials that compared the efficacy and safety of ACTs with other antimalarials with no restrictions to time or geographic location. The Malaria in Pregnancy Library (http://library.mip-consortium.org) is a comprehensive bibliographic database created by the Malaria in Pregnancy Consortium that is updated every 4 months using a standardized protocol to search over 40 sources, including PubMed, Web of Knowledge, and Google Scholar. Languages other than English were not excluded a priori. The search followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [19]; no prior protocol for the meta-analysis was developed. The following search terms were used: artemether OR artesunate OR artemisinin OR dihydroartemisinin OR ACT. No search terms were needed for malaria or pregnancy because the malaria in pregnancy library already screens for these terms. The references of retrieved articles were examined to identify additional studies.
Selection Criteria
A first screening was conducted based on title and abstracts; the remaining references were reviewed by 2 authors (R.J.B. and M.B.), and studies were included if they met the following inclusion criteria: (1) RCT in humans comparing oral treatment with ACTs vs non-ACTs; (2) inclusion of pregnant women in their second and/or third trimester with uncomplicated falciparum malaria; and (3) availability of data to assess the efficacy and safety per treatment arm. Studies or study arms that contained monotherapy of artemisinin derivatives or antimalarials or combination antimalarials that are currently no longer considered or are contraindicated for treatment of malaria in pregnancy (eg, sulfadoxine-pyrimethamine [SP] alone and chlorproguanil-dapsone) were excluded. Discrepancies between reviewers were resolved through discussion until consensus was reached.
Quality Assessment
The same authors (R.J.B. and M.B.) assessed the quality of the included studies using the Cochrane Collaboration's tool for assessing the risk of bias among randomized trials [20]. Quality was classified as “low” (high risk of bias), “high” (low risk of bias), or “unclear” (Supplementary 1, Tables 1.1 and 1.2). Details of the quality criteria used are included in Supplementary 1.
Data Extraction
Data extraction was undertaken independently by 2 authors (R.J.B. and M.B.) using spreadsheets. We extracted the following general information: author, year of publication, geographic location, population, details of treatment regimens used, and number of women treated per arm, and study characteristics such as mean age, gestational age, fever, bodyweight, gravidity, and parasite density at the time of enrollment. To assess efficacy, we extracted information on treatment efficacy outcomes based on the polymerase chain reaction (PCR)-adjusted failure rate by day 28, 42, or 63, parasite and fever clearance, mean birth weight, low birth weight, and mean gestational age. To assess safety, we extracted information on preterm births (<37 weeks), congenital malformations, stillbirths, and miscarriage. To assess tolerability, information on reported side-effects was extracted, with special reference to side-effects commonly associated with quinine, such as tinnitus, dizziness, vomiting, and hypoglycemia [6]. Any discrepancies were resolved by discussion until consensus was reached.
Outcomes
The primary outcome for the efficacy analysis was the PCR-adjusted failure rate by day 28 to day 63 (whichever was reported latest). Secondary outcomes included mean birth weight, prevalence of low birth weight, mean gestational age, and prevalence of prematurity. The outcomes for the safety analysis were prevalence of congenital abnormalities, stillbirth, and miscarriage. We combined the outcomes of miscarriage and stillbirths into fetal loss because the number of women enrolled before 28 weeks gestation and at risk of miscarriage was not routinely reported. To assess tolerability, we assessed the prevalence of adverse effects, including tinnitus, dizziness, and vomiting. Low birth weight was defined as <2500 g, measured within 24 hours after delivery. Prematurity was defined as a delivery before 37 weeks of gestation.
Meta-Analysis
Two of the 6 included studies had more than 1 comparison drug. In the study by Mutabingwa et al [14], the 4 arms included SP, chlorproguanil-dapsone, SP-amodiaquine, and artesunate-amodiaquine. The SP-only and chlorproguanil-dapsone arms were excluded from the analysis because these drugs are no longer recommended for the case management of malaria (SP) or are no longer available (chlorproguanil-dapsone) [14]. The study by Kalilani et al [11] included 3 arms: SP, SP-azithromycin, and SP-artesunate, and again the SP-only arm was excluded. Whenever standard deviations for continuous variables were not available, these values were calculated using the reported t test value or the 95% confidence interval (CI) [21]. Data on efficacy, safety, and tolerability were summarized using forest plots, and meta-analyses were conducted when 3 or more studies had this outcome. We used both random effects (DerSimonian and Laird: primary method) and fixed-effects models to obtain pooled estimates (pooled risk ratios [PRR] or weighted mean differences and corresponding 95% CI [22]). Heterogeneity was evaluated using the τ2 and χ2 test, and I2 was used to measure the extent of heterogeneity across trials [23]. Data were analyzed using Stata (version 13). We conducted subgroup analysis by type of comparison drug (ACT vs quinine or ACT vs non-ACT) and continent (Asia vs Africa). We performed sensitivity analyses to examine the effect of study quality (high vs moderate-to-low).
RESULTS
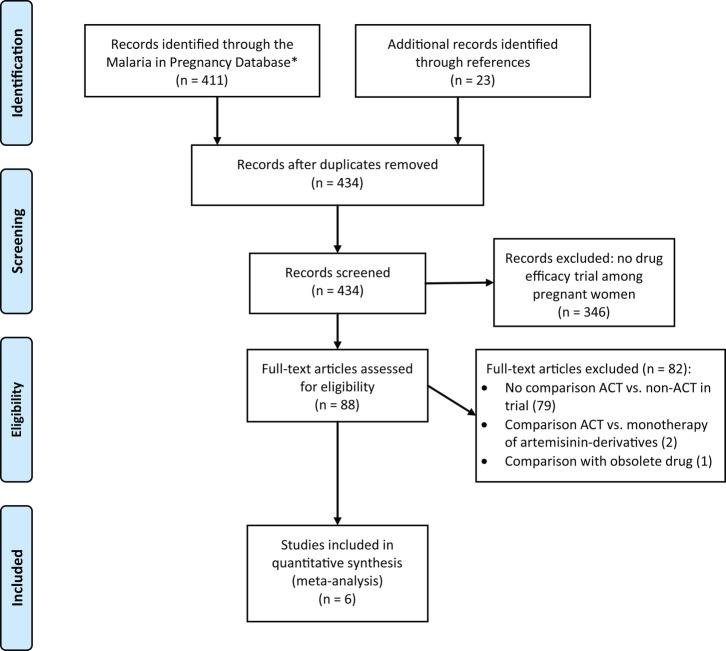
Of the 411 studies identified, 65 were included for review of the full text. An additional 23 studies were identified from a search of the references of full-text reviews. Of these 88 studies, 6 met the inclusion criteria: 3 from sub-Saharan Africa (Malawi, Tanzania, and Uganda) and 3 from Thailand (Figure 1). The studies were conducted between 1995 and 2009 (Table 1). Artemisinin-based combination therapies used in the trials included artemether-lumefantrine (1 study), mefloquine-artesunate (2 studies), amodiaquine-artesunate (1 study), SP-artesunate (1 study), and atovaquone-proguanil-artesunate (1 study; Table 1); duration of regimens were 3 days, except for 1 study in Thailand where artesunate was given for 5 days (in combination with mefloquine) [9–14]. Comparator drugs included the following: quinine for 7 days (4 studies: 3 in Asia, 1 in Africa); SP in combination with azithromycin for 2 days (1 study, Africa); or amodiaquine for 3 days (1 study, Africa; Table 1). A total of 807 women were enrolled; 415 women received ACTs, 265 received quinine, and the remaining 127 received the other non-ACTs.
Figure 1.
PRISMA flow diagram. Abbreviation: ACTs, artemisinin-based combination therapies.
Table 1.
Description of Included Studies Evaluating ACT vs Non-ACT in Pregnancy, Asia and Africa, 1995–2009
| Author [Ref.] | Country (Time Period) | Population | Experimental Treatment (First Arm) | No. of Women in First Arm of the Study | Comparison Treatment (Other Arm(s)) | No. of Women in Other Arm(s) of the Study | Efficacy Outcomes Reporteda | Safety Outcomes Reported (in Chronological Order) | Tolerability Reported (in Alphabetical Order) |
|---|---|---|---|---|---|---|---|---|---|
| Bounyasong [10] | Thailand (January 1995–December 1998) | Pregnant women with a gestational age of at least 28 wks infected by Plasmodium falciparum (<4% of RBC parasitized) | AS5 + MQ1 (2 mg/kg artesunate p.o. as a first dose with subsequent 1 mg/kg per 12 h p.o. for 5d + 25 mg/kg mefloquine p.o. on day 6)b Patients admitted for duration of treatment |
28 women | Q7 (10 mg/kg quinine sulphate p.o. t.i.d. for 7 d) Patients admitted for duration of treatment |
29 women |
Treatment failure Parasite clearance time Fever clearance time |
Hypoglycemia Hematocrit Pregnancy complications Fetal growth (biparietal diameter) Gestational age (ultrasound, LMP and fundal height) Apgar scores; arterial blood pH Birthweight within 24 h Head and abdominal circumference Neurological test scores infant Neonatal jaundice (follow-up: 28 d) Neurological and physical development up to 24 mo |
Blurred vision Nausea Palpitations Tinnitus Vertigo Vomiting (Follow up 28 d) |
| Kalilani [11] | Malawi (September 2003–September 2004) | Pregnant women (EGA 14–26 wks) between 15 and 49 y old, with peripheral P falciparum parasitemia | AS3 + SP1 (3× 500 mg sulfadoxine/25 mg pyrimethamine p.o. once + 200 mg/day artesunate p.o. for 3 d) All doses supervised |
47 women | SP1 + AZ2 (3× 500 mg sulfadoxine/25 mg pyrimethamine p.o. once + 1 g/day azithromycin p.o. for 2 d) All doses supervised |
47 women | Treatment failure Parasite clearance time Fever clearance time |
Hemoglobin Pregnancy outcome: Abortion (<28 wks of gestation)Stillbirth (>28 wks of gestation) Birth weight within 24 h Prematurity (Ballard score within 24 h) Neonatal death Note: women received the same drug again during the rest of the pregnancy |
Adverse events Abdominal pain Coughing Diarrhoea Fever General body pain Headache Nausea Skin rash Vomiting (follow up: 28 d after first dose) |
| McGready [13] | Thai-Burmese border (October 1995–July 1997) | Pregnant women in their 2nd or 3rd trimester with a microscopy-confirmed uncomplicated P falciparum infection | AS3 + MQ2 (4 mg/kg per day artesunate p.o. on day 0, 1, and 2 + 15, respectively; 10 mg/kg mefloquine p.o. on day 1 and 2) All doses supervised |
66 women | Q7 (10 mg/kg quinine sulphate p.o. t.i.d. for 7 d) All doses supervised |
42 women |
Treatment failure (for P falciparum) Parasite clearance Fever clearance Gametocyte carriage Other species than P falciparum |
Hematocrit Pregnancy outcome: Abortion Stillbirths Congenital abnormalities Birth weight (Salter scale) Placental weight Gestational age (Dubowitz within 3 d) Neonatal deaths Infant development up to 12 mo |
Anorexia Dizziness Headache Muscle and joint pain Tinnitus. (follow-up: 28 d) |
| McGready [12] | Thai-Burmese border (December 2001–July 2003) | Otherwise healthy pregnant women (14–32 wks) with their first episode of uncomplicated P falciparum or mixed infection, and hematocrit ≥20% | AS3 + ATP3 (4 mg/kg per day artesunate + 20 mg/kg per day atovaquone + 8 mg/kg per day proguanil p.o. for 3 d) All doses supervised |
39 women | Q7 (10 mg/kg quinine sulphate p.o. t.i.d. for 7 d) All doses supervised |
42 women |
PCR-adjusted cumulative cure rate
Median time to PCR-confirmed parasite recrudescence Median time from admission to a novel infection Parasite clearance Fever clearance Gametocyte carriage Other species than P falciparum |
Hematocrit Pregnancy outcomes: Abortion (<28 wks) Stillbirth (>28 wks) Birth weight within 24 h Growth parameters (newborn head, height and arm circumference) Gestational age (ultrasound, fundal height or Dubowitz) Congenital abnormalities Infant follow-up (neonatal death, growth parameters, neurodevelopmental scores up to 1 y) |
Urticaria Vomiting Tinnitus (follow up: 42 d) |
| Mutabingwa [14] | Tanzania (January 2004–September 2006) | Pregnant women (14–34 wks of gestation) with mild-moderate, slide proven, falciparum malaria under 38 y old with an uncomplicated pregnancy | AS3 + AQ3 (10 mg/kg amodiaquine + 4 mg/kg artesunate p.o. for 3 d) All doses supervised |
83 women | SP1 + AQ3 (3× 500 mg sulfadoxine/25 mg pyrimethamine p.o. once + 10 mg/kg amodiaquine for 3 d) All doses supervised |
80 women |
Parasitological failure by day 28 Parasitological failure by day 14 Clinical failure at day 14 and 28 Gametocytes on day 14 |
Hemoglobin Stillbirth Birth weight Gestational age (Dubowitz score) Congenital abnormalities Perinatal and neonatal mortality |
Abdominal pain Dermatological Diarrhoea Dizziness Nausea/vomiting Respiratory complaints. (follow up: 28 d) |
| Piola [9] | Uganda (October 2006–May 2009) | Women with a viable pregnancy with an estimated gestation of 13 wks or longer and a mild-moderate malaria infection detected by microscopy (P falciparum mixed or monoinfection). Women were excluded if they had P falciparum parasitemia >250 000 parasites/µL or severe anemia (hemoglobin <7 g/dL) | AM3 + L3 (80 mg artemether/480 mg lumefantrine p.o. b.i.d. for 3 d) All doses supervised |
152 women | Q7 (10 mg/kg quinine sulphate p.o. t.i.d. for 7 d) All doses supervised |
152 women |
PCR-corrected adequate clinical and parasitological response at day 42 PCR-uncorrected adequate clinical response on day 42 or delivery (whichever came last) Parasite clearance Fever clearance Gametocyte carriage Other species than P falciparum |
Hemoglobin Abnormal laboratory parameters (ALT, bilirubin, creatinine, full blood count) Abnormal QTc interval on ECG Gestational age (ultrasound, LMP if >24 wks) Birthweight Congenital abnormalities Perinatal, neonatal, and infant death |
Abdominal pain Anorexia Dizziness Headache Influenza-like syndrome Nausea Tinnitus Vomiting Weakness (follow up: 42 d) |
Abbreviations: ACT, artemisinin-based combination therapy; AQ, amodiaquine; AL, artemether-lumefantrine; ALT, alanine aminotransferase; AS, artesunate; ATP, atovaquone-proguanil; AZ, azithromycin; d, days; ECG, electrocardiography; EGA, estimated gestational age; MQ, mefloquine; PCR, polymerase chain reaction; p.o, per os; Q, quinine; L, lumefantrine; LMP, last menstrual period; RBC, red blood cells; Ref., reference; SP, sulfadoxine-pyrimethamine; tid, 3 times per day.
a Primary efficacy outcomes are shown in bold type.
b Artesunate doses might have been extended until parasite clearance and clinical improvement in this study.
Study Quality
All studies were open label; 4 studies reported blinding of the microscopist [9, 11, 12, 14], but none of the studies reported whether the person who assessed the newborn was blinded (Supplementary 1). The quality of 2 studies was assessed as “low-to-moderate” due to the lack of information on the methods used for the random sequence generation and/or how allocation concealment was assured [10, 13], or due to possible reporting bias [12, 13] or incomplete reporting of outcomes [9, 12, 13]. All doses of treatment were directly observed in all studies.
Characteristics of Study Participants
Most women were enrolled between 20 and 30 weeks gestation (Table 2). Documented fever ranged from 4% to 100% at enrollment. The mean or median bodyweights in 2 studies from the Thai-Burmese border and 1 study in Malawi were lower than in the other studies. Two studies screened for human immunodeficiency virus (HIV) status; HIV prevalence was 27% in Malawi, with a potential difference by study arm (21% vs 34%), but not all participants agreed to be tested [11]. Only 1 of 161 participants in the study in Tanzania tested positive for HIV [14]. Although HIV status in the study in Uganda was not assessed, this was likely approximately 8% based on regional HIV data in pregnant women around that time [9, 24, 25].
Table 2.
Characteristics of Participants at Enrolment of Included Studies Evaluating ACT vs Non-ACT in Pregnancy, Asia and Africa, 1995–2009
| Study | Age |
Gestational Age |
Fever |
Maternal Weight |
Gravidity |
Parasitemia |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACT | Non-ACT | ACT | Non-ACT | ACT | Non-ACT | ACT | Non-ACT | ACT | Non-ACT | ACT | Non-ACT | |
| Bounyasong [10] | Mean 26.1 y, sd 7.5, n = 28 | Mean 27.2 y, sd 7.5, n = 29 | Mean 26.7 wks, sd 7.6, n = 28 | Mean 27.4 wks, sd 7.6, n = 29 | Mean 40.4°C, sd 0.6, n = 28 | Mean 40.6°C, sd 0.6, n = 29 | Mean 57.1 kga, sd 4.1, n = 28 | Mean 58.7 kga, sd 4.1, n = 29 | Mean 2.4, sd 0.6, n = 28 | Mean 2.6, sd 0.6, n = 29 | Mean1330/200 WBC, sd 209, n = 28 | Mean 1313/200 WBC, sd 209, n = 29 |
| Kalilani [11] | Median 20 y, IQR 17–24 | Median 20 y, IQR 18–23 | Median 22 wks, IQR 20–24 | Median 24 wks, IQR 20–24 | >37.5°C: 2 of 49 (4.1%) | >37.5°C: 3 of 49 (6.1%) | Mean 52.9 kg, sd 5.9, n = 47 | Mean 52.2 kg, sd 6.1, n = 47 | Median 1, IQR 1–3 PG: 28 of 47 (59.6%) | Median 1, IQR 1–3 PG: 25 of 47 (53.1%) | Geometric mean 687/µL, range 120–4260 | Geometric mean 1184/µL, range 150–22 500 |
| McGready [13] | Median 24 y, range 15–37, n = 66 | Median 23 y, range 16–36, n = 42 | Median 24 wks, range 12–40, n = 66 | Median 24 wks, range 15–38, n = 42 | >37.5°C: 10 of 66 (15.2%) | >37.5°C: 9 of 42 (21.4%) | Median 48 kg, range 36–68, n = 66 | Median 50 kg, range 41–67, n = 42 | PG: 18 of 66 (27.3%) | PG: 12 of 42 (28.6%) | Median 11 651/µL, range 32–241 127, n = 66 |
Median 19 086/ µL, range 79–149 389, n = 42 |
| McGready [12] | Mean 26 y, sd 6, n = 39 | Mean 26 y, sd 7, n = 42 | Mean 21 wks, sd 5.3, n = 39 | Mean 21 wks, sd 4.5, n = 42 | Fever b 7 of 39 (17.9%) |
Fever b 7 of 42 (16.7%) |
Mean 49 kg, sd 7, n = 39 | Mean 50 kg, sd 7, n = 42 | PG: 12 of 39 (30.8%) | PG: 11 of 42 (26.2%) | Geometric mean 2596/ µL, range 33–123 027 | Geometric mean 2084/µL, range 33–109 648 |
| Mutabingwa [14] | Median 21 y, IQR 19–26, n = 83 | Median 20 y, IQR 19–25, n = 80 | Median 6 mo, IQR 5–7, n = 83 | Median 7 mo, IQR 5–7, n = 80 | NR | NR | NR | NR | PG: 20 of 34 (59%) | PG: 14 of 38 (37%) |
Median181/200 WBC range 62–628, n = 83 |
Median 25/200 WBC range 51–578, n = 80 |
| Piola [9] | Mean 22.5 y, range 15–38, n = 152 | Mean 22.6 y, range 17–38, n = 152 | Mean 22.3 wks, range 9–38, n = 152 | Mean 24.7 wks, range 10–39, n = 152 | >37.5°C: 35 of 152 (23.0%) |
>37.5°C: 30 of 152 (19.7%) |
Mean 58 kg, sd 10, n = 152 | Mean 58 kg, sd 10, n = 152 | Median 2, range 1–8 | Median 2, range 1–7 | Geometric mean 1418/µL, sd 4727, n = 152 | Geometric mean 1995/µL, sd 9771, n = 152 |
Abbreviations: ACT, artemisinin-based combination therapy; IQR, interquartile range; kg, kilogram; NR, not reported; PG, primigravidae; sd, standard deviation; WBC, white blood cells.
a Bounyasong [10]: Weight before pregnancy.
b Fever threshold not defined.
Meta-Analysis of Efficacy
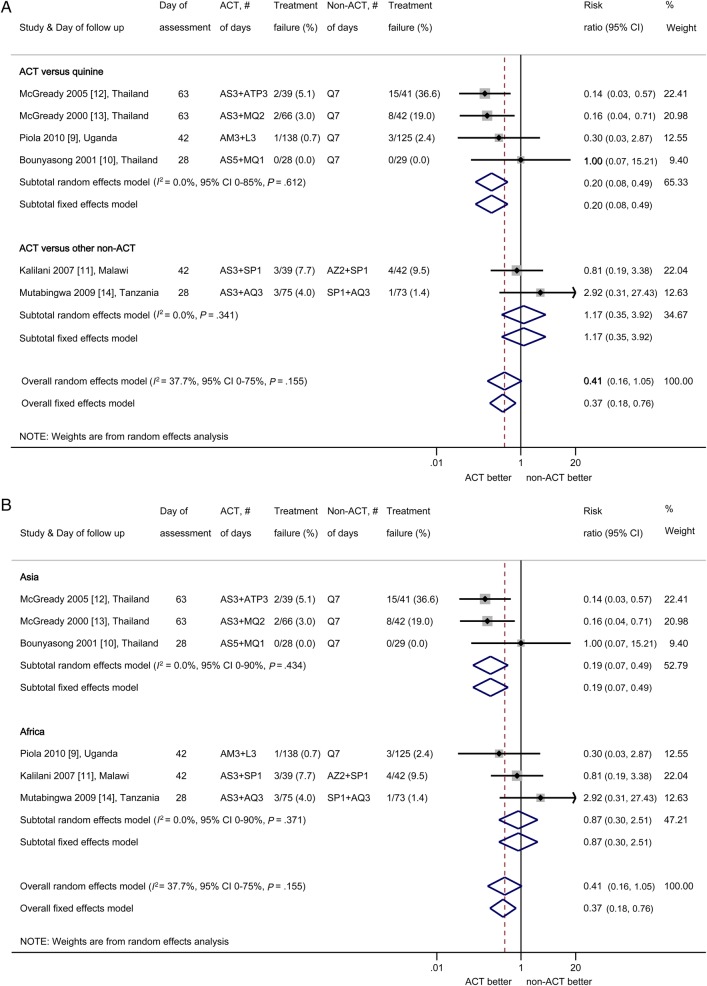
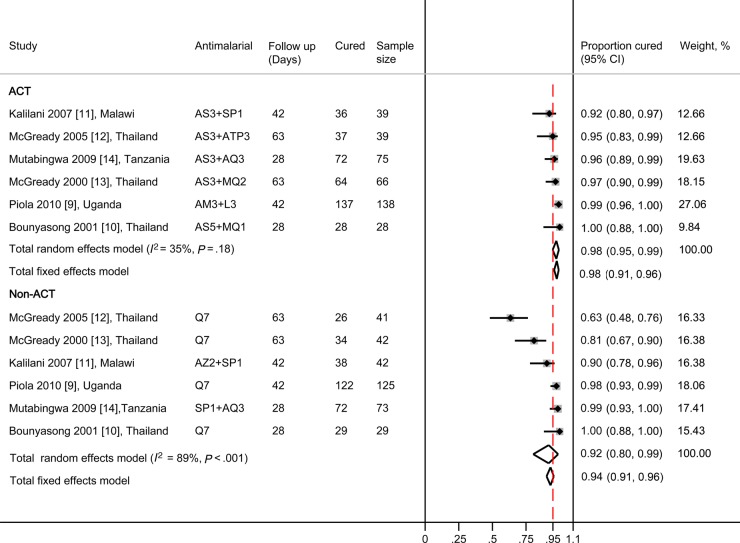
The median PCR-adjusted failure rate by days 28 to 63 in the non-ACT group was 6 (range, 0–37) per 100 women: meta-analysis showed that the PCR-adjusted failure rates with ACTs were lower than with non-ACTs, but the difference overall was not statistically significant (PRR random-effects model 0.41, 95% CI, 0.16–1.05; I2 = 38%, 95% CI, 0%–75%, 6 trials; Figure 2A). Subgroup analysis by comparator drug showed that ACTs were significantly more effective than oral quinine and reduced the risk of treatment failure by 80% with no heterogeneity between trials (PRR 0.20, 95% CI, 0.08–0.49; I2 = 0%, 95% CI, 0%–85%, 4 studies; Figure 2A). Analysis by continent showed significantly lower failure rates with ACTs than non-ACTs in Thailand (PRR 0.19; 95% CI, 0.07–0.49), but not in Africa (PRR 0.87; 95% CI, 0.30–2.51; Figure 2B). Artemisinin-based combination therapies were associated with ≥95% cure rates in 5 of the 6 studies; the exception was the SP-artesunate arm in the study from Malawi, which had a cure rate of 92% after 42 days (Figure 3). The parasite clearance rates were significantly faster with ACTs than with quinine (Supplementary Table 2.1) [9, 10, 12, 13] with a lower risk of being positive by day 2 (PRR 0.23, 95% CI, 0.11–0.45; I2 = 50%, 3 studies). Results of other efficacy outcomes that were not combined by meta-analysis because of differences between studies in units of measure (eg, hemoglobin vs hematocrit) or time points are given in Supplementary 2; these include hemoglobin, fever clearance, effect on gametocytes, other species than P falciparum and infant follow up. Gametocyte carriage overall was significantly lower in the ACT groups compared with quinine in 2 studies in Thailand, and in the study in Uganda gametocyte clearance was faster in the ACT group compared with quinine (Supplementary Table 2.2) [9, 12, 13].
Figure 2.
(A) Meta-analysis of PCR-adjusted parasitological treatment failure risk comparing artemisinin-based combination therapies (ACTs) vs. non-ACTs, stratified by comparator drug. (B) Meta-analysis of PCR-adjusted parasitological treatment failure risk comparing ACTs versus non-ACTs, stratified by geographical region. (Note: Because statistical programs cannot handle 0% treatment failure for both arms in meta-analysis, information from Bounyasong [10] was entered as 1/28 for each arm, to attain a balanced risk ratio (RR) of 1 [26].) The shaded areas around the estimates are proportional to the weight of each study in the analysis. Abbreviations: AM, artemether; AQ, amodiaquine; AS, artesunate; ATP, atovaquone-proguanil; AZ, azithromycin; CD, chlorproguanil-dapsone; CI, confidence interval; L, lumefantrine; MQ, mefloquine; Q, quinine; SP, sulfadoxine-pyrimethamine.
Figure 3.
Polymerase chain reaction (PCR)-adjusted cure rates (proportions) after treatment with artemisinin-based combination therapies (ACTs) and non-ACTs for uncomplicated malaria in pregnancy. The number after the drug indicates the number of days the drug was given. The red dashed vertical bar indicates the 0.95 proportion mark, which is the cut-off recommended by the World Health Organization (WHO) as the minimum efficacy for antimalarial medicines to be adopted as part of national malaria programs [27]. Abbreviations: AM, artemether; AQ, amodiaquine; AS, artesunate; ATP, atovaquone-proguanil; AZ, azithromycin; CI, confidence interval; L, lumefantrine; MQ, mefloquine; Q, quinine; SP, sulfadoxine-pyrimethamine.
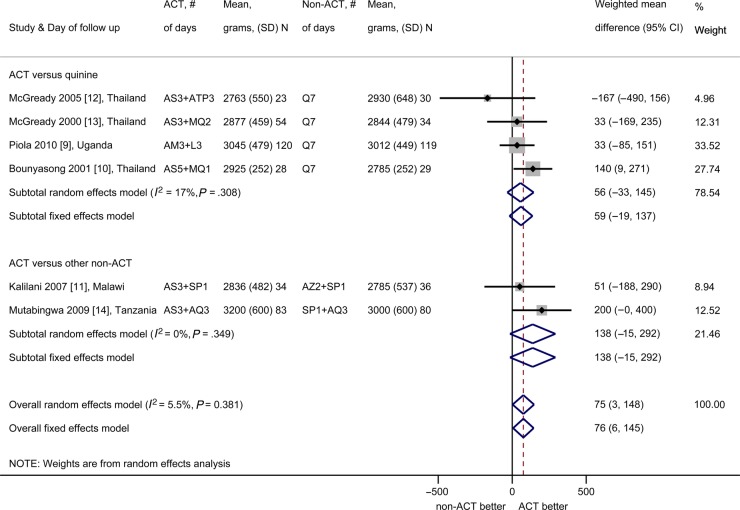
Meta-Analysis of Birth Outcomes
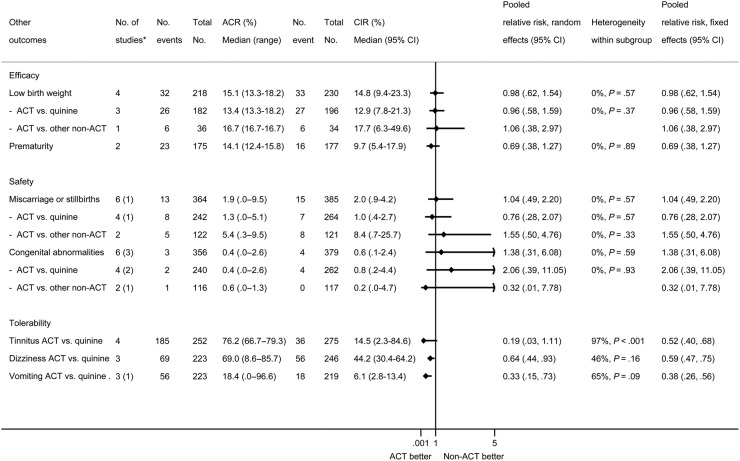
Mean birth weight was significantly higher in children born to ACTs recipients compared with non-ACTs recipients (mean weighted difference 75 g, 95% CI, 3–148 g; I2 = 6%, 95% CI, 0%–76%, 6 studies; Figure 4). The prevalence of low birth weight was not different (PRR 0.98, 95% CI, 0.62–1.54; I2 = 0%, 4 studies; Figure 5), and neither were the estimated mean gestational age (mean weighted difference 0.11 weeks, 95% CI, −0.26 to 0.48 weeks, I2 = 0%, 4 studies, all ACT vs quinine) or the prevalence of preterm births (PRR 0.69, 95% CI, 0.38–1.27; I2 = 0%, 2 studies; Figure 5). The occurrence of fetal loss (ACTs: 15 of 385 [3.9%], non-ACTs: 13 of 364 [3.6%], PRR 1.04, 95% CI, 0.49–2.20; I2 = 0%, 5 studies; Figure 5) and congenital abnormalities detected at birth were not significantly different (ACTs: 4 of 377 [1.1%], non-ACTs: 3 of 356 [0.8%], PRR 1.38, 95% CI, 0.31–6.08; I2 = 0%, 3 studies; Figure 5). Three studies (all in Thailand) observed infants for more than 5 months and reported an absence of developmental differences by treatment arm (Supplementary Table 2.3) [10, 12, 13].
Figure 4.
Mean birth weight (grams) of newborns born to artemisinin-based combination therapy (ACT) and non-ACT recipients. (Note: All participants in the study by Kalilani et al [11] received the same treatment again at least 4 weeks after the first treatment, regardless of whether they were parasitemic at that subsequent visit.) The shaded areas around the estimates are proportional to the weight of each study in the analysis. Abbreviations: AM, artemether; AQ, amodiaquine; AS, artesunate; ATP, atovaquone-proguanil; AZ, azithromycin; CD, chlorproguanil-dapsone; CI, confidence interval; L, lumefantrine; MQ, mefloquine; N, sample size; Q, quinine; SD, standard deviation; SP, sulfadoxine-pyrimethamine.
Figure 5.
Other efficacy, safety, and tolerability outcomes and subgroup analyses by control drug. The Assumed control-group risk (ACR) represents the observed median risk (expressed per 100 women) and range for each subgroup in the control-drug arm. The range for the ACR is only provided to illustrate high‐ and low‐risk populations, whereas the median risk is illustrative of a population with a moderate risk. The Corresponding intervention-group risk ([CIR] 95% confidence interval [CI]) is the corresponding risk in the ACT group computed as the ACR × risk ratio ([RR] 95% CI) [28]. Heterogeneity relating to the extent that the RRs vary between subgroups are shown as the I2 statistic, which depicts the percentage of the between‐study or between subgroup heterogeneity that is attributable to variability in the effect, rather than sampling variation. The corresponding P value is based on the χ2 statistic. *The number in brackets depicts the number of studies that were excluded in the analysis because of 0 events in both arms. †Heterogeneity between subgroups (quinine vs other non-ACT as control drug): low birth weight I2 0%, P = .89; miscarriage or stillbirth I2 0%, P = .39; congenital abnormalities I2 0%, P = .48.
Meta-Analysis of Tolerability
A lower prevalence of tinnitus, dizziness, and vomiting was reported among women using ACTs compared with women using quinine, and this result was statistically significant for dizziness and vomiting (tinnitus PRR 0.19, 95% CI, 0.03–1.11, 4 studies; dizziness PRR 0.64, 95% CI, 0.44–0.93, 3 studies, vomiting PRR 0.33, 95% CI, 0.15–0.73, 2 studies [1 study zero events]; Figure 5). Only 1 of the 4 studies that used quinine reported on hypoglycemia, with a significantly higher proportion in the quinine arm compared with ACT (quinine 21 of 29, 72.4%, vs ACT 3 of 28, 10.7%, risk ratio [RR] 6.76, 95% CI, 2.27–20.15, P < .01) [10].
DISCUSSION
In our meta-analyses, ACTs were better tolerated and much more effective than oral quinine for the treatment of uncomplicated falciparum malaria in pregnancy, and they were associated with faster asexual and sexual parasite clearance and lower PCR-corrected treatment failures and less gametocyte carriage. No differences were seen in the risk of fetal death or congenital abnormalities, although the number of events was low and the power to detect differences was limited.
Across all studies, treatment with ACTs was associated with a consistently higher mean birth weights (75 grams) relative to the non-ACT recipients. The size of the effect on birth weight is remarkable given the variety in timing of treatments by gestation across pregnancy. Artemisinin-based combination therapies may be more effective in providing radical cure and clearing parasites from the placenta than non-ACTs, as suggested by the trial in Uganda, which showed a lower prevalence of placental hemozoin deposition in the ACT arm compared with women who received quinine [29]. Alternative explanations include a chance finding, given that there was no difference in the frequency of low birth weight, or observer bias due to the absence of blinding among staff who weighed newborns.
A difference was noted in efficacy by geographical region. The studies in Asia were conducted in the same region in Thailand using the same comparator drug (quinine), and this will have added to the more uniform results in Asia, whereas the studies in Africa were conducted in 3 different countries (with different malaria epidemiology) with 3 different ACTs and comparator drugs, which will have added to the variability in results in Africa.
A review of artemisinin use in pregnancy conducted in 2007 concluded that ACTs have an excellent efficacy profile in the second and third trimester, but researchers also stressed the importance of more research into the safety of ACTs in pregnancy [30]. The current review adds to this limited database and shows that the risk of congenital abnormalities and fetal death (spontaneous miscarriage or stillbirth) is similar among women with acute malaria treated with ACTs or other antimalarials when used for the case management of uncomplicated malaria in the second and third trimester (Figure 4).
Artemisinin-based combination therapies were much better tolerated than the comparison drugs, in particular quinine, which is associated with the symptom complex of cinchonism, which combined with the fact that it has to be taken 3 times daily for 7 days is a major determinant of the low adherence to the quinine regimen [5, 31]. This was illustrated in one of the included studies from Uganda where quinine recipients were more likely to stop their treatment prematurely (6 of 152 or 3.9%) than artemether-lumefantrine recipients (1 of 152 or 0.7%, P = .06) [9]. Hypoglycemia is a well known side-effect of quinine therapy, especially when given parenterally in patients with severe disease; 1 of the 4 studies using oral quinine monitored for hypoglycemia [32] and found a higher risk in the quinine arm (RR 6.76, 95% CI, 2.27–20.15, P < .01) [10].
This review is subject to several limitations, which may inform the design and conduct of future studies. First, the 6 included studies were heterogeneous in study design with regards to treatment regimen, outcomes, and population. Each study used a different ACT, and the timing of the measurement of the primary efficacy endpoint, the PCR-adjusted failure rate, varied from day 28 to 63; none of the studies was blinded. Tolerability indicators varied between studies as well as the methods and timing of assessment of many of the secondary efficacy measures, making it difficult to pool the information. The small number of studies limited the ability to explore determinants of the heterogeneity that was observed (such as effect of HIV infection, differences in mean maternal weight, or time trends) and the evaluation of rarer adverse events. All 3 studies in Asia were conducted on the Western border of Thailand with Myanmar, which may limit the generalizability of the results. Three studies were conducted in countries where intermittent preventive treatment with SP was the national policy at the time of the study. In one study, the same case-management drug regimen was repeated after at least 4 weeks as an alternative to intermittent preventive treatment with SP [11]; in another study, it was reported that women did not receive intermittent preventive treatment when they participated in the study [9], whereas in the last study women were treated according to the National Tanzanian policy and could have received SP during follow up [14]. The design of a standardized protocol for the assessment of the efficacy and safety of antimalarials for the case management of malaria in pregnancy would help to reduce some of these limitations in future trials.
CONCLUSIONS
In summary, this meta-analysis suggests that 3-day ACT regimens are more effective and better tolerated than 7 days of oral quinine for the treatment of uncomplicated P falciparum malaria in the second and third trimester of pregnancy. Artemisinin-based combination therapies should be the preferred treatment option for uncomplicated malaria in second and third trimester of pregnancy, and countries should make sure this treatment policy is adhered to by health workers [5].
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Drs. Mutabingwa and Kalilani for providing additional information for this review, and we thank Esperanca Sevene and Stephanie Kovacs for their useful comments.
Author contributions. F. O. t. K. and J. H. conceived the study and participated in its design. R. J. B., M. B., and A. M. v. E. conducted the initial data screening, extraction, and analysis. R. J. B. and M. B. wrote the first draft of the manuscript. A. M. v. E. conducted additional analyses and revised the manuscript. All authors critically reviewed the manuscript. All authors read and approved the final manuscript.
Disclaimer. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Financial support. This study was funded by the Malaria in Pregnancy Consortium through a grant from the Bill & Melinda Gates Foundation (46099) to the Liverpool School of Tropical Medicine.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Desai M, ter Kuile FO, Nosten F et al. . Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Guidelines for the Treatment of Malaria. Geneva: World Health Organization; 2006. [Google Scholar]
- 3.World Health Organization. Guidelines for the Treatment of Malaria. 3rd ed Geneva: World Health Organization; 2015. [Google Scholar]
- 4.World Health Organization. World Malaria Report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 5.Hill J, D'Mello-Guyett L, Hoyt J et al. . Women's access and provider practices for the case management of malaria during pregnancy: a systematic review and meta-analysis. PLoS Med 2014; 11:e1001688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AR Scientific INC. Quilaquin®, quinine sulfate, capsules USP, 324 mg. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021799s008lbl.pdf Accessed 13 June 2015.
- 7.Kaye DK, Nshemerirwe R, Mutyaba TS, Ndeezi G. A randomized clinical trial comparing safety, clinical and parasitological response to artemether-lumefantrine and chlorproguanil-dapsone in treatment of uncomplicated malaria in pregnancy in Mulago hospital, Uganda. J Infect Dev Ctries 2008; 2:135–9. [PubMed] [Google Scholar]
- 8.McGready R, Tan SO, Ashley EA et al. . A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated Plasmodium falciparum treatment in pregnancy. PLoS Med 2008; 5:e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piola P, Nabasumba C, Turyakira E et al. . Efficacy and safety of artemether-lumefantrine compared with quinine in pregnant women with uncomplicated Plasmodium falciparum malaria: an open-label, randomised, non-inferiority trial. Lancet Infect Dis 2010; 10:762–9. [DOI] [PubMed] [Google Scholar]
- 10.Bounyasong S. Randomized trial of artesunate and mefloquine in comparison with quinine sulfate to treat P. falciparum malaria pregnant women. J Med Assoc Thai 2001; 84:1289–99. [PubMed] [Google Scholar]
- 11.Kalilani L, Mofolo I, Chaponda M et al. . A randomized controlled pilot trial of azithromycin or artesunate added to sulfadoxine-pyrimethamine as treatment for malaria in pregnant women. PLoS One 2007; 2:e1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGready R, Ashley EA, Moo E et al. . A randomized comparison of artesunate-atovaquone-proguanil versus quinine in treatment for uncomplicated falciparum malaria during pregnancy. J Infect Dis 2005; 192:846–53. [DOI] [PubMed] [Google Scholar]
- 13.McGready R, Brockman A, Cho T et al. . Randomized comparison of mefloquine-artesunate versus quinine in the treatment of multidrug-resistant falciparum malaria in pregnancy. Trans R Soc Trop Med Hyg 2000; 94:689–93. [DOI] [PubMed] [Google Scholar]
- 14.Mutabingwa TK, Muze K, Ord R et al. . Randomized trial of artesunate+amodiaquine, sulfadoxine-pyrimethamine+amodiaquine, chlorproguanal-dapsone and SP for malaria in pregnancy in Tanzania. PLoS One 2009; 4:e5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowunmi A, Oduola AM, Ogundahunsi OA et al. . Randomised trial of artemether versus artemether and mefloquine for the treatment of chloroquine/sufadoxine-pyrimethamine-resistant falciparum malaria during pregnancy. J Obstet Gynaecol 1998; 18:322–7. [DOI] [PubMed] [Google Scholar]
- 16.Orton LC, Omari AA. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2008; CD004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malaria in Pregnancy Consortium. Malaria in Pregnancy Library. Available at: http://library.mip-consortium.org Accessed 2 April 2015.
- 18.van Eijk AM, Hill J, Povall S, Reynolds A et al. . The Malaria in Pregnancy Library: a bibliometric review. Malar J 2012; 11:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gotzsche PC et al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Deeks JJ. Selecting studies and collecting data. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions: The Cochrane Collaboration. Chichester, England: John Wiley & Sons Ltd, 2011:151–83. [Google Scholar]
- 22.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28:105–14. [DOI] [PubMed] [Google Scholar]
- 23.Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions: The Cochrane Collaboration. Chichester, England: John Wiley & Sons Ltd, 2011:243–93. [Google Scholar]
- 24.Ministry of Health Uganda, ORC Macro. Uganda HIV/AIDS Sero-behavioural Survey 2004–2005. Calverton, MD: Ministry of Health and ORC Macro, 2006. [Google Scholar]
- 25.Ministry of Health Uganda, ICF International, Centers for Disease Control and Prevention, US Agency for International Development, WHO Uganda. Uganda AIDS Indicator Survey 2011. Kampala, Uganda: Ministry of Health Uganda, 2012. [Google Scholar]
- 26.Higgins JP, Deeks JJ, Altman DG. Special topics in statistics. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions: The Cochrane Collaboration. Chichester, England: John Wiley & Sons Ltd, 2011:481–524. [Google Scholar]
- 27.World Health Organization. Antimalarial drug efficacy. Available at: http://www.who.int/malaria/areas/treatment/drug_efficacy/en/ Accessed 18 January 2015.
- 28.Schunemann HJ, Oxman AD, Higgins JP et al. . Presenting results and ‘Summary of findings’ tables. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions: The Cochrane Collaboration. Chichester, England: John Wiley & Sons Ltd, 2011:335–57. [Google Scholar]
- 29.Muehlenbachs A, Nabasumba C, McGready R et al. . Artemether-lumefantrine to treat malaria in pregnancy is associated with reduced placental haemozoin deposition compared to quinine in a randomized controlled trial. Malar J 2012; 11:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dellicour S, Hall S, Chandramohan D, Greenwood B. The safety of artemisinins during pregnancy: a pressing question. Malar J 2007; 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith Paintain L, Antwi GD, Jones C et al. . Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: provider knowledge and acceptability. PLoS One 2011; 6:e24035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thien HV, Kager PA, Sauerwein HP. Hypoglycemia in falciparum malaria: is fasting an unrecognized and insufficiently emphasized risk factor? Trends Parasitol 2006; 22:410–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.