Abstract
A 22-year-old woman presented to the emergency room of a local hospital with pleuritic chest pain. She regularly worked out and admitted to taking performance-enhancing drugs (PEDs). Clinical findings and further diagnostic work up revealed a diagnosis of perimyocarditis, and adequate therapy was initiated. During the course of the first day, the patient had to be intubated and mechanically ventilated. A diagnosis of bilateral pneumonia and acute respiratory distress syndrome (ARDS) due to an infection by rhinovirus spp was made. A smoking habit, the intense physical training and the use of PED's may have exacerbated the course of the viral pneumonia. After 12 days the patient could be extubated. The length of stay in the intensive care unit was 16 days. After hospital discharge, the patient went to a pulmonary rehabilitation facility for 2 weeks. The outcome was favourable and the patient resumed her strength and endurance training.
Background
Prolonged use of anabolic-androgenic steroids (AAS) is associated with endocrine, cardiovascular and immunological side effects.1 Fatal outcomes including sudden cardiac death have been reported in AAS abusers. The increased risk of adverse outcomes when these patients need general anaesthesia is well known. Extended preoperative work up, different ventilator settings, cautious use of muscle relaxants and different dosing of anaesthetic agents are advised to improve their perioperative outcomes.2 However, no such recommendations exist for emergency departments, or for internal or surgical wards. The health risks of self-administered cocktails of performance-enhancing drugs (PEDs) are largely unknown and often unrecognised. The increasing prevalence of substance abuse due to easy access and its potentially fatal consequences reinforce the need for heightened awareness among healthcare providers.3 PEDs may increase the risk for infection.1 Low virulent viruses such as the rhinovirus4 may cause substantial morbidity in these patients, who should be considered as potentially immunosuppressed.
Case presentation
A 22-year-old woman presented to the emergency room of a local hospital with pleuritic chest pain and shortness of breath. The symptoms started suddenly during a car drive lasting 5 h. Ibuprofen did not alleviate the pain. A mild upper respiratory tract infection had started 3 days previously, and was apparently getting worse. She was on a special diet and had eaten little and had only taken small amounts to drink in the past several days.
She had no known medical condition and initially denied regular intake of any medication. She smoked cigarettes but did not use recreational drugs. On further inquiry, the patient, who regularly worked out, admitted to taking thyroid hormones (liothyronine and levothyroxine), oestrogen-antagonists (anastrozole), anabolic steroids (dehydrochlormethyltestosterone, stanozolole), sympathomimetic drugs (clenbuterole), insulin-like growth factor (IGF-1) and diuretics (aldactone and torasemide) for muscle build-up and performance enhancement. However, exact dosing and duration of intake of the PEDs could not be determined. The patient used a hormonal contraceptive vaginal ring.
Investigations and treatment
On admission, the patient's pulse rate was 101 bpm, blood pressure was 129/69 mm Hg and her body temperature was 37.4°C. The oxygen saturation measured transcutaneously was 99% while breathing room air. The neck veins were flat when examined in the supine position, consistent with an extracellular volume deficit. Faint bilateral basal crackles suggested a lower respiratory tract infection. Moreover, the patient had diffuse epigastric pain on palpation. Her clitoris was hypertrophied. Laboratory tests showed increased markers of inflammation (C reactive protein 16 mg/L (normal <4 mg/L) and leucocytes of 11.8 G/L (normal <9.8 G/L), with mild relative monocytosis of 9.5% (normal 3–8%)). The liver enzymes were slightly elevated (aspartate transaminase (AST) 34 U/L (normal <31 U/L), alanine transaminase (ALT) 45 U/L (normal <34 U/L)) and cardiac enzymes were increased (troponin 3.55 µg/L (normal <0.04 µg/L), creatine kinase 246 U/L (normal <142 U/L)). Haemoglobin concentration on admission was 127 g/L (normal 123–163 g/L) and haematocrit was 37% (normal 36–45%). Initial chest X-ray revealed no abnormalities of the lung parenchyma, and no signs of pulmonary congestion (figure 1A). The D-dimers were within normal limits, suggesting a low probability of a pulmonary embolism. With the history of a preceding common cold, typical findings in the ECG (positive Spodick's sign) and small pericardial effusion in an otherwise normal abdominal sonography, we established the diagnosis of a peri-myocarditis. A transthoracic echocardiogram revealed a diffusely hypokinetic left ventricle with a near-normal left-ventricular ejection fraction of 50–55% (normal 55–65%), and confirmed the presence of a small pericardial effusion without haemodynamic relevance. There were no signs of right-ventricular dysfunction and no pulmonary hypertension.
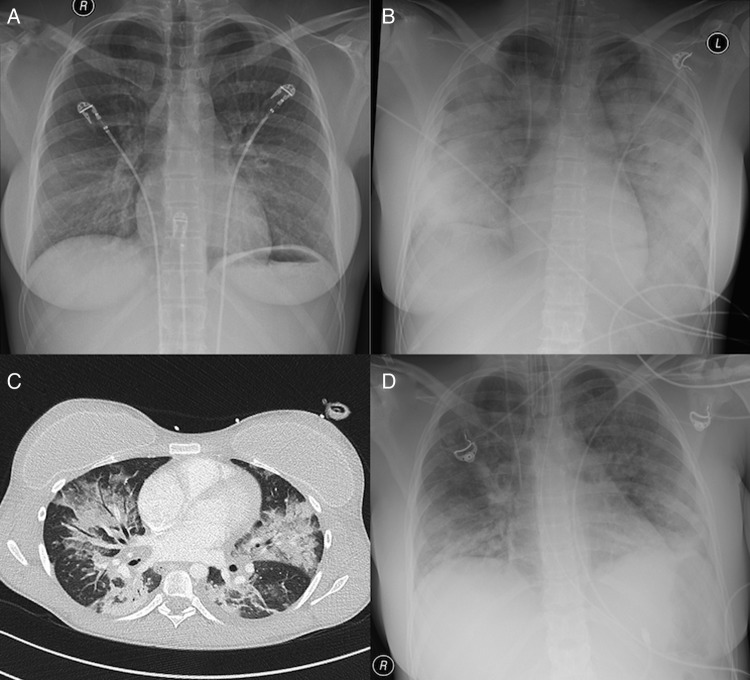
Figure 1.
Chest X-rays obtained in the emergency room on admission (A), in the intensive care unit 15 h later (B) and 1 day before extubation (D). The patchy infiltrates in (B) are consistent with a bilateral pneumonia, gradually disappearing within 14 days (D). In (C) an image obtained by CT on day 7 is shown. Bilateral extensive patchy infiltrates are shown with focal ground-glass opacities and multiple air bronchograms, again consistent with bilateral pneumonia. The patient has bilateral breast implants. There is abundant oedema fluid in the subcutaneous tissue.
The low thyroid-stimulating hormone (TSH) of 0.15 mU/L (normal 0.34–5.60 mU/L), increased free T4 of 36.1 pmol/L (normal 8.0–20.0 pmol/L) and normal free T3 concentrations of 5.1 pmol/L (normal 3.8–6.0 pmol/L) were compatible with drug-induced hyperthyroidism, possibly contributing to the tachycardia, which persisted at 125/min despite 2.5 mg bisoprolol given orally later on.
The patient was volume resuscitated and transferred to the intensive care unit for heart rhythm monitoring. There she became profoundly hypoxic and was intubated and mechanically ventilated 15 h after admission to the hospital. The chest X-ray showed bilateral infiltrates (figure 1B). The patient had a systemic inflammatory response syndrome necessitating pressure support with norepinephrine for 3 days. The pneumonia severity index was low (52 points), however, corresponding to risk class II and a predicted mortality of 0.6%. The simplified acute physiology score was 18 points, confirming a low predicted mortality of 2.9%. After extensive sampling, empiric antibiotic therapy with amoxicillin/clavulanic acid and clarithromycin was started, and later switched to piperacillin/tazobactam. Bronchoscopy revealed chronic bronchitis, and cytological analysis of bronchial secretions showed abundant neutrophilic, but no eosinophilic, granulocytes. An RNA sequence of rhinovirus could be amplified by reverse transcription PCR in three different tracheal secretion specimens. Therefore, we established the diagnosis of severe rhinoviral bilateral pneumonia as a cause for the acute respiratory distress syndrome (ARDS). The time course of the Horovitz index is shown in figure 2, confirming a severe lung injury by the pneumonia. A CT of the chest was consistent with the diagnosis of an ARDS (figure 1C). There were no signs of a pulmonary peliosis. The following viral infections were excluded by qualitative PCR: influenza (A and B), parainfluenza, respiratory syncytial, cytomegaly, adeno, boca, corona, entero, metapneumonia and parecho viruses. There was no antibody response to hantaviruses. Testing for the severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) corona viruses was not performed. Bacterial cultures of blood, urine and tracheal secretions remained sterile, including those taken before starting the antibiotic therapy. No antibodies to the HIV were found. Titres for antinuclear, antineutrophil cytoplasmic and antidouble strand-DNA antibodies were negative.
Figure 2.
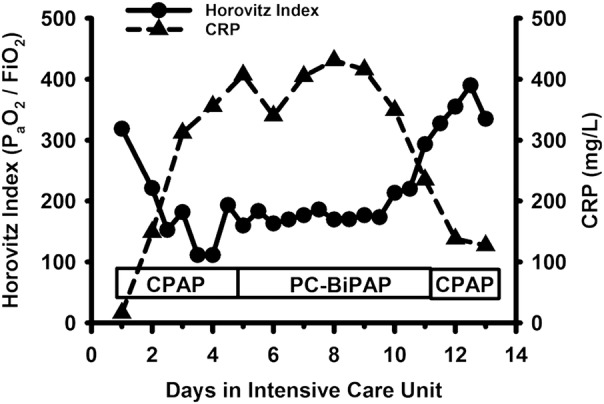
The time course of the Horovitz index and the plasma concentration of the C reactive protein (CRP) are depicted while the patient was intubated and mechanically ventilated in the intensive care unit. A Horovitz index <200 is consistent with a substantial lung injury. CPAP, continuous positive airway pressure support ventilation; PC-BiPAP, pressure controlled biphasic positive airway pressure ventilation.
Outcome and follow-up
The course of the illness was favourable: the gas exchange improved over time, the C reactive protein concentration diminished (figure 2) and a chest X-ray showed regression of the opacities (figure 1D). The patient was extubated on the 13th day. Four days later she was transferred to the ward. A 2-week pulmonary rehabilitation programme was started after a hospital stay of 26 days. Outpatient follow-up visits confirmed that the patient was doing well, and she resumed her physical training sessions.
Discussion
Rhinoviruses genetically comprise three different groups (A, B and C), and belong to the genus Enterovirus of the Picornaviridae family.4 They are a frequent cause of upper respiratory tract infections including the common cold, accounting for approximately 50% of cases. The incubation period is 2–4 days, the viral shedding may last as long as 1–3 weeks. Infections may occur throughout the year. Patients with asthma, chronic obstructive pulmonary disease or cystic fibrosis are at high risk of having an exacerbation in case of a rhinoviral infection.4 In addition, rhinoviruses can cause lower respiratory tract infections, especially in the elderly5 or in immunocompromised hosts. Using reverse transcription PCR, rhinoviruses were repeatedly found in respiratory secretions of patients with community-acquired and healthcare-associated pneumonias in intensive care units6–8 (table 1). Human rhinovirus was detected in 24–58% of all virus positive specimens, and was among the most frequently isolated viruses. Very often (up to 88%), the patients with rhinoviral pneumonia had a severe underlying disease, including malignant tumours, or were immunosuppressed.6 Therefore, the mortality of rhinoviral pneumonia may be very substantial (52.9%).6 There is no established treatment or prevention strategy for rhinoviral pneumonias in humans.4
Table 1.
Incidence of (rhino-)viral infections in patients with severe pneumonia in the intensive care unit
| Author | Adult patients enrolled (mechanically ventilated) | Mean age, years (smokers) | Specimens analysed | Bacteria detected | Viruses detected | Bacterial–viral co-infection | Detection rate | Mortality of viral pneumonia | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Choi6 | 198 (92%) | 65 (12.1%) | 115 BAL, 159 NP swabs | 71 (36%) | 72 (36%) | 18 (9%) | 17 (24%) | 26.5% in viral infection; 33.3% in bacterial–viral co-infection | Rhinovirus most common identified virus; mortality rate 52.9% |
| Wiemken7 | 393 (NR) | 52 (NR) | NP swabs | NR | 92 (23%) | NR | 33 (36%) | NR | Rhinovirus second most frequently detected virus |
| Karhu8 | 49 (100%) | 55 (45%) | 31 BAL, 18 bronchial aspirates | 21 (43%) | 5 (10%) | 19 (39%) | 15 (58%) | 0% in pure viral group; 15.8% in mixed bacterial–viral infection | Rhinovirus most common identified virus |
BAL, bronchoalveloar lavage; NP, nasopharyngeal; NR, not reported.
Human rhinoviruses also play a role in the pathogenesis of pericarditis.9 In particular, group C rhinoviruses were reported to cause severe lower respiratory tract infections and haemorrhagic pericarditis.10 Our patient had rapidly progressive, severe bilateral rhinoviral pneumonia, leading to the development of an ARDS, necessitating airway intubation and mechanical ventilation in the intensive care unit. Her smoking habit, the excessive physical exercise and the ingestion of PEDs negatively impacted on her immune system (figure 3).
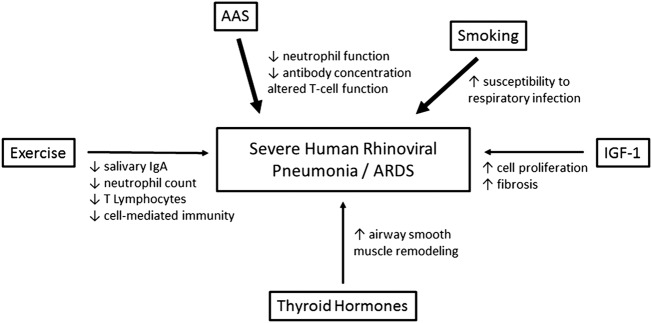
Figure 3.
Scheme showing the immunomodulation induced by heavy exercise, smoking and performance-enhancing drug (PED) abuse, and their potential impact on the development of a severe viral pneumonia. The thickness of the arrows reflects the supposed importance of the parameter shown (AAS, anabolic-androgenic steroids; ARDS, acute respiratory distress syndrome; IGF-1, insulin-like growth factor 1).
Smokers with pneumonia are about three times more likely to be admitted to an intensive care unit than non-smokers,11 indicating a more severe course of the illness in smokers, to which they are more prone.12 Strenuous exercise can reduce immunological defence mechanisms and may increase susceptibility to infections (reviewed in ref. 13). Athletes were found to have an increased incidence of respiratory tract infections during intense training periods, which may be due to decreased salivary IgA production.14 Moreover, exhaustive training sessions lower the neutrophil and T-cell lymphocyte counts in the peripheral blood and therefore may compromise both, the innate and the adaptive immune functions.15 16
The ideal of an athletic, highly performant body, and easy availability of AAS, even from the internet, without prescription by a physician, have propagated their use not only among elite athletes, but also among casual fitness enthusiasts.17 The estimated prevalence of PED use among members of fitness centres in the Netherlands is 8.2%.18 Known severe side effects including premature death do not discourage fitness aficionados from regular intake of PEDs.19 AAS are interloped with the immune system in manifold ways. However, few studies exist addressing their long-term immunomodulatory effects. The exact type of drug used, the dose and the timing of administration often remain elusive. A pathogenetic role was ascribed to their use in the development of severe infections,20 including pneumonias.
In vitro, the oxidative capacity of neutrophilic granulocytes was reduced by androgenic steroids, which negatively impacts on their phagocytic function.21 Moreover, antibody concentrations (IgA, IgM) were lower in bodybuilders using AAS,22 suggesting an enhanced risk of infection. Nandrolone increased production of interleukin 1β and tumour necrosis factor α by human peripheral blood lymphocytes, while the interferon γ production by a human cell line was diminished.23 The latter may jeopardise the antiviral response in vivo, and may be due to an altered T helper cell function by androgenic steroids.21
In addition to the desired effect on muscle and bone function, IGF-1 may have an important pathogenetic role in pulmonary diseases supporting airway inflammation and fibrosis.24 25 Thyroid hormones may contribute to airway smooth muscle remodelling under certain circumstances.26 Taken together, the immunomodulatory effects of smoking, heavy work-outs and chronic ingestion of PEDs may have contributed to the severe pneumonia in our patient.
Learning points.
Human rhinoviruses can cause severe pneumonias, including the development of an acute respiratory distress syndrome.
In viral pneumonias, deterioration of the gas exchange necessitating intubation and mechanical ventilation may be very rapid.
The treatment for rhinoviral pneumonia is entirely supportive.
Consumption of performance-enhancing drugs probably has immunomodulatory effects.
Acknowledgments
The authors thank the following physicians from the Limmattal Hospital for expert patient care and critical review of the manuscript: Tobias Bischof, Thomas Kratt, Andreas Weiss and Marcos Oberacher. They especially thank Jochen Schwarz for figure 1C.
Footnotes
Contributors: KNM initiated and drafted the initial manuscript, and approved the final manuscript as submitted. DW critically reviewed the drafted manuscript and approved the final manuscript as submitted. DS critically reviewed the drafted manuscript and approved the final manuscript as submitted. TH supervised the drafting of the manuscript, designed the illustrations, critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kersey RD, Elliot DL, Goldberg L et al. National Athletic Trainers’ Association position statement: anabolic-androgenic steroids. J Athl Train 2012;47:567–88. 10.4085/1062-6050-47.5.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kam PC, Yarrow M. Anabolic steroid abuse: physiological and anaesthetic considerations. Anaesthesia 2005;60:685–92. 10.1111/j.1365-2044.2005.04218.x [DOI] [PubMed] [Google Scholar]
- 3.Clement CL, Marlowe DB, Patapis NS et al. Nonprescription steroids on the Internet. Subst Use Misuse 2012;47:329–41. 10.3109/10826084.2011.630225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs SE, Lamson DM, St George K et al. Human rhinoviruses. Clin Microbiol Rev 2013;26:135–62. 10.1128/CMR.00077-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louie JK, Yagi S, Nelson FA et al. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis 2005;41:262–5. 10.1086/430915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SH, Hong SB, Ko GB et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med 2012;186:325–32. 10.1164/rccm.201112-2240OC [DOI] [PubMed] [Google Scholar]
- 7.Wiemken T, Peyrani P, Bryant K et al. Incidence of respiratory viruses in patients with community-acquired pneumonia admitted to the intensive care unit: results from the Severe Influenza Pneumonia Surveillance (SIPS) project. Eur J Clin Microbiol Infect Dis 2013;32:705–10. 10.1007/s10096-012-1802-8 [DOI] [PubMed] [Google Scholar]
- 8.Karhu J, Ala-Kokko TI, Vuorinen T et al. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis 2014;59:62–70. 10.1093/cid/ciu237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spodick DH. The pericardium: a comprehensive textbook. New York: Marcel Dekker, Inc., 1997. [Google Scholar]
- 10.Tapparel C, L'Huillier AG, Rougemont AL et al. Pneumonia and pericarditis in a child with HRV-C infection: a case report. J Clin Virol 2009;45:157–60. 10.1016/j.jcv.2009.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrie TJ, Shariatzadeh MR. Community-acquired pneumonia requiring admission to an intensive care unit: a descriptive study. Medicine (Baltimore) 2007;86:103–11. 10.1097/MD.0b013e3180421c16 [DOI] [PubMed] [Google Scholar]
- 12.Dye JA, Adler KB. Effects of cigarette smoke on epithelial cells of the respiratory tract. Thorax 1994;49:825–34. 10.1136/thx.49.8.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleeson M. Immune function in sport and exercise. J Appl Physiol (1985) 2007;103:693–9. 10.1152/japplphysiol.00008.2007 [DOI] [PubMed] [Google Scholar]
- 14.Neville V, Gleeson M, Folland JP. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med Sci Sports Exerc 2008;40:1228–36. 10.1249/MSS.0b013e31816be9c3 [DOI] [PubMed] [Google Scholar]
- 15.Hack V, Strobel G, Weiss M et al. PMN cell counts and phagocytic activity of highly trained athletes depend on training period. J Appl Physiol (1985) 1994;77:1731–5. [DOI] [PubMed] [Google Scholar]
- 16.Steensberg A, Toft AD, Bruunsgaard H et al. Strenuous exercise decreases the percentage of type 1 T cells in the circulation. J Appl Physiol (1985) 2001;91:1708–12. [DOI] [PubMed] [Google Scholar]
- 17.Hakansson A, Mickelsson K, Wallin C et al. Anabolic androgenic steroids in the general population: user characteristics and associations with substance use. Eur Addict Res 2012;18:83–90. 10.1159/000333037 [DOI] [PubMed] [Google Scholar]
- 18.Stubbe JH, Chorus AM, Frank LE et al. Prevalence of use of performance enhancing drugs by fitness centre members. Drug Test Anal 2014;6:434–8. 10.1002/dta.1525 [DOI] [PubMed] [Google Scholar]
- 19.Pope HG Jr, Wood RI, Rogol A et al. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr Rev 2014;35:341–75. 10.1210/er.2013-1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herr A, Rehmert G, Kunde K et al. A thirty-year old bodybuilder with septic shock and ARDS from abuse of anabolic steroids. Anaesthesist 2002;51:557–63. 10.1007/s00101-002-0335-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenu EW, McNaughton L, Marshall-Gradisnik SM. Is there a potential immune dysfunction with anabolic androgenic steroid use? A review. Mini Rev Med Chem 2011;11:438–45. 10.2174/138955711795445907 [DOI] [PubMed] [Google Scholar]
- 22.Calabrese LH, Kleiner SM, Barna BP et al. The effects of anabolic steroids and strength training on the human immune response. Med Sci Sports Exerc 1989;21:386–92. 10.1249/00005768-198908000-00008 [DOI] [PubMed] [Google Scholar]
- 23.Hughes TK, Fulep E, Juelich T et al. Modulation of immune responses by anabolic androgenic steroids. Int J Immunopharmacol 1995;17:857–63. 10.1016/0192-0561(95)00078-X [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Kim SR, Oh Y et al. Targeting insulin-like growth factor-I and insulin-like growth factor-binding protein-3 signaling pathways. A novel therapeutic approach for asthma. Am J Respir Cell Mol Biol 2014;50:667–77. 10.1165/rcmb.2013-0397TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krein PM, Sabatini PJ, Tinmouth W et al. Localization of insulin-like growth factor-I in lung tissues of patients with fibroproliferative acute respiratory distress syndrome. Am J Respir Crit Care Med 2003;167:83–90. 10.1164/rccm.2201012 [DOI] [PubMed] [Google Scholar]
- 26.Dekkers BG, Naeimi S, Bos IS et al. L-thyroxine promotes a proliferative airway smooth muscle phenotype in the presence of TGF-beta1. Am J Physiol Lung Cell Mol Physiol 2015;308:L301–6. 10.1152/ajplung.00071.2014 [DOI] [PubMed] [Google Scholar]