Abstract
A Chinese man who had undergone a curative high anterior resection for sigmoid cancer was administrated XELOX (capecitabine and oxaliplatin) as postoperative adjuvant chemotherapy. He subsequently developed sinusoidal obstruction syndrome (SOS) that resolved on discontinuation of XELOX treatment. Genetic evaluation determined that he had the GSTT1-null and GSTM1-null genotype, known to be an independent risk factor for developing oxaliplatin-induced SOS.
Background
This is the first reported case of oxaliplatin-induced sinusoidal obstruction syndrome (SOS) among the Chinese. Subsequent genetic testing revealed that this patient had the GSTT1-null and GSTM1-null genotype, known to be an independent risk factor for developing oxaliplatin-induced SOS.1
There is no known difference in the frequency of GSTM1-null genotype between patients of Chinese and non-Chinese ethnicities.1–9 Interestingly, however, is the reported prevalence of GSTT1-null genotype being slightly higher in people of Chinese (45–50%) compared to those of non-Chinese (6–26%) origin. It is unclear if this translates to differences in risk of SOS in different ethnic groups. Our report highlights the association of a genetic predisposition for SOS and will hopefully increase clinicians’ vigilance for this possibility in oxaliplatin-treated patients. Our finding, if validated, could potentially enable us to formulate a plausible genetic risk profile, together with other risk factors, to predict a patient's risk of developing SOS, before starting oxaliplatin-based chemotherapy. The choice of chemotherapy could thereafter be adjusted based on the patient's unique risk profile, or closer monitoring (if necessary, discontinuation) could be given, should oxaliplatin still be the choice of drug administered.
Case presentation
A 56-year-old man of ethnic Chinese descent initially presented to his primary care physician, in June 2014, with per-rectal bleeding for 2 months. He had no significant medical history, no family history of colorectal cancer, no known drug allergies, denied any previous intake of alcohol, smoking and herbal agents, and was unemployed. Colonoscopy with biopsy showed moderately differentiated adenocarcinoma of the rectosigmoid. CT did not show metastases. Blood test of liver function was normal. The patient underwent a high anterior resection of the sigmoid tumour. Histology confirmed adenocarcinoma with involvement of 1 of 16 regional lymph nodes. After surgery, the patient was given adjuvant chemotherapy using a combination of the XELOX regimen, comprising oral capecitabine 1000 mg/m2 two times per day on days 1–14, plus intravenous oxaliplatin 130 mg/m2 on day 1. The first cycle was uneventful but, 20 days after the second cycle of oxaliplatin, he presented to the clinic with a 1-day duration of acute abdominal distension. Clinically, he had facial swelling, generalised ascites and bilateral lower limb oedema. He was not taking any medications, other than dexamethasone as an antiemetic on the first 2 days of chemotherapy.
Investigations
Liver function test showed mild elevation of liver enzymes and hypoalbuminaemia (table 1). Urea and electrolytes were normal.
Table 1.
Patient's liver function test results
| Time of measurement | Aspartate aminotransferase (IU/L) | Alanine aminotransferase (IU/L) | Alkaline phosphatase (IU/L) | γ-Glutamyl transferase (IU/L) | Total bilirubin (μmol/L) | Total protein (g/L) | Albumin (g/L) |
|---|---|---|---|---|---|---|---|
| Preoperation | 25 | 33 | 101↑ | 53 | 6 ↓ | 76 | 45 |
| Postoperation; before #1 XELOX | 20 | 20 | 98 | NM | 5 ↓ | 74 | 43 |
| After #1 XELOX before #2 XELOX | 43↑ | 33 | 91 | NM | 10 | 74 | 45 |
| After #2 XELOX (at time of admission for ascites) | 33 | 55 | 99 | NM | 17 | 52↓ | 28↓ |
| During admission | |||||||
| Day 2 | 51↑ | 47 | 108↑ | 64 | 14 | 55↓ | 29↓ |
| Day 3 | 47↑ | 41 | 118↑ | 66 | 11 | 57↓ | 30↓ |
| Day 4 | 53↑ | 44 | 125↑ | NM | 11 | 61↓ | 31↓ |
| Day 8 | 65↑ | 45 | 121↑ | NM | 13 | 67↓ | 35↓ |
| Day 9 | 48↑ | 36 | 109↑ | 74 | 11 | 62↓ | 33↓ |
| At follow-up (6 days postdischarge) | 36 | 28 | 130↑ | NM | 10 | 69 | 37↓ |
NM, not measured.
Haemoglobin count was 13.2 g/dL and serial white cell counts were not raised (on admission: 5.2×109/L; before discharge: 4.24×109/L).
Serum carcinoembryonic antigen was 3.2 µg/L, from the previous 2.1 µg/L at postoperation follow-up.
As nephrotic syndrome was considered, 24 h urine protein (0.18 g/day) and urine protein:creatinine ratio (0.13) were normal. Total cholesterol was 3.33 mmol/L.
Serological tests for hepatitis B and C viruses were negative.
CT of the chest/abdomen/pelvis showed moderate ascites, heterogeneous liver parenchyma and periportal oedema (possibly representing hepatic venous congestion) with no liver metastases. There were no features of superior or inferior vena cava obstruction. Ultrasound of the hepatic vein revealed no evidence of veno-occlusive disease.
Differential diagnosis
Our working diagnosis was oxaliplatin-induced SOS; differential diagnoses were liver metastases, nephrotic syndrome, viral hepatitis and sepsis.
Clinically, the onset of acute SOS is within 1–3 weeks of exposure to the offending medication. Patients present with signs of portal hypertension (ascites, oedema and varices), weight gain and abdominal pain. Jaundice is generally mild or absent, but may develop if the injury is severe. Blood tests typically show hepatic transaminase elevations following a hepatocellular pattern and mild or minimal alkaline phosphatase (ALP) increase.
Our patient developed facial swelling and ascites with bilateral lower limb oedema 20 days after the second course of XELOX was administered. His aspartate aminotransferase (AST), alanine aminotransferase (ALT) and ALP were only mildly raised, and total bilirubin was normal.
Liver ultrasound can suggest SOS by demonstrating hepatomegaly, ascites and liver texture changes suggestive of congestion, and can help exclude acute Budd-Chiari syndrome and portal vein thrombosis by demonstrating patency of hepatic and portal veins. Our patient's ultrasound hepatic vein was not suggestive of veno-occlusive disease, but CT scan showed heterogeneous liver parenchyma and periportal oedema, possibly representing hepatic venous congestion.
Malignant infiltration of the liver was excluded by the absence of liver metastases on CT scan. Nephrotic syndrome was excluded by normal 24 h urine protein, urine protein:creatinine ratio and total cholesterol. Viral hepatitis was unlikely as serological tests for hepatitis B and C viruses were negative. Normal white cell counts and absence of fever ruled out sepsis.
Our patient was treated symptomatically with oral frusemide. Subsequently, he was not given further oxaliplatin, and had no further episodes of ascites. The acute onset of his symptoms and their resolution on discontinuation of oxaliplatin raised the clinical suspicion of oxaliplatin-induced SOS.
Treatment
The patient's ascites and oedema subsided after a short course of oral frusemide.
At follow-up 1 week later, he was well, with no liver tenderness, and no longer had ascites and limb swelling. Blood test showed that AST and ALT had normalised.
He was advised against further exposure to oxaliplatin, and did not have recurrence of symptoms.
Outcome and follow-up
The patient was offered genetic testing to identify genotypic predisposition to oxaliplatin-induced SOS.
Peripheral whole blood was obtained from the patient and healthy volunteers, with informed written consent. This study was approved by the SingHealth Institutional Review Board (CIRB Ref: 2011/826/B).
Genomic DNA was extracted from peripheral whole blood by standard column purification using Blood and Cell Culture kit (Qiagen, Hilden, Germany) according to manufacturer's protocol. Purified DNA was quantified on Nanodrop2000 (Thermo Scientific, USA).
Seminested PCR for GSTM1 and GSTT1 genes was performed on purified genomic DNA from the patient, with β-globin as an internal control, as previously described. Briefly, a 219–290 bp product was first amplified from 40–50 ng genomic DNA using Platinum Taq polymerase (Life Technologies, Carlsbad, California, USA) on a Geneamp 9700 Thermocycler (Applied Biosystems). The first PCR product was then diluted 100 times and subsequently used as a template for the second PCR. The same cycling conditions were used for both PCR cycles: initial activation at 95°C for 10 min; followed by 35 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 1 min; and final extension at 72°C for 10 min. Primers used were as listed by Vreuls et al.1 Gel electrophoresis on 1% agarose with SYBR Safe (Life Technologies), with 2 µl of total reaction volume was used to visualise the PCR products. For positive control, PCR on genomic DNA from a healthy volunteer with no colorectal disease was concurrently performed.
The seminested technique was adopted for its more accurate ability to amplify long DNA, as the fragment to be amplified had 219–290 bp. The primer sequences of both, first and second PCR, are listed in table 2.1 Subsequently, electrophoretic analysis was performed, visualising the amplified product by SYBR Safe in a 1% agarose gel. As one of the primers is located in the deleted sequence, this technique enabled us to identify the homozygous null genotype.
Table 2.
Primer sequences of GSTM1 and GSTT1 genotype1
| Primer designation | Sequences | Product length (bp) |
|---|---|---|
| GSTM1 | ||
| First amplification | ||
| Forward | 5′-GAACTCCCTGAAAAGCTAAAGC-3′ | 219 |
| Reverse | 5′-GTTGGGCTCAAATATACGGTGG-3′ | |
| Second amplification | ||
| Forward | 5′-CAGAGTTTCTGGGGAAGCGG-3′ | 191 |
| Reverse | Idem first amplification | |
| GSTT1 | ||
| First amplification | ||
| Forward | 5′-TTCCTTACTGGTCCTCACATCTC-3′ | 290 |
| Reverse | 5′-AAGACTTGGCAGCCAGCACC-3′ | |
| Second amplification | ||
| Forward | Idem first amplification | 249 |
| Reverse | 5′-TACAGACTGGGGATGGATGG-3′ | |
| β-globin | ||
| First amplification | ||
| Forward | 5′-GAAGAGCCAAGGACAGGTAC-3′ | 268 |
| Reverse | 5′-CAACTTCATCCACGTTCACC-3′ | |
| Second amplification | ||
| Forward | 5′-GGCTGGGCATAAAAGTCAGG-3′ | 162 |
| Reverse | Idem first amplification | |
GSTM, glutathione S-transferase enzyme μ; GSTT, glutathione S-transferase enzyme θ.
The primers designed by Vreuls et al,1 or GSTM1 and GSTT1, respectively, were such that one primer of the pair was located in the deleted sequence of the null genotypes. There was no product from the seminested PCR of GSTM1 and GSTT1 genes in the patient (figure 1), indicating that he had a homozygous null genotype for these two alleles.
Figure 1.
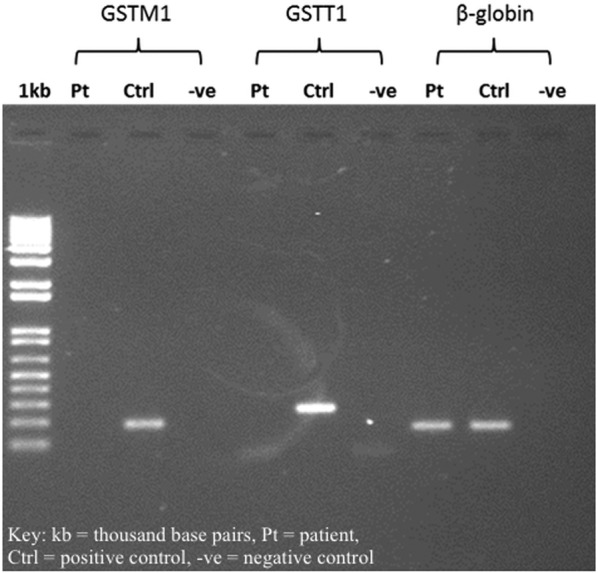
Gel electrophoresis results.
Discussion
Oxaliplatin-based chemotherapeutic regimens such as FOLFOX (5-fluorouracil, leucovorin and oxaliplatin) and XELOX (capecitabine and oxaliplatin) are commonly used in patients with colorectal cancer as adjuvant systemic chemotherapy postresection. While these drugs have demonstrated efficacy, they have also been associated with various degrees of hepatotoxicity.
SOS is often associated with oxaliplatin. It has also been described in association with drugs such as azathioprine, 6-mercaptopurine, 6-thioguanine, busulfan, cyclophosphamide, doxorubicin, etoposide, etc.10
Rubbia-Brandt et al11 found that 34 of 43 (78%) patients treated with oxaliplatin developed hepatic sinusoidal dilation. SOS is characterised by sinusoidal dilation and congestion, centrilobular vein fibrosis and obstruction, perisinusoidal fibrosis, necrosis of pericentral hepatocytes, parenchymal extinction lesions, hepatocyte plate disruption and nodular regenerative hyperplasia.10 12–16
Oxaliplatin and other platinum compounds lead to the generation of reactive oxygen species that can deplete glutathione from sinusoidal endothelial cells (SECs).17 18 The toxic injury to these SECs causes damage to the sinusoidal wall, hence marked sinusoidal dilation and extravasation of erythrocytes into the Disse's spaces through the endothelial lining's discontinuities. Moreover, collagen matrix deposits in perisinusoidal spaces and terminal hepatic venules because of the activation of hepatic stellate cells.11 13 As a result, there is circulatory compromise of centrilobular hepatocytes, fibrosis and obstruction of liver blood flow.
A number of other surgical series supported the findings of Rubbia-Brandt et al. Mehta et al19 demonstrated that 61% of 70 patients receiving regimens containing oxaliplatin developed sinusoidal dilation. Three other studies found that 9.7%–23% of patients treated with oxaliplatin developed grade 2–3 sinusoidal dilation.12 20 21 Kweekel et al22 summarised a case series reporting an association between oxaliplatin and sinusoidal dilation (table 3).
Table 3.
A summary of case series reporting the association between oxaliplatin and sinusoidal dilation
| Study | Number of patients | Sinusoidal dilation | Greater morbidity | Greater mortality |
|---|---|---|---|---|
| Vauthey et al20 | 79 | 15 of 79 patients (19%) | No | No |
| Mehta et al19 | 70 | 43 of 70 patients (61%) | No | No |
| Aloia et al | 52 | 10 of 52 patients (19%) | Yes* | No |
| Kandutsch et al12 | 47 | 11 of 47 patients (23%) | No | No |
| Rubbia-Brandt et al25 | 43 | 34 of 43 patients (78%) | Not available | Not available |
| Pawlik et al21 | 31 | 3 of 31 patients (10%) | No | No |
| Nakano et al16 | 90 | 38 of 90 patients (42%) | Yes | No |
*Aloia et al found a non-statistically significant trend towards increased morbidity.
A diagnosis of SOS can often be made based on clinical symptoms and signs, and routine laboratory tests. Liver histology is diagnostic but not always practical, due to chemotherapy-induced thrombocytopenia and neutropaenia. Transjugular liver biopsy and concurrent measurement of the hepatic venous pressure gradient can also be used for accurate diagnosis and grading for severity, based on degree of portal hypertension.
Monitoring for symptoms and signs of SOS for early recognition is perhaps most crucial in patients who receive oxaliplatin-based chemotherapy. Management of SOS is aimed at avoiding further injury by stopping oxaliplatin, providing support for complications such as pain and hypotension, and maintaining intravascular volume and renal perfusion while limiting third-space fluid accumulation.23 24 Correcting electrolyte and acid–base imbalance and pulmonary failure, and managing infectious complications, are also essential.
Prevention of SOS is best achieved by keeping SOS in consideration, and thereafter withholding oxaliplatin or at least closely monitoring for signs and symptoms, if using oxaliplatin.
Genetic expression profiling performed by Brandt et al in 2011 revealed that, compared with controls, in oxaliplatin-induced SOS livers, gene expression analysis showed that upregulated genes included those involved in acute phase response (notably interleukin 6), coagulation system (Serpine1, THBD and VWF), hepatic fibrosis/hepatic stellate cell activation (COL3a1, COL3a2, PDGF-A, TIMP1, and MMP2), oxidative stress (JUN, SOD2), angiogenesis (VEGF-C) and hypoxic factors (HIF1A). The most significant increase was observed in CCL20 mRNA.25
At present, the only known and validated germline predisposition gene associated with SOS is with GSTM1.1 Inactivation of the platinum compound by glutathione and other antioxidants helps prevent against toxicity.22 26 27 Glutathione and platinum form an adduct that leads to detoxification of oxaliplatin. This adduct formation is catalysed by glutathione S-transferase enzyme (GST).22 Several subclasses of GST are known; θ (GSTT), μ (GSTM), α (GSTA), π (GSTP) and ω (GSTO), each with their own polymorphism.28 GSTT and GSTM have null genotypes—GSTT1-null and GSTM1-null. The null genotypes result in no enzymatic GST function hence decreased adduct formation between glutathione and platinum, hence decreased defence mechanism against oxaliplatin.22 27 A patient may therefore be susceptible to SOS if there are deficiencies in the detoxification process.11 29
Consideration for genetic testing was made for our patient as SOS seemed the most likely diagnosis to account for the acute onset of his ascites and lower limb swelling after the second cycle of XELOX, and the fact that his symptoms did not reappear after XELOX was stopped. He was found to have the GSTT1-null and GSTM1-null genotype. This finding could potentially allow us, in association with other risk factors, to conceive a plausible genetic risk profile predicting whether a patient is at risk of developing SOS, before starting oxaliplatin, and subsequently might result in discontinuation of oxaliplatin on an individual patient basis. Further prospective research will be helpful to evaluate this.
Learning points.
In patients who need oxaliplatin-based chemotherapy, monitoring for signs and symptoms of sinusoidal obstruction syndrome (SOS) after the administration of oxaliplatin is crucial for prompt management should the need arise: discontinuing oxaliplatin and supportive management.
Prevention of SOS is best achieved by keeping SOS in consideration.
Our patient with oxaliplatin-induced SOS was found to have the GSTT1-null and GSTM1-null genotype, the latter of which has been found to be an independent risk factor for oxaliplatin-induced SOS.1
Genetic testing can be useful in predicting a patient's risk of developing oxaliplatin-induced SOS before starting oxaliplatin, and hence can enable suitable adjustments to the cumulative amount of oxaliplatin treatment given. Further research is necessary to develop this possibility.
Acknowledgments
The authors would like to thank the patient and his family for their participation in our study.
Footnotes
Contributors: SXK and SHC performed the experiments and collated data. JN provided scientific oversight and designed the study.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vreuls CPH, Olde Damink SWM, Koek GH et al. Glutathione S-transferase M1-null genotype as risk factor for SOS in oxaliplatin-treated patients with metastatic colorectal cancer. Br J Cancer 2013;108:676–80. 10.1038/bjc.2012.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin HJ, Han CY, Bernstein DA et al. Ethnic distribution of the glutathione transferase Mu 1–1 (GSTM1) null individuals and application to bladder cancer susceptibility. Carcinogenesis 1994;15:1077–81. 10.1093/carcin/15.5.1077 [DOI] [PubMed] [Google Scholar]
- 3.London SJ, Daly AK, Cooper J et al. Polymorphism of glutathione S-transferase M1 and lung cancer risk among African-Americans and Caucasians in Los Angeles County, California. J Natl Cancer Inst 1995;87:1246–53. 10.1093/jnci/87.16.1246 [DOI] [PubMed] [Google Scholar]
- 4.Setiawan VW, Zhang ZF, Yu GP et al. GSTT1 and GSTM1 null genotypes and the risk of gastric cancer: a case-control study in a Chinese population. Cancer Epidemiol Biomarkers Prev 2000;9:73–80. [PubMed] [Google Scholar]
- 5.Losi-Guembarovski R, Luciana Paula Grégio D, Mara de Syllos CI et al. Glutathione S-transferase Mu (GSTM1) null genotype in relation to gender, age and smoking status in a healthy Brazilian population. Genet Mol Biol 2002;25;357–60. 10.1590/S1415-47572002000400001 [DOI] [Google Scholar]
- 6.Balmukhanov TS, Khanseitova AK, Nigmatova VG et al. Polymorphisms at GSTM 1, GSTP 1, GSTT 1 detoxification genes loci and risk of breast cancer in Kazakhstan population. Adv Breast Cancer Res 2013;2:114–8. [Google Scholar]
- 7.Tsabouri SE, Georgiou I, Alamanos I et al. Increased prevalence of GSTM(1) null genotype in patients with myelodysplastic syndrome: a case-control study. Acta Haematol 2000;104;169–73. doi:46510 [DOI] [PubMed] [Google Scholar]
- 8.Doney AS, Lee S, Leese GP et al. Increased cardiovascular morbidity and mortality in type 2 diabetes is associated with the glutathione S transferase theta-null genotype: a Go-DARTS study. Circulation 2005;111:2927–34. 10.1161/CIRCULATIONAHA.104.509224 [DOI] [PubMed] [Google Scholar]
- 9.Zhu H, Bao J, Liu S et al. Null genotypes of GSTM1 and GSTT1 and endometriosis risk: a meta-analysis of 25 case-control studies. PLoS ONE 2014;9:e106761 10.1371/journal.pone.0106761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanless IR. Vascular disorders of the liver. In: Saxena R, ed. Practical hepatic pathology—a diagnostic approach. Philadelphia: Saunders, 2011:443–54. [Google Scholar]
- 11.Rubbia-Brandt L, Audard V, Sartoretti P et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 2004;15:460–6. 10.1093/annonc/mdh095 [DOI] [PubMed] [Google Scholar]
- 12.Kandutsch S, Klinger M, Hacker S et al. Patterns of hepatotoxicity after chemotherapy for colorectal cancer liver metastases. Eur J Surg Oncol 2008;34:1231–6. 10.1016/j.ejso.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Nam SJ, Cho JY, Lee HS et al. Chemotherapy-associated hepatopathy in korean colorectal cancer liver metastasis patients: oxaliplatin-based chemotherapy and sinusoidal injury. Korean J Pathol 2012;46:22–9. 10.4132/KoreanJPathol.2012.46.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson SM, Wilson CH, Burt AD et al. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol 2012;19:4287–99. 10.1245/s10434-012-2438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan P, Nanji S, Pollett A et al. Chemotherapy-induced liver injury in metastatic colorectal cancer: semiquantitative histologic analysis of 334 resected liver specimens shows that vascular injury but not steatohepatitis is associated with preoperative chemotherapy. Am J Surg Pathol 2010;34:784–91. 10.1097/PAS.0b013e3181dc242c [DOI] [PubMed] [Google Scholar]
- 16.Nakano H, Oussoultzoglou E, Rosso E et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 2008;247:118–24. 10.1097/SLA.0b013e31815774de [DOI] [PubMed] [Google Scholar]
- 17.Laurent A, Nicco C, Chéreau C et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res 2005;65:948–56. [PubMed] [Google Scholar]
- 18.Alexandre J, Nicco C, Chéreau C et al. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J Natl Cancer Inst 2006;98:236–44. 10.1093/jnci/djj049 [DOI] [PubMed] [Google Scholar]
- 19.Mehta NN, Ravikumar R, Coldham CA et al. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol 2008;34:782–6. 10.1016/j.ejso.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 20.Vauthey JN, Pawlik TM, Ribero D et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006;24:2065–72. 10.1200/JCO.2005.05.3074 [DOI] [PubMed] [Google Scholar]
- 21.Pawlik TM, Olino K, Gleisner AL et al. Preoperative chemotherapy for colorectal liver metastases: Impact on hepatic histology and postoperative outcome. J Gastrointest Surg 2007;11:860–8. 10.1007/s11605-007-0149-4 [DOI] [PubMed] [Google Scholar]
- 22.Kweekel DM, Gelderblom H, Guchelaar HJ. Pharmacology of oxaliplatin and the use of pharmacogenomics to individualize therapy. Cancer Treat Rev 2005;31:90–105. 10.1016/j.ctrv.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 23.Livertox. (n.d.). Retrieved 20 April 2015. http://livertox.nih.gov/Phenotypes_sinus.html
- 24.Veno-occlusive disorders. In: Levy A, Mortele K, Yeh B, eds. Gastrointestinal imaging. 1st edn Oxford University Press, 2015:354. [Google Scholar]
- 25.Rubbia-Brandt L, Tauzin S, Brezault C et al. Gene expression profiling provides insights into pathways of oxaliplatin-related sinusoidal obstruction syndrome in humans. Mol Cancer Ther 2011;10:687–96. 10.1158/1535-7163.MCT-10-1072 [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Kanel GC, DeLeve LD. Support of sinusoidal endothelial cell glutathione prevents hepatic veno-occlusive disease in the rat. Hepatology 2000;31:428–34. 10.1002/hep.510310224 [DOI] [PubMed] [Google Scholar]
- 27.Furuta T. Pharmacogenomics in chemotherapy for GI tract cancer. J Gastroenterol 2009;44:1016–25. 10.1007/s00535-009-0124-9 [DOI] [PubMed] [Google Scholar]
- 28.Di Pietro G, Magno LA, Rios-Santos F. Flutathione S-transferases: an overview in cancer research. Expert Opin Drug Metab Toxicol 2010;6:153–70. 10.1517/17425250903427980 [DOI] [PubMed] [Google Scholar]
- 29.Ward J, Guthrie JA, Sheridan MB et al. Sinusoidal obstructive syndrome diagnosed with superparamagnetic iron oxide-enhanced magnetic resonance imaging in patients with chemotherapy-treated colorectal liver metastases. J Clin Oncol 2008;26:4304–10. 10.1200/JCO.2008.16.1893 [DOI] [PubMed] [Google Scholar]


