Abstract
We present a case of a 60-year-old Caucasian man recently returned from Angola, where he had been successfully treated for a severe (non-cerebral) falciparum malaria infection. He was presented to the emergency room, with a subacute onset encephalopathy, ataxia and a generalised tonic–clonic seizure. Cerebrospinal fluid (CSF) analysis revealed lymphocytic pleocytosis (123 cells/µL) and hyperproteinorrhachia (188 mg/dL). Brain MRI and EEG were unremarkable. CSF PCR testing for neurotropic viruses was negative as were CSF and blood cultures. The patient was treated with ceftriaxone and acyclovir, with full recovery on the second day of treatment. We believe post-malaria neurological syndrome, a rare self-limited encephalopathy, should be considered in the differential diagnosis. Nevertheless, the presentation, lack of changes on brain MRI and EEG, along with possible false-negative CSF viral PCR, could still represent a viral encephalitis, which brings to question the treatment approach to adopt (conservative vs wide spectrum antiviral plus antibiotics).
Background
Malaria is a highly prevalent parasitic disease, clustering around tropical and subtropical areas, such as Africa, the Amazon region and Southeast Asia.1 In Portugal, ‘imported malaria’ from travellers returning from endemic countries is still a relevant part of our clinical daily life. According to recently published data, there has actually been an increase in annual hospitalisations due to malaria infections in Portugal.2 The reasons for this are largely unknown but may be related to the economic shifts increasing travel to endemic regions (especially African countries, such as Angola and Mozambique, which have a historic relationship with Portugal).2 This increase in incidence is important not only regarding the different systemic complications, but also because clinicians in general should be aware of more rare complications of such an infection. Specifically, in the past 20 years, there have been growing reports of a different clinical syndrome that presents after the clearance of parasites from blood. According to Nguyen et al,3 up to 2 months after a successful antibiotic course with clearance of the parasites from the bloodstream, a few patients (22 of 18124 in Nguyen et al’s original case series) undergo what is referred to as a post-malaria neurological syndrome (PMNS). It is usually a self-limited diffuse encephalopathy with a myriad of symptoms (seizures, ataxia, tremor, encephalopathy, psychosis and/or visual hallucinations), usually following a severe malaria infection.3 4 Viral encephalitis could have a presentation similar to that described for PMNS, but it would have a completely different treatment approach and prognosis. As there are no reliable markers of PMNS, clinical suspicion remains the most important factor in identifying this disorder. The similarity of the presentation to other potentially devastating disorders could lead to difficulties in the establishment of proper treatment.
Case presentation
A 60-year-old Caucasian man with no previous relevant medical history presented with a 3-day period of progressive behavioural and gait disturbance. His wife reported that, during this period, he was increasingly agitated and irritable, barely sleeping, and displaying increasingly inappropriate behaviour (repeatedly getting up during the night to get dressed and undressed, with no clear purpose). He was also getting progressively more disoriented, with confused speech, and exhibited clumsy movements and poor balance. He was seen at the emergency room, where he had a generalised tonic–clonic seizure prior to observation. He had returned from Angola 2 weeks earlier, where he had been treated with quinine for a severe (non-cerebral) malaria falciparum infection, and discharged with full recovery and clearance of parasitaemia from the blood. Two blood smears were performed during the 2 weeks before the acute presentation, which were negative for malaria. On admission, 19 days after clearance of parasitaemia, his general and neurological examination (following post-ictal phase), revealed psychomotor agitation, aggressiveness (requiring multiple sedatives), temporospatial disorientation, effortful speech with word-finding difficulty and bilateral upper limb ataxia. There was a low fever (37.6°C tympanic temperature) and no other symptoms.
Investigations
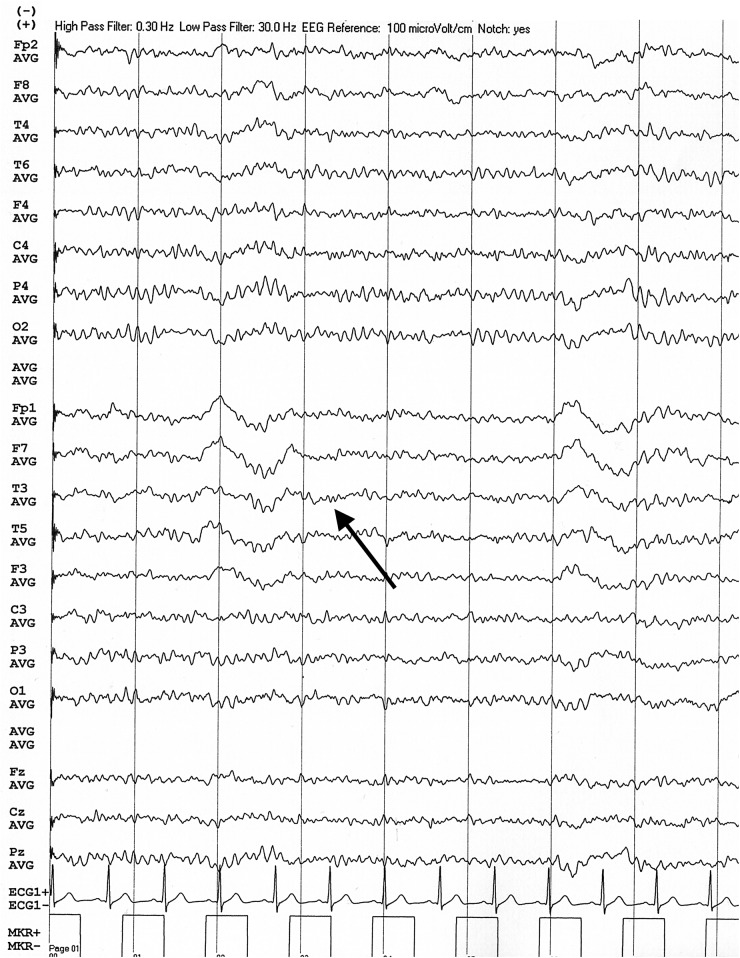
On admission, a third blood smear was negative for parasites. Laboratory results revealed a high white cell count with slight lymphocytosis (16.9×109/L leucocytes with 44% lymphocytes); C reactive protein was 0.29 mg/dL (normal: <0.5 mg/dL). Head CT revealed no changes. Brain MRI performed at day 4 (figure 1) was also normal and an EEG performed at day 4 (figure 2) showed occasional left temporal slow waves. Lumbar puncture performed at day 4 revealed lymphocytic pleocytosis (123 cells/µL) and hyperproteinorrhachia (188 mg/dL). Subsequent results for cerebrospinal fluid (CSF) PCR for common neurotropic viruses (herpes simplex virus 1 and 2; cytomegalovirus; human herpes virus 6 and 7; varicella zoster virus) were negative, as were CSF and blood cultures.
Figure 1.
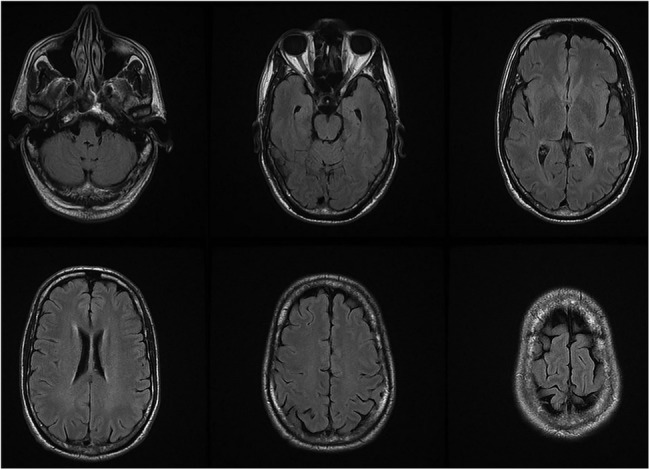
Brain MRI, axial T2 fluid-attenuated inversion recovery with no evident changes.
Figure 2.
EEG showing left temporal slow waves (black arrow).
Differential diagnosis
Initially, we included infectious (meningo) encephalitis and autoimmune encephalitis in the differential diagnosis, however, none of the ancillary tests (especially CSF PCR and cultures) were suggestive of those diagnoses, which led us to consider post-malaria neurological syndrome as the most probable diagnosis.
Treatment
As, at onset, a working diagnosis of infectious encephalitis was not excluded, the patient was empirically started on acyclovir and ceftriaxone, which were continued for 21 days.
Outcome and follow-up
The patient's symptoms remitted abruptly 2 days after admission and he remained asymptomatic until discharge 25 days after admission. The patient remains completely asymptomatic 2 years later and there have been no relapses or symptoms suggestive of central nervous system (CNS) dysfunction.
Discussion
PMNS, similar in presentation to diffuse encephalitis, is a self-limited syndrome, the mechanisms of which remain to be clarified. Some authors suggest it could be an immune-mediated disease somehow related with acute disseminated encephalomyelitis, given that they share many characteristics: a postinfectious nature, with multiple neurological signs and symptoms (and multiple white matter changes in MRI in some cases), monophasic course and good overall response after steroid therapy.4
In fact, according to a recent literature review,4 its main features are presentation after recovery from a severe (and not-necessarily cerebral) falciparum malaria, onset after clearance of parasitaemia from the blood (usually up to 60 days later), a self-limited course (2–14 days); presentation as a diffuse postinfectious encephalopathy (sometimes with fever), characterised by several neurological changes such as aphasia, seizures, confusion, ataxia and psychosis; inflammatory CSF values (pleocytic lymphocytosis 10–76 cells/μL; hyperproteinorrhachia 31–252 mg/dL); and an EEG consistent with mild to severe encephalopathy but without epileptiform activity. Brain MRI can be unremarkable or it can reveal signal changes in several different areas of the brain. There is no specific treatment, but steroids can be used in the most severe cases as they may have a role in the recovery process.4
All reported cases of PMNS have been associated with a previous infection of Plasmodium falciparum. Even if this is the most common cause of malaria in sub-Saharan Africa, in Asia, falciparum and vivax malaria have similar prevalence.5 This suggests that a specific, unclarified relation of PMNS with falciparum malaria exists. Factors associated with the development of PMNS after malaria were studied by Nguyen et al.3 From 18 124 patients with falciparum malaria (1176 of whom had a severe infection), 22 patients developed PMNS. From the 22 patients, 21 had a history of severe infection. PMNS developed in 16 of the 412 known mefloquine recipients compared with 4 of 764 non-recipients (data are not clear for one of the patients). Besides being more common in severe infection, a synergy between disease severity and drug regimen has been proposed. Interestingly, 10 of the 22 patients were at some time treated with quinine, as was the patient we report. Additionally, Markley et al4 also reported patients who developed PMNS after quinine treatment. We report the case of a patient who had fully recovered from a severe falciparum malaria infection and soon developed a diffuse encephalopathy with no particular defining characteristics, but with CSF results suggestive of CNS inflammation. We considered the possibility of a PMNS, especially taking into account the patient’s recent history and sharing many of the characteristics previously defined in PMNS, such as inflammatory CSF, onset after recent severe falciparum infection and the absence of structural abnormalities despite neurological symptoms, but no specific features support this diagnosis. We also considered the possibility of co-infection or later infection with a neurotropic virus known to cause encephalitis, similar to the question raised by Nguyen et al,3 however, both CSF (and blood) cultures and CSF PCR for herpes virus (and other neurotropic viruses) were negative, so there were also no features supporting a viral (or otherwise infectious) encephalitis diagnosis either. We know that too early CSF PCR testing for neurotropic viruses can yield negative results,6 and there have been reports of (repeatedly) false-negative CSF PCR testing in some cases of herpes simplex encephalitis,7 which is why, given the lack of specific markers for PMNS and the diagnostic uncertainty, we decided on full 21-day antiviral and antibiotic treatment despite a full recovery on the second day after admission.
Although the pathophysiology of PMNS is still unclear and there is a considerable amount of uncertainty surrounding it, we believe it is the most reasonable explanation for the patient we report, especially considering the time frame and relationship with a recent malaria infection.
Learning points.
Post-malaria neurological syndrome is a rare encephalopathy following clearance of malaria parasites from blood, with an unknown pathophysiology.
There is no direct treatment approach and it is usually self-limited (steroids may have a role in the recovery process).
Cerebrospinal fluid studies can show lymphocytic pleocytosis and hyperproteinorrhachia, similar to viral encephalitis.
Infectious (mainly viral) encephalitis should be considered in the differential diagnosis.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.[No authors listed] Severe malaria. Trop Med Int Health 2014;19(Suppl 1):7–131. 10.1111/tmi.12313_2 [DOI] [PubMed] [Google Scholar]
- 2.Fonseca AG, Dias SS, Baptista JL et al. The burden of imported malaria in Portugal 2003 to 2012. J Travel Med 2014;21:354–6. 10.1111/jtm.12141 [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TH, Day NP, Ly VC et al. Post-malaria neurological syndrome. Lancet 1996;348:917–21. 10.1016/S0140-6736(96)01409-2 [DOI] [PubMed] [Google Scholar]
- 4.Markley JD, Edmond MB. Post-malaria neurological syndrome: a case report and review of the literature. J Travel Med 2009;16:424–30. 10.1111/j.1708-8305.2009.00349.x [DOI] [PubMed] [Google Scholar]
- 5.White NJ, Pukrittayakamee S, Hien TT et al. Malaria. Lancet 2014;383:723–35. 10.1016/S0140-6736(13)60024-0 [DOI] [PubMed] [Google Scholar]
- 6.Davies N, Brown LJ, Gonde J et al. Factors influencing PCR detection of viruses in cerebrospinal fluid of patients with suspected CNS infections. J Neurol Neurosurg Psychiatry 2005;76:82–7. 10.1136/jnnp.2004.045336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler A, Kadimi S, Apaloo C et al. Herpes simplex encephalitis with two false-negative cerebrospinal fluid PCR tests and review of negative PCR results in the clinical setting. Case Rep Neurol 2011;3:172–8. 10.1159/000330298 [DOI] [PMC free article] [PubMed] [Google Scholar]