The authors wish to indicate two corrections in the article referenced above:
-
•
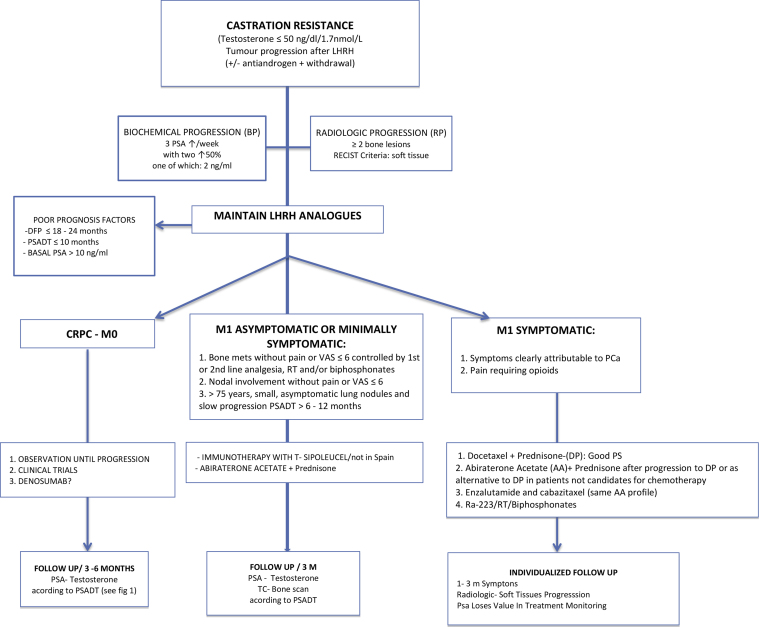
Fig. 3. Where it says “M1 symptomatic or minimally symptomatic”, should say “M1 asymptomatic or minimally symptomatic”.
-
•
Table 3. Where it says “COU-AA-301: CRPC with metastasis, asymptomatic or minimally symptomatic prior to chemotherapy”, should say “COU-AA-302: CRPC with metastasis, asymptomatic or minimally symptomatic prior to chemotherapy”.
Fig. 3.
Imaging, treatment and follow-up algorithm for patients with castration-resistance prostate cancer.
Table 3.
Results of phase III trials in patients with mCRPC and new drug combinations.
| Study | Control arm | Active drug arm | Months difference in OS | |
|---|---|---|---|---|
| Ra 223 | ALSYMPCA: CRPC with bone metastases and symptoms | SUPPORT TREATMENT + PLACEBO 307 patients |
Ra 223 + SUPPORT TREATMENT 614 patients |
11.2 vs. 14 3.8 months |
| MDV 3100 | AFFIRM: CRPC in progression after chemotherapy PREVAIL: mCRPC IN CHEMOTHERAPY NAIVE |
PLACEBO ± CORTICOSTEROIDS 399 patients PLACEBO: 845 patients |
MDV3100 ± CORTICOSTEROIDS 800 patients MDV3100 872 patients |
13.6 vs. 18.4 4.8 months Enzalutamide reduced the risk of death by 29%. Study unblinded in 2013 |
| ABIRATERONE | COU-AA-302: CRPC with metastasis, asymptomatic or minimally symptomatic prior to chemotherapy | PLACEBO + CORTICOSTEROIDS 542 patients |
ABIRATERONE + CORTICOSTEROIDS 546 patients |
30.1 vs. 35.3 5.2 months |
| ABIRATERONE | COU-AA-301: CRPC with metastasis after chemotherapy | PLACEBO + CORTICOSTEROIDS 398 patients |
ABIRATERONE + CORTICOSTEROIDS 797 patients |
11.2 vs. 15.8 months 4.6 months |
| CABAZITAXEL | TROPIC: CRPC with metastasis after chemotherapy | MITOXANTRONE + CORTICOSTEROIDS 377 patients |
CABAZITAXEL + CORTICOSTEROIDS 378 patients |
12.7 vs. 15.1 2.4 months |
| SIPULEUCEL-T | IMPACT 301: CRPC with metastasis prior to chemotherapy | PLACEBO 171 patients |
SIPULEUCEL T 341 patients |
21.7 vs. 25.8 4.1 months |
The authors apologise for these errors.