Abstract
A seven-year-old male underwent surgical resection and chemoradiation for average risk medulloblastoma; twelve years later, the presence of a necrotic and infiltrative mass in the same area and invading the brainstem prompted a subtotal resection. Pathology was indicative of glioblastoma. He was then treated with concurrent temozolomide and using biologically effective dose calculations for gross residual tumor tissue in the brainstem as well as brainstem tolerance, a radiotherapy dose of 3750 cGy was chosen, fractionated in twice-daily fractions of 125 cGy each. The gross tumor volume was expanded with a 5 mm margin to the planning target volume, which was also judiciously subtracted from the normal brainstem. He completed his radiotherapy course with subsequent imaging free of residual tumor and continued adjuvant temozolomide and remains under follow-up surveillance. This case underscores the rarity of metachronous medulloblastoma and glioblastoma, of which only five known cases heretofore have been described. We discuss the technicalities of radiotherapy planning in this patient, including common hurdles for radiation oncologists in similar patients.
Keywords: Medulloblastoma, Glioblastoma, Re-irradiation, Brainstem tolerance, Toxicity
1. Background
Metachronous brain tumors are defined as the development of at least two histologically distinct primary intracranial neoplasms, not related to local recurrence of the initial tumor. Local recurrences are often difficult to differentiate from a de novo tumor.1 Sometimes, the development of a second primary brain tumor is a result of previous radiation, especially in pediatric patients.2, 3 The presence of certain risk factors, such as familial cancer syndromes, may increase the chance of developing secondary neoplasms after radiotherapy.4
While many radiation-induced intracranial neoplasms have been reported within fifteen years of radiotherapy,2, 3 other studies have seen the occurrence of secondary tumors as late as 20–25 years after radiation.5, 6 However, as the interval between initial and second tumor occurrence increases, it is often difficult to ascertain whether the second tumor was caused by radiotherapy or de novo.
Metachronous primary intracranial neoplasms are relatively scarce in the literature, especially in pediatric patients. Although there have been reports of metachronous glioblastomas, medulloblastomas and anaplastic astrocytomas in adults,5, 6, 7 there are rare reports on metachronous medulloblastoma and glioblastoma to date.8 Re-irradiation to the posterior fossa and brainstem for secondary glioblastoma after radiotherapy for medulloblastoma has even fewer cases reported to guide treatment. We report a case of glioblastoma in a pediatric patient after chemoradiotherapy for medulloblastoma, and discuss technical issues of radiotherapy planning and treatment for re-irradiation.
2. Case presentation
A 7-year-old otherwise healthy male experienced several days of severe bioccipital headaches with nausea and a few episodes of non-bloody emesis. He had no significant past medical history and received all appropriate immunizations. Physical examination was remarkable for papilledema on fundoscopy, and the absence of neurologic deficits. Initial computed tomography (CT) scan of the head revealed a large mass in the posterior fossa (Fig. 1). It measured 5 cm in the greatest dimension and displaced the fourth ventricle anteriorly, causing secondary hydrocephalus in the lateral and third ventricles. It was highly suspicious for medulloblastoma. Lumbar puncture did not show any evidence of tumor cells. Modified Chang's staging of the tumor was T3a, M0. He underwent gross total resection without residual disease evident on postoperative imaging (Fig. 2A). Pathology revealed a classic/biphasic medulloblastoma with focal anaplasia (Fig. 2B). There was vague neuroblastic nodule formation without internodular desmoplasia. Synaptophysin immunostaining was positive. He was then classified as standard (average) risk given the absence of visible residual disease, initial M0 status, and the age of 7 years.
Fig. 1.
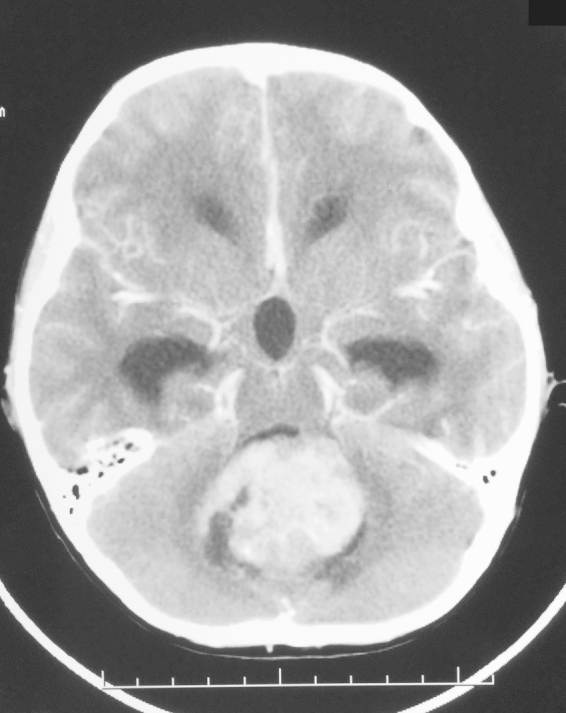
Head CT scan with contrast of patient on initial presentation.
Fig. 2.
Postoperative T1 with contrast MRI after gross tumor resection (Panel A, left); histopathology of resected brain tumor, indicating medulloblastoma (Panel B, right).
The patient then underwent postoperative chemoradiation on the Children's Cancer Group A9961 protocol9 which consisted of concurrent vincristine and cranio-spinal irradiation (CSI) to 2430 cGy followed by a posterior fossa (PF) boost to 5580 cGy, and adjuvant cisplatin, vincristine, and lomustine. Radiation was performed at another hospital using two-dimensional treatment planning (Fig. 3). He lost tactile sensation on the left half of his face and tongue from the time of surgery and developed continually worsening sensorineural hearing loss (for which left-sided cochlear implant was later placed) after chemoradiation therapy.
Fig. 3.
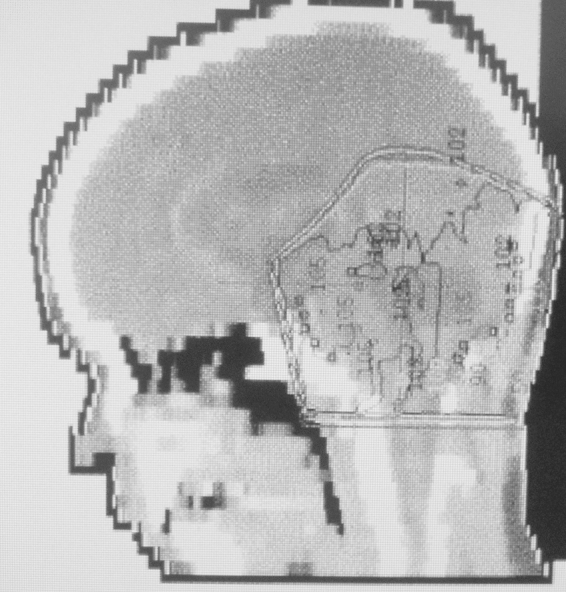
Two-dimensional radiotherapy treatment planning image of adjuvant posterior fossa radiotherapy for medulloblastoma.
He was followed with serial brain magnetic resonance imaging (MRI), without evidence of recurrence. Twelve years after initial diagnosis, the patient began having several days of sudden-onset diplopia and gait ataxia. Physical examination revealed bilateral lateral gaze nystagmus as well as gross abnormalities in gait and postural balance. He also demonstrated some mild left-sided dysmetria and dysdiadochokinesia. Strength testing was unremarkable, and he was without any new cranial nerve defects.
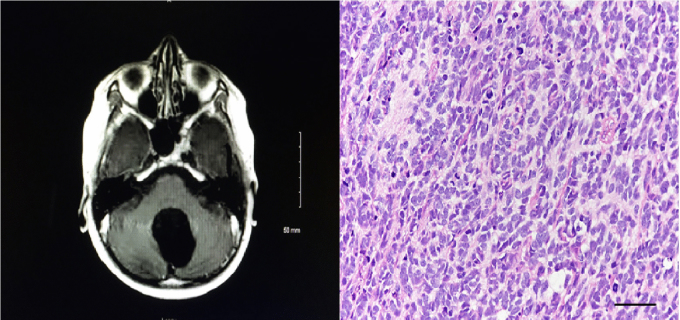
MRI of the brain performed at that time (Fig. 4) revealed a large, heterogeneously enhancing mass in the posterior fossa, anatomically near the site of resection and radiation, with local extension to the brainstem. He underwent subtotal resection with residual disease in the brainstem (Fig. 5A). Pathology demonstrated an infiltrating small cell glioblastoma with high mitotic activity and necrosis with pseudopalisading, and immunoreactivity for OLIG2 but not for synaptophysin; this was confirmed at St. Jude's Hospital (Fig. 5B).
Fig. 4.
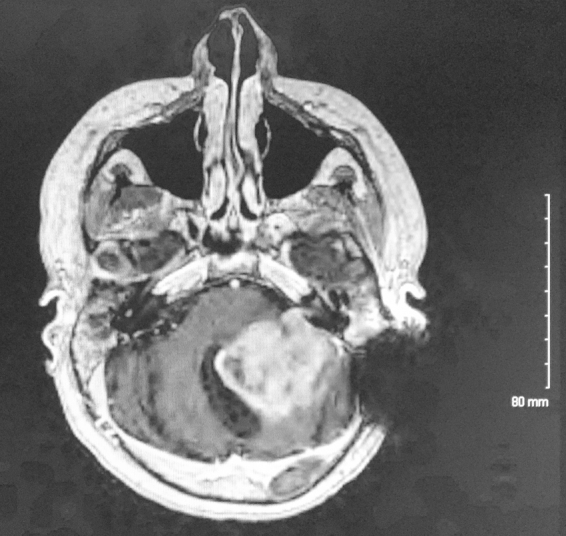
T1 with contrast MRI of patient twelve years after initial presentation, showing a heterogeneously enhancing posterior fossa mass invading the brainstem. Left sided dark signal indicates an artifact from cochlear implant.
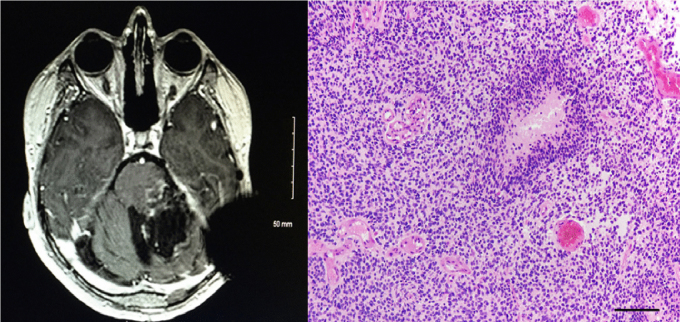
Fig. 5.
Postoperative T1 with contrast MRI revealing residual gross disease in the left side of brainstem along with the resection cavity (Panel A, left); histopathology of the second brain tumor resection, demonstrating glioblastoma (Panel B, right).
Three weeks after resection, the radiation planning MRI showed interval enlargement since the postoperative MRI (Fig. 6); treatment with concurrent temozolomide was in place. Using the linear-quadratic formula of equivalent 2 Gy dose (EQD2), nd(d/α/β)/(2/α/β), and assuming a brainstem α/β ratio of 3, it was calculated that the EQD2 to the brainstem from prior radiotherapy was 5360 cGy (biologically effective dose (BED) of 8930 cGy). It was hence decided to treat the patient's tumor bed and residual tumor in the brainstem to 3750 cGy, fractionated in twice-daily fractions of 125 cGy each to reduce the risk of radiation damage to the brainstem. Radiotherapy was done with intensity-modulated radiotherapy (IMRT). The gross tumor volume (GTV) was contoured as areas of enhancement on the T1 with contrast MRI images, with a 5 mm margin to the planning target volume (PTV) to account for setup uncertainties. The margin was subtracted from the normal brainstem as much as possible, in efforts to avoid excessive brainstem dose.
Fig. 6.
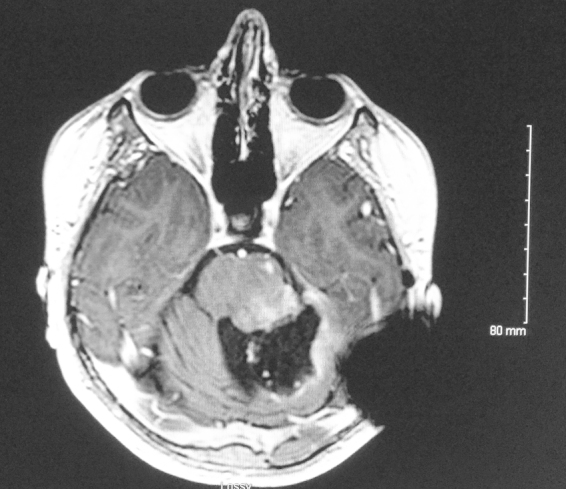
T1 with contrast MRI used for treatment planning, taken three weeks after postoperative MRI (Fig. 5A) demonstrating increased interval enhancement along the tumor resection bed and brainstem.
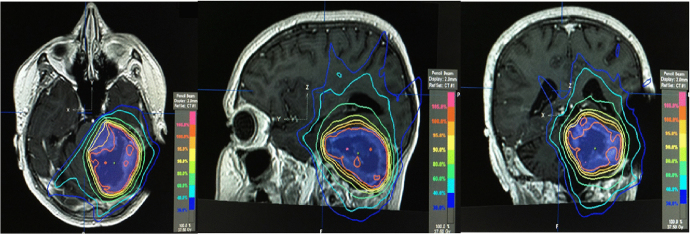
Fig. 7 shows the treatment plan which consisted of a maximum dosage of 4020 cGy and a minimum of 3550 cGy to the PTV outside of the brainstem. The maximum dose to the PTV inside the brainstem was 3731 cGy, and the minimum was 3300 cGy. The maximum dose to the brainstem not included in the PTV was 3670 cGy; 0.3 cubic centimeters (cc) of the brainstem received 3400 cGy, 10 cc received 1750 cGy, and 20 cc received 675 cGy. The patient's radiotherapy course was uneventful, with near-complete resolution of his diplopia and improvement in his gait. A repeat brain MRI taken after completing radiotherapy demonstrated no contrast enhancement in the tumor bed, indicative of no further gross residual tumor (Fig. 8). He received adjuvant temozolomide and continued to be followed up with unremarkable serial imaging and stably improved clinical reassessments, most recently eleven months after treatment.
Fig. 7.
Axial (left), sagittal (center), coronal (right) MRI images with treatment plan for adjuvant radiotherapy for glioblastoma. Violet volume indicates PTV; isodose line distributions per colors on the right side of images.
Fig. 8.
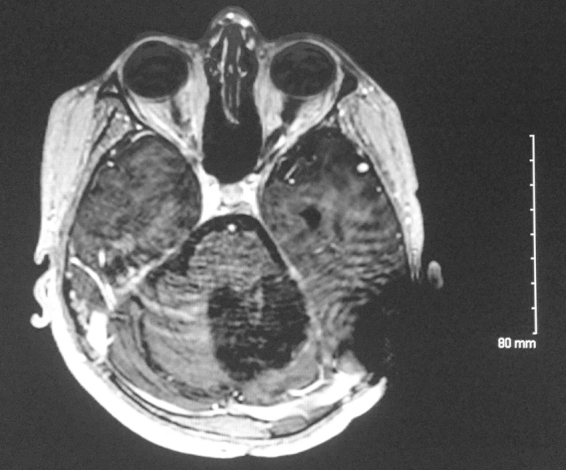
T1 with contrast MRI after completion of radiotherapy with interval resolution of previous contrast enhancement along the rim of tumor bed.
3. Discussion/conclusions
This case underscores both the rarity and complexity of re-irradiating a patient with a secondary glioblastoma after chemoradiation for medulloblastoma. This case lends experiential evidence of re-irradiation of the posterior fossa and brainstem in a pediatric patient with two metachronous intracranial neoplasms.
It is key to mention that although there are long-term survivors of glioblastoma10 (and adult medulloblastoma,11 which is beyond the scope of this paper) the intent of radiotherapy in this patient was not curative, but aimed to decrease the local tumor burden and to continue a symptomatic relief. It is also important to communicate this to patients in a sensitive and caring manner at the time of consultation, in conjunction with the risks of re-irradiation to critical structures such as the brainstem. Although similar trials have not been conducted in children, a trial in adults with glioblastoma did note modest survival benefit of radiotherapy as compared to supportive care alone.12
Packer et al.8 performed an analysis of secondary tumors in 421 patients enrolled in the Children's Cancer Group A9961 trial. Four patients had secondary glioblastoma, which occurred at a median time of 7.3 years (range, 3.7–9.2) from initial diagnosis, with a median follow-up of 9.7 years. Three of the four secondary glioblastoma patients died. The authors postulate that every one of the fifteen patients that developed secondary tumors did so at a location that would have received at least scatter radiation dose. Although the report does not specify how recurrences were treated, we offer our experience in re-irradiation of one of five known cases of metachronous medulloblastoma and glioblastoma, highlighting the intricate balance of tumor control and dose-limiting organ toxicity.
There were several difficult issues for us to consider when planning re-treatment in this patient. First, the decision to use hyperfractionation and provide as much conformality near the brainstem was quite imperative for re-treatment of the brainstem. According to the initial data by Emami et al.,13 and later in QUANTEC,14 there is a <5% chance of brainstem necrosis with maximum doses <54 Gy to the brainstem, or when limiting 1–10 cc to ≤59 Gy. Another report published at the same time added that the risk of symptomatic brainstem necrosis markedly increases at doses above 64 Gy.15 As decreasing the dose per fraction decreases the EQD2 to the brainstem, combined with the previous radiation dosage to 5580 cGy, a twice-daily fractionation scheme was certainly a necessity. Further research delineating contouring of the corticospinal tract as an organ at risk could be useful for similar concerning cases, and further study is certainly warranted.16
There have been several trials in pediatric brainstem glioma patients in which primary radiotherapy exceeded 70 Gy. Although a limitation of all these trials was that poor survival did not afford the assessment of long-term complications, the two trials that provided the highest BED3 are as follows. The Pediatric Oncology Group17 went to doses of 7560 cGy in 126 cGy twice-daily fractions (BED of 10,735 cGy; EQD2 of 6440 cGy), and the Children's Cancer Group trial18 went to doses of 7800 cGy in 100 cGy twice-daily fractions (BED3 of 10,400 cGy, EQD2 of 6240 cGy). Both studies used parallel-opposed fields and two-dimensional planning, with no significant toxicity experienced during the last follow-up of two to three years. Although more research is needed to determine pediatric brainstem dose tolerances, currently there is no evidence to suggest that the limits differ from those of adults.
Another important issue to consider for treatment planning was that the patient's initial treatment used two-dimensional treatment planning to a total PF dose of 5580 cGy. This implies that the maximum dose to the brainstem could very well have reached an unknown value less than 5580 cGy. However, when deciding on dosage, the maximum of 5580 cGy had to be assumed. Based on a previous EQD2 of 5360 cGy (as above), addition of 3750 cGy in 125 cGy fractions would add 3190 cGy EQD2 (5250 cGy BED3), for a cumulative EQD2 of 8550 cGy (BED3 of 14,170 cGy) to the brainstem. This is consistent with a report that specifically assessed reirradiation of eight pediatric brain tumors with 16-month median follow-up.19 The median cumulative BED2 was 144 Gy, with a range of 126–181 Gy. Radiotherapy was tolerated by all eight patients without significant re-irradiation complications, though the median survival is too low in these patients to assess late complications. Additionally, Merchant et al.20 described a series of 38 patients with recurrent ependymoma for which re-irradiation resulted in total brainstem doses of 90–120 Gy, with only one treatment-related brainstem toxicity causing death at median follow-up of 30 months. The authors indicate that a longer time interval between the initial and second radiotherapy courses may contribute to greater intrinsic brainstem repair, thus potentially being able to escalate brainstem dose without severe toxicity. Brainstem repair capacity is incompletely understood, although studies on the spinal cord suggest that at least 25% of the initial dose is repaired in humans at substantial time intervals.21
Similarly, the dose to the optic chiasm is a concern. Anatomically, the optic chiasm would be just anterior to the rostralmost treated areas of many medulloblastoma patients, and retreatment would pose a similar obstacle. Although based on the previous two-dimensional treatment plan, the optic chiasm may have been at the border of the field, we had to assume it received the full initial dose of 5580 cGy. One analysis measuring optic chiasm toxicity showed that the risk of increased substantially at doses over 60 Gy.22 Thus, based on our fields and contoured volumes, the optic chiasm received minimal dose. The rostral extent of our PTV did not overlap with the most caudal portion of the optic chiasm. Subtracting our PTV contour from the brainstem as much as possible certainly aided in decreasing dose to the optic chiasm. One report23 went as high as 7650 cGy equivalent uniform dose to the optic chiasm without any complications noted.
Lastly, the patient's cochlear implant was another area of concern. Multiple studies have noted intact function of the implant after radiotherapy in pediatric patients.21 Attenuation correction must be taken into account when planning.24 Of note, the back and side scatter contributions were negligible.25
In summary, we present a rare case of metachronous medulloblastoma status post chemoradiotherapy and development of a secondary glioblastoma for which there remains a need for more experiential data regarding re-irradiation treatment planning.
Contributors
V.V. obtained radiological and chart review data and drafted the manuscript. R.R. obtained radiological and chart review data and assisted in drafting the manuscript. A.R.B. and N.R.B. provided assistance in drafting the manuscript and all revisions to the manuscript. R.D.M. provided histopathological data and edited the manuscript and participated in supervision of the project. C.L. conceived of the study and participated in its design and coordination and helped to draft the manuscript and supervised the entire project. All authors read and approved the final manuscript.
Conflict of interest
None declared.
Financial disclosure
None declared.
Contributor Information
Vivek Verma, Email: vivek.verma@unmc.edu.
Chi Lin, Email: clin@unmc.edu.
References
- 1.Eom K.S., Kim J.M., Kim T.Y. Mixed germ cell tumors in septum pellucidum after radiochemotherapy of suprasellar germinoma: de novo metachronous or recurrent neoplasms? Childs Nerv Syst. 2008;24:1355–1359. doi: 10.1007/s00381-008-0654-0. [DOI] [PubMed] [Google Scholar]
- 2.Makidono A., Kobayashi N., Saida Y. Metachronous gliomas following cranial irradiation for mixed germ cell tumors. Childs Nerv Syst. 2009;25:713–718. doi: 10.1007/s00381-009-0829-3. [DOI] [PubMed] [Google Scholar]
- 3.Sasayama T., Nishihara M., Tanaka K. Two metachronous tumors induced by radiation therapy: case report and review of the literature. J Neurooncol. 2008;88:315–320. doi: 10.1007/s11060-008-9570-0. [DOI] [PubMed] [Google Scholar]
- 4.Limacher M., Frebourg T., Natarajan-Ame S. Two metachronous tumors in the radiotherapy fields of a patient with Li-Fraumeni syndrome. Int J Cancer. 2001;96:238–242. doi: 10.1002/ijc.1021. [DOI] [PubMed] [Google Scholar]
- 5.Hope A.J., Mansur D.B., Tu P.H. Metachronous secondary atypical meningioma and anaplastic astrocytoma after postoperative craniospinal irradiation for medulloblastoma. Childs Nerv Syst. 2006;22:1201–1207. doi: 10.1007/s00381-006-0062-2. [DOI] [PubMed] [Google Scholar]
- 6.Jinguji S., Okamoto K., Yoshimura J. Occurrence of metachronous pure germinomas long after treatment of a mixed germ cell tumor containing yolk sac tumor and germinoma. J Neurosurg Pediatr. 2013;11:68–73. doi: 10.3171/2012.9.PEDS12151. [DOI] [PubMed] [Google Scholar]
- 7.Martinez R., Schackert H.K., von Kannen S. Independent molecular development of metachronous glioblastomas with extended intervening recurrence-free interval. Brain Pathol. 2003;13:598–607. doi: 10.1111/j.1750-3639.2003.tb00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Packer R.J., Zhou T., Holmes E., Vezina G., Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children's Oncology Group trial A9961. Neuro Oncol. 2013;15:97–103. doi: 10.1093/neuonc/nos267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packer R.J., Gajjar A., Vezina G. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly-diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 10.Urbanczyk H., Straczynska-Niemiec A., Glowacki G., Lange D., Miszczyk L. Case presentation – a five-year survival of the patient with glioblastoma brain tumor. Rep Pract Oncol Radiother. 2014;19:347–351. doi: 10.1016/j.rpor.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buglione M., Ghirardelli P., Triggiani L. Radiotherapy for adult medulloblastoma: long term result from a single institution. A review of prognostic factors and why we do need a multi-institutional cooperative program. Rep Pract Oncol Radiother. 2015;20:284–291. doi: 10.1016/j.rpor.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keime-Guibert F., Chinot O., Taillandier L. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 13.Emami B., Lyman J., Brown A. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 14.Marks L.B., Yorke E.D., Jackson A. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo C., Yorke E., Merchant T.E. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010;76:S36–S41. doi: 10.1016/j.ijrobp.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igaki H., Sakumi A., Mukasa A. Corticospinal tract-sparing intensity-modulated radiotherapy treatment planning. Rep Pract Oncol Radiother. 2014;19:310–316. doi: 10.1016/j.rpor.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman C.R., Krischer J.P., Sanford R.A. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 1993;27:197–206. doi: 10.1016/0360-3016(93)90228-n. [DOI] [PubMed] [Google Scholar]
- 18.Packer R.J., Boyett J.M., Zimmerman R.A. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Children's Cancer Group Phase I/II trial. Cancer. 1994;74:1827–1834. doi: 10.1002/1097-0142(19940915)74:6<1827::aid-cncr2820740628>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Chojnacka M., Skowronska-Gardas A., Pedziwiatr M. Reirradiation of relapsed brain tumors in children. Rep Pract Oncol Radiother. 2012;17:32–37. doi: 10.1016/j.rpor.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merchant T.E., Boop F.A., Kun L.E., Sanford R.A. A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys. 2008;71:87–97. doi: 10.1016/j.ijrobp.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Kirkpatrick J.P., van der Kogel A.J., Schultheiss T.E. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76:S42–S49. doi: 10.1016/j.ijrobp.2009.04.095. [DOI] [PubMed] [Google Scholar]
- 22.Mayo C., Martel M.K., Marks L.B. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76:S28–S35. doi: 10.1016/j.ijrobp.2009.07.1753. [DOI] [PubMed] [Google Scholar]
- 23.Niyazi M., Karin I., Sohn M. Analysis of equivalent uniform dose (EUD) and conventional radiation treatment parameters after primary and re-irradiation of malignant glioma. Radiat Oncol. 2013;8:287. doi: 10.1186/1748-717X-8-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy K., Cook B., Shaw C. Intact performance of a cochlear implant following radiotherapy in a child with acute lymphoblastic leukemia. Pract Radiat Oncol. 2012;2:233–236. doi: 10.1016/j.prro.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Gossman M.S., Treaba C.G., Kirk J.R. Radiation therapy in cochlear implant recipients. Otol Neurotol. 2011;32:553–557. doi: 10.1097/MAO.0b013e3182138793. [DOI] [PubMed] [Google Scholar]