Abstract
Aim
The aim of this study is to assess the effect of the compositions of various soft tissues and tissue-equivalent materials on dose distribution in neutron brachytherapy/neutron capture therapy.
Background
Neutron brachytherapy and neutron capture therapy are two common radiotherapy modalities.
Materials and methods
Dose distributions were calculated around a low dose rate 252Cf source located in a spherical phantom with radius of 20.0 cm using the MCNPX code for seven soft tissues and three tissue-equivalent materials. Relative total dose rate, relative neutron dose rate, total dose rate, and neutron dose rate were calculated for each material. These values were determined at various radial distances ranging from 0.3 to 15.0 cm from the source.
Results
Among the soft tissues and tissue-equivalent materials studied, adipose tissue and plexiglass demonstrated the greatest differences for total dose rate compared to 9-component soft tissue. The difference in dose rate with respect to 9-component soft tissue varied with compositions of the materials and the radial distance from the source. Furthermore, the total dose rate in water was different from that in 9-component soft tissue.
Conclusion
Taking the same composition for various soft tissues and tissue-equivalent media can lead to error in treatment planning in neutron brachytherapy/neutron capture therapy. Since the International Commission on Radiation Units and Measurements (ICRU) recommends that the total dosimetric uncertainty in dose delivery in radiotherapy should be within ±5%, the compositions of various soft tissues and tissue-equivalent materials should be considered in dose calculation and treatment planning in neutron brachytherapy/neutron capture therapy.
Keywords: Neutron brachytherapy, Neutron capture therapy, Tissue composition, Dose rate, 252Cf
1. Background
Neutron brachytherapy is a type of high linear energy transfer (LET) radiotherapy which is effective in killing radioresistant cancer cells. This method has also been proven to be effective for treatment of intracavitary cancers of the cervix when it is used in combination with external beam radiotherapy. Californium-252 (252Cf) is a neutron-emitting radionuclide recently applied in 252Cf-based neutron brachytherapy in China.1
Boron neutron capture therapy (BNCT) is a noninvasive treatment which is an alternative for invasive treatment of malignant tumors. BNCT is used for treatment of a number of tumors such as glioma, melanoma, liver, cerebral metastasis, colon, sarcoma, head and neck, lung tissue and chest. This type of treatment consists of two steps: injection of a local medicine to the patient and irradiation of the patient with thermal neutrons. The cross-section of the neutron capture interaction with the injected drug is higher than that with other elements, such as hydrogen, nitrogen, and oxygen, which are present in tissue. When the patient is irradiated by thermal neutrons with energy of 0.025 eV, these neutrons interact with the capture agent (the injected drug) and this leads to biological damage to the tumor.2
10B is a common isotope which is designed to be present in the injected drugs. This isotope is absorbed by tumor cells and consecutively the interaction of thermal neutrons with 10B nuclei in tumor cells leads to production of 7Li and α products which have a high linear energy transfer values (LETs). The resulting lithium ions and alpha particles carry the energy away.3, 4 The average energy in this case is 2.33 MeV.5 Due to the limited ranges of the reaction products in tissue (5–9 μm), the destructive effects of the resulting projectiles are limited to cells containing 10B.6 Some other isotopes for use in neutron capture therapy (NCT) which have been introduced in recent studies are 157Gd and 33S.
Various neutron sources used in NCT consist of a reactor, an accelerator and a 252Cf source. Recently 252Cf has been used as a brachytherapy source for treatment of late stage cancers. Brachytherapy with a neutron source such as 252Cf is more effective than brachytherapy with photon sources in treatment of tumors resistant to radiation, such as huge tumors, late stage tumors, melanomas, and glioblastomas.5
In the dosimetric formalism presented in the updated report by task group No. 43 (TG-43U1) of the American Association of Physicists in Medicine (AAPM), the dosimetric parameters are recommended to be calculated in a water phantom as tissue-equivalent media. Since the 252Cf source which is used in neutron brachytherapy/neutron capture therapy is a neutron emitter, it is reasonable that the difference between neutron cross-sections in water and soft tissue would lead to differences between the absorbed doses in these two media. Since treatment planning systems (TPSs) calculations are routinely performed for water, ignorance of these differences in TPSs could lead to error in dose calculation and therefore dose delivery in neutron brachytherapy/neutron capture therapy.
There are some literatures which have focused on dose calculation in BNCT with 252Cf source in various phantom media.7, 8, 9 These studies investigated various media, such as muscle (skeletal), brain (grey/white matter), skin, blood (whole), pancreas, lung tissue, bone, A-150 plastic, plexiglass, human head phantom, and water. These studies demonstrated differences among the doses in various media. However, to the best of our knowledge, there is not a comprehensive study which evaluated a wide range of soft tissues and tissue-equivalent materials from various points of view. For example, in some studies (as an example in a study by Rivard10) the primary and secondary photon dose components were ignored in dose calculations and only neutron kerma rate was reported for various phantom materials. Since current calculations of TPSs are based on dose measurements in a water phantom, it is important to perform a quantitative study on various soft tissues and tissue-equivalent materials to calculate the differences between the doses in water and other soft tissues and media. The purpose of the current study is to assess the effect of the composition of various soft tissues and tissue-equivalent materials on dose distribution in neutron brachytherapy/neutron capture therapy.
2. Materials and methods
2.1. 252Cf source geometry
In this study, an Applicator Tube (AT) model 252Cf source, which has been constructed by Oak Ridge National Laboratory (ORNL),11 was simulated using MCNPX (version 2.6.0) Monte Carlo code.12 In the AT 252Cf source, the cylindrical active core is Pd:Cf2O3 ceramic metal with a 1.5 cm length and a 0.615 mm radius. The source also has two capsules: primary and secondary. The primary capsule is composed of an alloy of 90% platinum and 10% iridium with an inner diameter of 1.35 mm and an outer diameter of 1.75 mm. The secondary capsule has the same composition as the primary one, but its dimensions differ from the primary capsule. In a previous study,13 dosimetric parameters for the AT 252Cf source were calculated and compared with those reported by the other studies. These parameters included air kerma strength conversion factor, dose rate constant, radial dose function, and total dose rate. Good agreement was observed between the data of 252Cf source simulations in the previous study and the other reported data, therefore, the source simulations were approved. In the present study the verified simulations were used in evaluation of the effect of compositions of various media.
2.2. Soft tissues and tissue-equivalent materials
Seven soft tissues and three tissue-equivalent materials were used as the phantom material separately. The studied tissues were 4-component soft tissue, muscle (skeletal), brain (grey/white matter), adipose, blood (whole), lung tissue, and 9-component soft tissue. The tissue-equivalent materials were A-150 plastic, plexiglass, and water. The elemental compositions and mass densities of the materials were adopted from report No. 44 of the International Commission on Radiation Units and Measurements (ICRU).14 For each element, various natural isotopes were also included in the calculations.15 Compositions of various soft tissues and tissue-equivalent materials including various natural isotopes are listed in Table 1. It should be noted that each material was assessed individually in a separate simulation. The 252Cf source was defined at the center of a spherical phantom with a radius of 20.0 cm in the simulations.
Table 1.
Isotope compositions of soft tissues and tissue-equivalent materials.
| Element | Atomic number | Natural isotopes | Weight fraction |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soft tissue (9-component) | Soft tissue (4-component) | Muscle (skeletal) | Brain (grey/white matter) | Adipose tissue | Blood (whole) | Lung tissue | A-150 | Plexiglass | Water | |||
| Hydrogen | 1 | 1H | 0.1019883 | 0.1011624 | 0.1019883 | 0.1069877 | 0.1139869 | 0.1019883 | 0.1029882 | 0.1013183 | 0.0805317 | 0.1118851 |
| 2H | 0.0000117 | 0.0000116 | 0.0000117 | 0.0000123 | 0.0000131 | 0.0000117 | 0.0000118 | 0.0000116 | 0.0000093 | 0.0000129 | ||
| Carbon | 6 | 12C | 0.1414699 | 0.1098123 | 0.1414699 | 0.1434485 | 0.5916014 | 0.1088230 | 0.1038765 | 0.7672002 | 0.5934276 | |
| 13C | 0.0015301 | 0.0011877 | 0.0015301 | 0.0015515 | 0.0063986 | 0.0011770 | 0.0011235 | 0.0082978 | 0.0064183 | |||
| Nitrogen | 7 | 14N | 0.0338749 | 0.0259043 | 0.0338749 | 0.0219190 | 0.0069742 | 0.0328786 | 0.0308859 | 0.0349280 | ||
| 15N | 0.0001251 | 0.0000957 | 0.0001251 | 0.0000810 | 0.0000258 | 0.0001214 | 0.0001141 | 0.0001290 | ||||
| Oxygen | 8 | 16O | 0.7062796 | 0.7599748 | 0.7082747 | 0.7102698 | 0.2773245 | 0.7431896 | 0.7471799 | 0.0521879 | 0.3188363 | 0.8859440 |
| 17O | 0.0002690 | 0.0002895 | 0.0002698 | 0.0002706 | 0.0001057 | 0.0002831 | 0.0002846 | 0.0000199 | 0.0001214 | 0.0003375 | ||
| 18O | 0.0014514 | 0.0015617 | 0.0014555 | 0.0014596 | 0.0005699 | 0.0015272 | 0.0015354 | 0.0001072 | 0.0006552 | 0.0018206 | ||
| Fluorine | 9 | 19F | 0.0174223 | |||||||||
| Sodium | 11 | 23Na | 0.002 | 0.001 | 0.002 | 0.001 | 0.001 | 0.002 | ||||
| Phosphorus | 15 | 31P | 0.003 | 0.002 | 0.004 | 0.001 | 0.002 | |||||
| Sulfur | 16 | 32S | 0.0028479 | 0.0028479 | 0.0018986 | 0.0009493 | 0.0018986 | 0.0028479 | ||||
| 33S | 0.0000228 | 0.0000228 | 0.0000152 | 0.0000076 | 0.0000152 | 0.0000228 | ||||||
| 34S | 0.0001287 | 0.0001287 | 0.0000858 | 0.0000429 | 0.0000858 | 0.0001287 | ||||||
| 36S | 0.0000006 | 0.0000006 | 0.0000004 | 0.0000002 | 0.0000004 | 0.0000006 | ||||||
| Chlorine | 17 | 35Cl | 0.0015156 | 0.0007578 | 0.0022734 | 0.0007578 | 0.0022734 | 0.0022734 | ||||
| 37Cl | 0.0004844 | 0.0002422 | 0.0007266 | 0.0002422 | 0.0007266 | 0.0007266 | ||||||
| Potassium | 19 | 39K | 0.0027977 | 0.0037303 | 0.0027977 | 0.0018652 | 0.0018652 | |||||
| 40K | 0.0000003 | 0.0000005 | 0.0000003 | 0.0000002 | 0.0000002 | |||||||
| 41K | 0.0002019 | 0.0002692 | 0.0002019 | 0.0001346 | 0.0001346 | |||||||
| Calcium | 20 | 40Ca | 0.0178148 | |||||||||
| 42Ca | 0.0001189 | |||||||||||
| 43Ca | 0.0000248 | |||||||||||
| 44Ca | 0.0003833 | |||||||||||
| 46Ca | 0.0000007 | |||||||||||
| 48Ca | 0.0000344 | |||||||||||
| Iron | 26 | 54Fe | 0.0000584 | |||||||||
| 56Fe | 0.0009175 | |||||||||||
| 57Fe | 0.0000212 | |||||||||||
| 58Fe | 0.0000028 | |||||||||||
2.3. Dose distribution evaluations
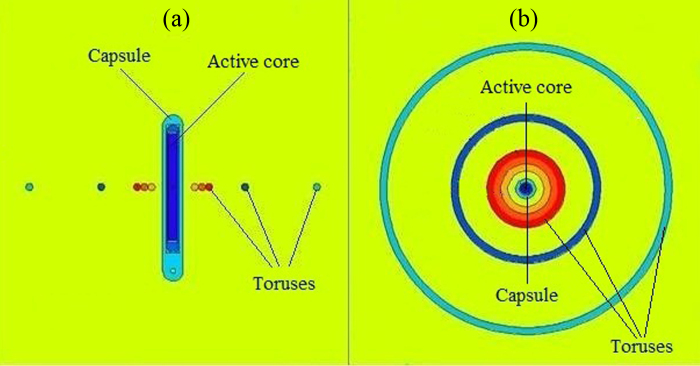
The phantom has a spherical shape with a 20.0 cm radius. The 252Cf source was defined, including its active core and capsules, at the center of the phantom. Relative total dose rate, relative neutron dose rate, total dose rate, and neutron dose rate were calculated around the 252Cf source at various radial distances of 0.3–15.0 cm from the source in 1.0 mm thick toruses. Longitudinal and transverse views of the 252Cf source, the toruses, and the phantom plotted by MCNPX code, are illustrated in Fig. 1(a) and (b), respectively. In this figure (Fig. 1) the toruses are corresponded to 0.3, 0.4, 0.5, 1.0, and 2.0 cm distances from the source center. Due to the low magnification of this figure in the source region, the primary and secondary capsules are not easily distinguishable. The Maxwellian spontaneous neutron distribution was used in the definition of the spectrum of neutrons emitted by the 252Cf source.
Fig. 1.
Geometry representing arrangement of 252Cf source, toruses, and the phantom plotted by MCNPX MC code. (a) Longitudinal view; (b) transverse view.
In this study, 9-component soft tissue, introduced by report No. 44 of ICRU, was adopted to compare with other soft tissues and the relative dose rates in a specific material were calculated as the ratio of dose rate in that material to the dose rate in 9-component soft tissue. The relative dose was calculated for total dose and neutron dose rates. The total dose in this case is the sum of the dose components from primary photons, secondary photons, and neutrons. The primary photon dose herein was accounted as the dose originated from photons emitted by the 252Cf source and the secondary photon dose was the dose from photons produced through interaction of the source's neutrons by the medium. Each dose rate type was calculated in terms of (cGy/(h μg)).
In calculation of the total dose, two input files were run. The first input file had a photon source, defined with the photon spectrum emitted by 252Cf, and the photon tally was scored for the purpose of calculation of the primary photon dose component. In the second input file, the neutron spectrum of the 252Cf source was defined and the dose components from the secondary photons and neutrons were calculated. Energy cut off for electrons and photons in all input files was set as 10 keV. According to the MCNPX manual, when no energy cut off is defined for neutrons, the default cut off is zero which means the transport of neutrons up to zero energy in the phantom.
In order to calculate the absorbed doses from primary and secondary photons, a *F8 tally was determined and the output of the *F8 tally in each tally cell was divided by the mass of that cell. The absorbed dose from neutrons was calculated using a *F6 tally which determines kerma in the material of interest based on estimation of energy deposition and microscopic cross-sections. Transport of 3.0 × 107 particles was performed for each input file and the maximum type A uncertainty in Monte Carlo calculations for the tally cells was 5.3%. The only variance reduction technique employed for these calculations was the aforementioned energy cutoff. One microgram of 252Cf emits 1.3 × 107 photons/s16 and has a neutron yield of about 2.314 × 106 neutrons/s.17 These values were utilized in the process of conversion of the Monte Carlo output (MeV/g) to the dose rate in terms of (cGy/(h μg)).
3. Results
Relative total dose rate values for 4-component soft tissue, muscle (skeletal), brain (grey/white matter), adipose, blood (whole), lung tissue, 9-component soft tissue, A-150 plastic, plexiglass, and water at various radial distances are listed in Table 2. Furthermore, relative neutron dose rate values for these six soft tissues and three tissue-equivalent materials in various radial distances are presented in Table 3. The relative dose rate at a specific voxel for a material was calculated as the ratio of the dose rate at the voxel in that material to the dose rate at the same voxel in the 9-component soft tissue phantom. Table 4, Table 5 list the total dose rate (cGy/(h μg)) and neutron dose rate (cGy/(h μg)) at various radial distances ranging 0.3–15.0 cm from the source. The neutron dose rate values obtained in the current study and those reported by Rivard10 for the muscle, brain, adipose, blood, lung, A-150, plexiglass, and water for a selection of radial distances are presented in Table 6. Additionally, the percentage differences between these two data sets are listed in this table.
Table 2.
Relative total dose rate for soft tissues and tissue-equivalent materials relative to total dose rate in 9-component soft tissue.
| Radial distance (cm) | Soft tissue (4-component) | Muscle (skeletal) | Brain (grey/white matter) | Adipose tissue | Blood (whole) | Lung tissue | A-150 | Plexiglass | Water |
|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 0.993 | 1.001 | 1.026 | 1.084 | 1.002 | 1.009 | 1.021 | 0.895 | 1.051 |
| 0.4 | 0.999 | 1.001 | 1.030 | 1.081 | 0.999 | 1.009 | 1.025 | 0.900 | 1.054 |
| 0.5 | 0.990 | 0.999 | 1.024 | 1.074 | 0.998 | 1.004 | 1.020 | 0.900 | 1.046 |
| 1.0 | 0.994 | 1.001 | 1.019 | 1.084 | 0.992 | 1.002 | 1.025 | 0.903 | 1.043 |
| 2.0 | 1.004 | 1.005 | 1.028 | 1.078 | 1.002 | 1.006 | 1.021 | 0.918 | 1.048 |
| 3.0 | 0.994 | 0.992 | 1.015 | 1.057 | 0.998 | 0.997 | 0.998 | 0.897 | 1.031 |
| 5.0 | 1.009 | 0.995 | 1.004 | 1.056 | 1.000 | 0.999 | 1.021 | 0.920 | 1.023 |
| 7.0 | 0.957 | 0.977 | 1.000 | 1.021 | 1.005 | 0.982 | 0.946 | 0.883 | 1.005 |
| 10.0 | 0.971 | 0.977 | 1.036 | 1.042 | 1.024 | 1.008 | 0.958 | 0.896 | 1.026 |
| 12.0 | 1.010 | 1.002 | 1.013 | 1.031 | 1.005 | 1.028 | 0.935 | 0.914 | 0.993 |
| 15.0 | 1.029 | 1.003 | 1.039 | 1.033 | 1.000 | 1.050 | 0.919 | 0.854 | 0.997 |
Table 3.
Relative neutron dose rate for soft tissues and tissue-equivalent materials relative to neutron dose rate in 9-component soft tissue.
| Radial distance (cm) | Soft tissue (4-component) | Muscle (skeletal) | Brain (grey/white matter) | Adipose tissue | Blood (whole) | Lung tissue | A-150 | Plexiglass | Water |
|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 0.990 | 1.000 | 1.042 | 1.130 | 0.998 | 1.006 | 1.038 | 0.844 | 1.072 |
| 0.4 | 0.990 | 1.000 | 1.041 | 1.131 | 0.998 | 1.006 | 1.039 | 0.846 | 1.072 |
| 0.5 | 0.990 | 1.000 | 1.041 | 1.131 | 0.998 | 1.006 | 1.040 | 0.849 | 1.071 |
| 1.0 | 0.992 | 1.000 | 1.039 | 1.130 | 0.998 | 1.005 | 1.042 | 0.856 | 1.067 |
| 2.0 | 0.996 | 1.001 | 1.035 | 1.129 | 0.997 | 1.004 | 1.040 | 0.868 | 1.059 |
| 3.0 | 1.004 | 1.003 | 1.031 | 1.125 | 0.997 | 1.004 | 1.031 | 0.875 | 1.049 |
| 5.0 | 1.018 | 1.004 | 1.020 | 1.116 | 0.997 | 1.003 | 1.011 | 0.880 | 1.033 |
| 7.0 | 1.032 | 1.007 | 1.008 | 1.091 | 0.998 | 1.003 | 0.975 | 0.872 | 1.019 |
| 10.0 | 1.060 | 1.012 | 0.996 | 1.072 | 0.999 | 1.006 | 0.917 | 0.851 | 1.007 |
| 12.0 | 1.075 | 1.007 | 0.989 | 1.047 | 0.994 | 0.997 | 0.876 | 0.852 | 0.989 |
| 15.0 | 1.119 | 1.023 | 0.993 | 1.046 | 1.003 | 1.004 | 0.835 | 0.846 | 1.004 |
Table 4.
Total dose rate (cGy/(h μg)) for soft tissues and tissue-equivalent materials.
| Radial distance (cm) | Soft tissue (9-component) | Soft tissue (4-component) | Muscle (skeletal) | Brain (grey/white matter) | Adipose tissue | Blood (whole) | Lung tissue | A-150 | Plexiglass | Water |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 16.783 | 16.661 | 16.806 | 17.215 | 18.200 | 16.813 | 16.927 | 17.135 | 15.014 | 17.641 |
| 0.4 | 11.593 | 11.580 | 11.604 | 11.945 | 12.529 | 11.582 | 11.698 | 11.877 | 10.435 | 12.222 |
| 0.5 | 8.592 | 8.503 | 8.585 | 8.795 | 9.226 | 8.575 | 8.630 | 8.767 | 7.731 | 8.990 |
| 1.0 | 2.842 | 2.826 | 2.846 | 2.896 | 3.082 | 2.818 | 2.848 | 2.913 | 2.567 | 2.965 |
| 2.0 | 0.772 | 0.775 | 0.775 | 0.793 | 0.832 | 0.773 | 0.776 | 0.788 | 0.708 | 0.809 |
| 3.0 | 0.349 | 0.347 | 0.346 | 0.354 | 0.369 | 0.348 | 0.348 | 0.348 | 0.313 | 0.360 |
| 5.0 | 0.118 | 0.119 | 0.117 | 0.118 | 0.124 | 0.118 | 0.118 | 0.120 | 0.108 | 0.121 |
| 7.0 | 0.058 | 0.055 | 0.056 | 0.058 | 0.059 | 0.058 | 0.057 | 0.055 | 0.051 | 0.058 |
| 10.0 | 0.025 | 0.024 | 0.024 | 0.026 | 0.026 | 0.025 | 0.025 | 0.024 | 0.022 | 0.025 |
| 12.0 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.015 | 0.014 | 0.016 |
| 15.0 | 0.009 | 0.009 | 0.009 | 0.009 | 0.008 | 0.009 | 0.009 | 0.008 | 0.007 | 0.009 |
Table 5.
Neutron dose rate (cGy/(h μg)) for soft tissues and tissue-equivalent materials.
| Radial distance (cm) | Soft tissue (9-component) | Soft tissue (4-component) | Muscle (skeletal) | Brain (grey/white matter) | Adipose tissue | Blood (whole) | Lung tissue | A-150 | Plexiglass | Water |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 10.407 | 10.298 | 10.407 | 10.843 | 11.764 | 10.385 | 10.466 | 10.807 | 8.779 | 11.161 |
| 0.4 | 7.083 | 7.012 | 7.083 | 7.376 | 8.008 | 7.067 | 7.122 | 7.360 | 5.995 | 7.589 |
| 0.5 | 5.149 | 5.099 | 5.150 | 5.361 | 5.822 | 5.138 | 5.178 | 5.354 | 4.370 | 5.514 |
| 1.0 | 1.674 | 1.661 | 1.675 | 1.740 | 1.892 | 1.670 | 1.683 | 1.744 | 1.433 | 1.786 |
| 2.0 | 0.451 | 0.449 | 0.452 | 0.467 | 0.509 | 0.450 | 0.453 | 0.469 | 0.392 | 0.478 |
| 3.0 | 0.193 | 0.194 | 0.194 | 0.199 | 0.217 | 0.193 | 0.194 | 0.199 | 0.169 | 0.203 |
| 5.0 | 0.060 | 0.061 | 0.060 | 0.061 | 0.067 | 0.060 | 0.060 | 0.060 | 0.053 | 0.062 |
| 7.0 | 0.025 | 0.026 | 0.025 | 0.025 | 0.027 | 0.025 | 0.025 | 0.024 | 0.022 | 0.025 |
| 10.0 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.008 | 0.007 | 0.009 |
| 12.0 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.004 | 0.004 | 0.005 |
| 15.0 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
Table 6.
Neutron dose rate (cGy/(h μg)) at various radial distances in soft tissues and tissue-equivalent materials from the current study and the study by Rivard.10 The percentage differences between the two data sets are also listed.
| Radial distance (cm) | Muscle (skeletal) | Brain (grey/white matter) | Adipose tissue | Blood (whole) | Lung tissue | A-150 | Plexiglass | Water | |
|---|---|---|---|---|---|---|---|---|---|
| Current study | 0.5 | 5.150 | 5.361 | 5.354 | 5.514 | ||||
| 1 | 1.675 | 1.740 | 1.892 | 1.670 | 1.683 | 1.744 | 1.433 | 1.786 | |
| 2 | 0.452 | 0.467 | 0.469 | 0.478 | |||||
| 5 | 0.060 | 0.061 | 0.060 | 0.062 | |||||
| Rivard | 0.5 | 7.628 | 8.054 | 8.007 | 8.295 | ||||
| 1 | 1.907 | 2.009 | 2.183 | 1.928 | 1.943 | 2.005 | 1.654 | 2.064 | |
| 2 | 0.461 | 0.483 | 0.484 | 0.495 | |||||
| 5 | 0.058 | 0.059 | 0.058 | 0.061 | |||||
| Percentage difference (%) | 0.5 | −32.49 | −33.44 | −33.13 | −33.53 | ||||
| 1 | −12.17 | −13.39 | −13.33 | −13.38 | −13.38 | −13.02 | −13.36 | −13.47 | |
| 2 | −1.95 | −3.31 | −3.10 | −3.43 | |||||
| 5 | 3.45 | 3.39 | 3.45 | 1.64 | |||||
4. Discussion
In this study, dose distributions around a 252Cf source in various soft tissues and tissue-equivalent materials were calculated. It is evident, from the data presented in Table 2, Table 3, Table 4, Table 5, that dose distributions in various soft tissues and tissue-equivalent materials are different. Since the composition of the elements in different media is different and the cross-sections of the neutron interactions depend on the elements, the dose in various soft tissues and tissue-equivalent materials are different. Assuming the same composition for various soft tissues in treatment planning systems can cause errors in dose calculations. One should notice that this effect is only one of the various sources of errors that are potentially involved in dose delivery in neutron brachytherapy/neutron capture therapy and there are also other sources of uncertainties which should be addressed in radiotherapy with neutron sources. Accumulation of these errors may result in therapeutic side effects. Thus, any error mitigation is progress and this study aims to show the importance of limiting one source of error in neutron brachytherapy/neutron capture therapy.
Ghorbani et al.18 have shown that there is a difference between the dose distributions in various soft tissues in brachytherapy with four photon emitting sources. In both studies (the present and by Ghorbani et al.) it has been shown that tissue composition has an effect on dose distribution and should be incorporated in treatment planning. The amount of dose difference varies which can be related to the type of the sources and energies in the listed studies.
Among the soft tissues and tissue-equivalent materials presented in Table 2, adipose tissue and plexiglass demonstrate the greatest differences for total dose rate compared to 9-component soft tissue. The least total dose rate difference is related to the blood, since the relative total dose rate in the blood is very close to 1.000 for most distances. This effect can be related to the compositions and neutron cross-sections for these media.
As shown from the data in Table 2, among the soft tissues, relative total dose rate in adipose tissue generally decreases with increasing distance from the source. Furthermore, relative total dose rates in the tissue-equivalent materials evaluated generally decreased with distance from the source. This effect can be related to the phenomenon that the interaction of neutrons with tissues before a point in the phantom affects the neutron spectrum and the change in the neutron spectrum will result in change to the relative dose at farther points. The reduction in the intensity of the source's particles due to phantom attenuation can be the other reason for this effect.
Relative neutron dose rate varies with radial distance in the studied media (Table 3). Among the studied soft tissues, relative neutron dose rate in 4-component soft tissue increases with distance from the source while this value drops with distance from the source in the brain and adipose tissues. In blood and lung tissue, there is no uniform change in relative neutron dose rate with increasing the distance. Furthermore, 4-component soft tissue, adipose tissue, and A-150 plastic have the highest deviations in relative neutron dose rate among the studied materials. Among studied tissue-equivalent materials, relative neutron dose rate in A-150 and water decreases with increases in distance from the source. Relative neutron dose rate in plexiglass rises initially and then gradually diminishes with distance from the source. These differences between various media can be related to the different compositions, different neutron interactions, and neutron attenuations.
For all studied materials, with increasing distance from the center of the source, total dose rate and neutron dose rate are reduced normally. This decrease is a normal effect which is seen as a steep dose gradient in brachytherapy due to the inverse square law of distance and phantom attenuation.
As shown by a comparison of the relative total dose rate data from Table 2 with the relative neutron dose rate in Table 3, it is evident that the neutron dose rate differs from the total dose rate. This can be also seen from a comparison of the total dose rate data of Table 4 with neutron dose rate data of Table 5. For example, total dose rate in water at 0.3 cm distance is equal to 17.641 (cGy/(h μg)) and neutron dose rate at this distance is 11.161 (cGy/(h μg)). The values indicate that there is a considerable discrepancy between the total dose rate and neutron dose rate. At farther distances, these two dose rates have less absolute discrepancies. By considering the method of calculation of total dose, the remnant of the total dose rate belongs to the primary and secondary photon dose rates. In other words, since total dose means the sum of dose contributions of neutrons, primary photons from 252Cf, and secondary photons induced from neutron interactions, the difference in the total dose rate and neutron dose rate implies that the primary and secondary photon dose components can play a considerable role in the total dose rate and should be taken into account in neutron brachytherapy/neutron capture therapy treatment planning calculations.
The neutron dose rate data for a selection of soft tissues and distances from the present study and by Rivard10 are listed in Table 6. As can be seen from the data in this table, there are some differences between these two data series especially at 0.5 cm and 1.0 cm distances. The results of the present study are in some extent lower than those reported by Rivard. These discrepancies can be due to the differences in the phantom sizes, source geometries, energy cut offs, etc. The phantom in the present study was a sphere with a radius of 20.0 cm, while Rivard used a 15.0 cm diameter spherical phantom. This difference in phantom size can affect neutron dose rates at far distances from the source. It is not reasonable to attribute the differences in Table 6 solely to the phantom size. An example in this regard can be examined in the study by Rivard19 where large variations in the size of phantom had minimal differences in the fast neutron dose at distances close to the source. On the other hand, the differences in Table 6 of the current study may be due to the complete geometry definition of the AT 252Cf source in the current study in comparison to the point source simulated in the study by Rivard.10 Self-absorption inside the source core is not accounted for in point source modeling. Using the geometry function as described in Rivard,10 it appears that the dose rate fall off for a line source is more gradual than for a point source. Quantitatively, the differences in geometry functions between a 1.5 cm line source and a point source at 0.5, 1.0, 2.0, and 5.0 cm are −34.5%, −14.2%, −4.3%, and −0.7%, respectively. Within a few percent, these corrections match those observed in Table 6. Thus, it appears that results from the point source geometry from Rivard study10 can be corrected in this manner. Rationale for applying this adjustment to neutron dose was provided in Rivard et al.20 for a variety of active lengths.
The data from Table 2 demonstrate that the total dose rate in A-150 plastic, plexiglass, and water can, in some cases, have more than 5% difference compared to 9-component soft tissue. Since these materials are used commonly as tissue substitutes in dosimetry, there may be incorporated errors in dosimetric calculations due to the differences in the compositions in these materials. In radiotherapy dosimetry, it is normal to perform the dosimetry in a tissue-equivalent material, especially in water, and to apply the measured data to the human body. These errors are not routinely taken into account in treatment planning calculations in neutron brachytherapy/neutron capture therapy. In report No. 24 by ICRU it was recommended that the dosimetric uncertainty in dose delivery in radiotherapy should be within ±5.0%.21 It is proposed to use correction factors in the use of water in dosimetric systems to consider the differences between the dose in water and other soft tissues in neutron brachytherapy/neutron capture therapy. When one compares the obtained results for these tissue-equivalent materials, it can be evident that the use of water is the more accurate method as a substitute for 9-component soft tissue in dosimetry.
In the present study the effect of tissue composition on dose distribution was assessed in neutron brachytherapy/neutron capture therapy with a 252Cf brachytherapy source. Since beside the material composition, the neutron spectrum can also affect the dose distribution, it can be predicted that the present results cannot be simply extended for neutron therapy/neutron capture therapy with external neutron sources. Therefore, a similar study on various soft tissues and tissue-equivalent materials with external neutron sources can be a subject of further research in this field with the aim of quantification of the dose distribution uncertainties related to taking the same composition for various materials in neutron therapy/neutron capture therapy with external sources. Furthermore, in the present study, when the effect of tissue composition was being examined, boron was not defined in the tissue. It is obvious that the presence of boron will change the neutron spectrum and therefore the dose rate when compared to a simple situation (as defined in the present study). Therefore, the results obtained herein can be estimation for the effect of tissue composition in neutron capture therapy, they cannot be applied quantitatively in neutron capture therapy, since the dose rate depends on the existence and concentration of the capture agent (10B).
The aim of this study was only to express the existence of differences between the dose distributions in different tissues. In terms of treatment planning, the geometry used, taking a homogeneous spherical phantom made of a single tissue, for expression of this effect was simple and unrealistic. In a real situation in clinical patient dosimetry, the body is not a homogeneous medium with a single thickness and it includes various soft tissues, air gaps, and bone with different thicknesses. The thickness, density, and chemical composition of the tissue will affect the dose at each point. Therefore, in treatment planning, estimation of the errors due to taking the same compositions for different tissues, is not easy and may depend on the section and the region of the treatment. As a rule of a thumb, according to the results of this study, the dose differences for various soft tissues (excluding the tissue equivalent materials) compared to 9-composition soft tissue vary between zero and 8.4% (corresponding to adipose tissue at radial distances of 0.3 or 3.0 cm). In the use of the results of this study for clinical purposes, we cannot estimate precise correction factors easily to account for the differences in dose distributions in various soft tissues. However, it is proposed herein that these errors be corrected with the use of Monte Carlo based treatment planning systems in which the computed tomography scans of each patient are incorporated as input data into the treatment planning system in order to obtain the dose distribution. In these treatment planning systems, the mass densities and compositions of various soft tissues should be defined as programs with patient specific implementations. Challenges in achieving this goal will be the costs of high speed processing computers and the running time needed for the plans for each patient.
5. Conclusion
Based on the results of the present study on the effect of composition of soft tissue/tissue-equivalent material on dose in neutron brachytherapy/neutron capture therapy, dose distributions in various soft tissues and tissue-equivalent materials are different. The differences vary with the distance from the source. Therefore, assuming the same composition for various soft tissues in treatment planning systems can cause errors in dose calculations. Since the International Commission on Radiation Units and Measurements (ICRU) recommends that the total dosimetric uncertainty in dose delivery in radiotherapy should be within ±5%, the compositions of various soft tissues and tissue-equivalent materials should be considered in dose calculation and treatment planning in neutron brachytherapy/neutron capture therapy. It is also concluded that the neutron dose rate differs from the total dose rate and this should be taken into account in neutron brachytherapy and NCT with 252Cf source.
Conflict of interest
None declared.
Financial disclosure
Mashhad University of Medical Sciences (MUMS) has financially supported the work.
Acknowledgment
The authors would like to thank Mashhad University of Medical Sciences for financial support of this study.
Contributor Information
Mahdi Ghorbani, Email: mhdghorbani@gmail.com.
Nima Hamzian, Email: nh_mph84@yahoo.com.
References
- 1.Wang Q., Li T., Liu H. The safety and usefulness of neutron brachytherapy and external beam radiation in the treatment of patients with gastroesophageal junction adenocarcinoma with or without chemotherapy. Radiat Oncol. 2014;9:99. doi: 10.1186/1748-717X-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth R.F., Coderre J.A., Vicente M.G. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res. 2005;11(11):3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- 3.Ghassoun J., Chkillou B., Jehouani A. Spatial and spectral characteristics of a compact system neutron beam designed for BNCT facility. Appl Radiat Isot. 2009;67(4):560–564. doi: 10.1016/j.apradiso.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Voyant C., Roustit R., Tatje J. Therapeutic potential of atmospheric neutrons. Rep Pract Oncol Radiother. 2010;16(1):21–31. doi: 10.1016/j.rpor.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IAEA . International Atomic Energy Agency, IAEA-TECDOC-1223; Vienna, Austria: 2001. Current status of neutron capture therapy. [Google Scholar]
- 6.Abujamra A.L. Diagnostic techniques and surgical management of brain tumors. In: Deng L., Chen C., Ye T., Li G., editors. The dosimetry calculation for boron neutron capture therapy. In Tech; 2011. [Google Scholar]
- 7.Beach J.L., Schroy C.B., Ashtari M. Boron neutron capture enhancement of 252Cf brachytherapy. Int J Radiat Oncol Biol Phys. 1990;18(6):1421–1427. doi: 10.1016/0360-3016(90)90317-d. [DOI] [PubMed] [Google Scholar]
- 8.Ghassoun J., Mostacci D., Molinari V. Detailed dose distribution prediction of Cf-252 brachytherapy source with boron loading dose enhancement. Appl Radiat Isot. 2010;68(2):265–270. doi: 10.1016/j.apradiso.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Rivard M.J., Zamenhof R.G. Moderated 252Cf neutron energy spectra in brain tissue and calculated boron neutron capture dose. Appl Radiat Isot. 2004;61(5):753–757. doi: 10.1016/j.apradiso.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Rivard M.J. Dosimetry for 252Cf neutron emitting brachytherapy sources: protocol, measurements, and calculations. Med Phys. 1999;26(8):1503–1514. doi: 10.1118/1.598646. [DOI] [PubMed] [Google Scholar]
- 11.Rivard M.J., Wierzbicki J.G., Chuba P.C. The status of low dose rate and future of high dose rate Cf-252 brachytherapy. In: Ingersoll D.T., editor. American Nuclear Society Radiation Protection and Shielding Division Topical Conference Proceedings: Technologies for the New Century, Vol. 2. ANS; La Grange Park, IL: 1998. pp. 253–260. [Google Scholar]
- 12.Pelowitz D. Los Alamos National Laboratory; 2008. MCNPX users manual, LA-CP-07-1473 Version 2.6.0. [Google Scholar]
- 13.Khosroabadi M., Ghorbani M., Rahmani F. Neutron capture therapy: a comparison between dose enhancement of various agents, nanoparticles and chemotherapy drugs. Australas Phys Eng Sci Med. 2014;37(3):541–549. doi: 10.1007/s13246-014-0284-7. [DOI] [PubMed] [Google Scholar]
- 14.ICRU . ICRU; Bethesda, MD: 1989. ICRU Report No. 44. Tissue substitutes in radiation dosimetry and measurement. [Google Scholar]
- 15.Rosman K.J.R., Taylor P.D.P. Atomic weights of the elements 1997. Pure Appl Chem. 1999;71:1593–1607. [Google Scholar]
- 16.Fantidis J.G., Potolias C., Vordos N. Optimization study of a transportable neutron radiography system based on a 252Cf neutron source. Moldavian J Phys Sci. 2011;10(1):121–130. [Google Scholar]
- 17.Martin R.C., Miller J.H. Applications of californium-252 neutron sources in medicine, research, and industry. Americas Nuclear Energy Symposium (ANES 2002); Miami, FL, October 16–18; 2002. [Google Scholar]
- 18.Ghorbani M., Salahshour F., Haghparast A. Effect of tissue composition on dose distribution in brachytherapy with various photon emitting sources. J Contemp Brachyther. 2014;6(1):54–67. doi: 10.5114/jcb.2014.42024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivard M.J. Neutron dosimetry for a general 252Cf brachytherapy source. Med Phys. 2000;27(12):2803–2815. doi: 10.1118/1.1326445. [DOI] [PubMed] [Google Scholar]
- 20.Rivard M.J., Wierzbicki J.G., Van den Heuvel F. Clinical brachytherapy with neutron emitting 252Cf sources and adherence to AAPM TG-43 dosimetry protocol. Med Phys. 1999;26(1):87–96. doi: 10.1118/1.598472. [DOI] [PubMed] [Google Scholar]
- 21.International Commission on Radiation Units and Measurements (ICRU); Washington: 1976. ICRU Report No. 24. Determination of absorbed dose in a patient irradiated by beams of X or gamma rays in radiotherapy procedures. [Google Scholar]