Abstract
Aim
To evaluate the unintentional coverage of the internal mammary chain (IMC) with tangential fields irradiation to the breast, and its relation with the type of surgery employed.
Background
The dose distribution in regions adjacent to the treatment targets (mammary gland or chest wall), with incidental irradiation of the IMC, could translate into clinical benefit, due to the proximity of these regions.
Materials and methods
One hundred and twelve consecutive conformal radiotherapy plans were correlating the average dose to the IMC with the type of surgery employed, the extent of disease and the irradiation techniques.
Results
The mean doses to IMC after modified radical mastectomy (MRM), modified radical mastectomy with immediate reconstruction (MRM + R), and breast conservative surgery (BCS) were 30.34 Gy, 30.26 Gy, and 18.67 Gy, respectively. Significant differences were identified between patients who underwent MRM or MRM + R over BCS (p = 0.01 and 0.003, respectively), but not between MRM and MRM + R (p = 0.88). Mean doses to IMC were greater in patients with T3–T4 tumors when compared with more initial stages (≤T2) (p = 0.0096). The lymph node involvement also correlated with higher average doses to IMC (node positive: 26.1 Gy × node negative: 17.8 Gy, p = 0.0017).
Conclusions
The moderate dose level to the IMC in the unintentional irradiation scenario seems to be insufficient to treat the subclinical disease, although it could have an impact in patients undergoing mastectomy.
Keywords: Breast cancer, Internal mammary chain, Radiotherapy, Unintentional irradiation, 3DCRT
1. Background
The internal mammary chain (IMC) is an important route of lymphatic drainage of the breast gland, especially from the medial quadrants, constituting a probable route of tumoral dissemination.1, 2 Radiotherapy can control or eliminate cancer cells in this region, classically through the intentional inclusion of the IMC in the treatment fields.3
However, irradiation of the IMC is still a matter of much debate,3, 4 because even with a high incidence of pathological involvement of this nodal region,5 there are low rates of recurrence at this region even when not irradiated.6 While the results of most prospective randomized trials that assess the benefits of IMC irradiation have not yet been published (NCT00005957, NCT00002851), therapeutic decisions are guided by one prospective study,7 some retrospective series evaluating both oncological outcomes and clinical toxicity,6, 8, 9 or even dosimetric studies.10 The controversy regarding its potential benefit leads to differences in the profile of the IMC irradiation indication in different geographical regions worldwide,11, 12 with arguments both for and against intentional inclusion of IMC in the irradiation fields.13
A rationale for the exclusion of IMC from the irradiation fields is related to the dose distribution in regions adjacent to the treatment targets (mammary gland or chest wall), with incidental irradiation of the IMC, which could translate into clinical benefit, due to the proximity of these regions.14, 15
This study aims to estimate the degree of unintended irradiation of the IMC and the profile of patients receiving greater spatial and dosimetric IMC coverage, correlating with the type of surgery employed, the extent of disease and the irradiation technique, in a series of conformal radiotherapy (3D-CRT) plans for breast cancer.
2. Materials and methods
In the period of January–September 2013, 120 consecutive patients underwent adjuvant radiotherapy for breast malignancy in the department of radiation oncology. Among these, 112 had their CT simulation (CT-sim) selected for dosimetric evaluation. Eight were excluded from the study for the following reasons: hypofractionation (5 patients), bilateral chest wall irradiation (1 patient), and re-irradiation for chest wall recurrence (2 patients). Among these, the majority was treated with breast conservative surgery without prosthesis (BCS) and had Tis, T1 or T2 pathological stage (Table 1). Three other patients with previously esthetic mammary prosthesis had conservative surgery (BCS + P). Twenty-six percent of the cases were treated with modified radical mastectomy, with or without immediate reconstruction (MRM and MRM + R, respectively).
Table 1.
Patients and treatment characteristics.
| n | % | |
|---|---|---|
| Stage | ||
| Tis | 13 | 11.6 |
| T1 | 56 | 50 |
| T2 | 29 | 25.9 |
| T3 | 4 | 3.6 |
| T4 | 8 | 7.1 |
| Tx | 2 | 1.8 |
| N0 | 67 | 59.8 |
| N1 | 29 | 25.9 |
| N2 | 8 | 7.1 |
| N3 | 7 | 6.2 |
| Nx | 1 | 1 |
| Surgery | ||
| BCSa | 83 | 74.1 |
| MRMb | 15 | 13.4 |
| MRM + Rc | 14 | 12.5 |
| Radiotherapy | ||
| Breast | 12 | 10.7 |
| Breast + boost | 64 | 57.1 |
| Breast + SCFd | 1 | 1 |
| Chest Wall | 6 | 5.4 |
| Reconstruction | 8 | 7 |
| Breast + boost + SCFd | 6 | 5.4 |
| Chest Wall + SCFd | 9 | 8 |
| Reconstruction + SCFd | 6 | 5.4 |
BCS: breast conserving surgery.
MRM: modified radical mastectomy.
MRM + R: modified radical mastectomy plus immediate reconstruction
SCF: supraclavicular fossa.
All CT-sims were performed with venous contrast and 5 mm thick sections. The patients were immobilized with the use of own personal devices, Vac-fix™ (Par Scientific A/S [Odense, Denmark]).
The prescription doses are 45 Gy and 50.4 Gy to the breast and chest wall, respectively, delivered over 5 weeks, with tangential fields (two opposite parallel with up to two sub-fields). The dose of 45 Gy was used to the supraclavicular fossa (SCF) region, over 5 weeks, according to N stage, with a single anterior field (or combined anterior and posterior fields). The boost to the tumor cavity, when indicated, was performed with two tangential fields with dose of 10 Gy, in 1 week.
The clinical target volume (CTV) of the breast and SCF was delineated based on the Radiation Therapy Oncology Group (RTOG) consensus guideline (online at: http://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx). The planning target volume (PTV) margin was 5 mm to the CTV. The contouring of the IMC was performed by the same physician in all cases and followed the topography of the internal thoracic vessels and included the extension from the first to third intercostal space ipsilateral to the affected breast, due to the increased probability of secondary involvement in this location.1 An evaluation volume was also created, to consider the chest wall movement (PTV IMC), and consisted of an expansion of 5 mm from IMC (Fig. 1). All cases were planned using the Eclipse™ v8.6.23 system (Varian [Palo Alto, USA]) for a linear accelerator with energy of 6 MV photons and multi-leaf collimator.
Fig. 1.
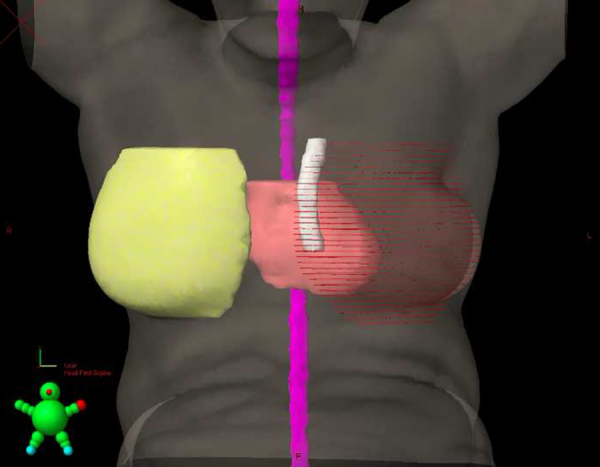
3D reconstruction of PTV IMC. White: IMC PTV.
It is expected that the small target (IMC) changes the position inside the PTV IMC during the respiratory movements, being exposed to regions of different dose levels during the treatment time. For this reason, the coverage of IMC was assessed as of the mean doses (Dmean) and dose ranges (Dmax–Dmin) in PTV IMC (represented in boxplot chart). Most of the irradiated volume with the isodose of reference (IR) is located in the breast or chest wall. Thus, it is not possible to apply the concept of Conformity Index,16 since the IR has a large volume outside of the unintended target (IMC). We present the dose–volume data for quality analysis of the unintended treatment. The geographic location of the PTV IMC was correlated with the PTV, being classified as: (a) inside, (b) partially in, and (c) outside the treatment field, based on the visualization of beam's eye view of the internal field.
The statistical significance of the median Dmean between groups was compared using the Mann–Whitney–Wilcoxon test (R software). A p value < 0.05 was considered significant. A comparison was made in the early tumor stages (T1 and T2/N0) group and the advanced tumor stages (T3 and T4/N+). The influence of the addition of the boost in breast and nodal irradiation in the region of the SCF was evaluated in patients undergoing BCS and modified radical mastectomy.
3. Results
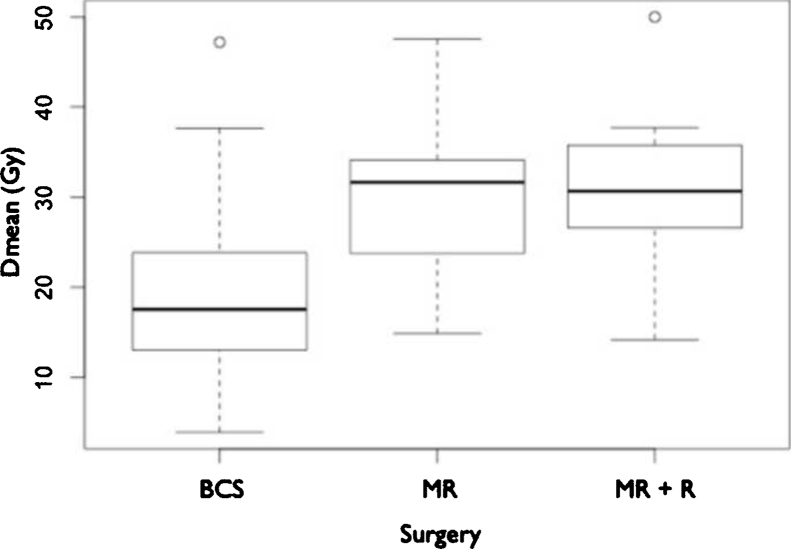
According to the surgery employed, the mean doses to IMC in patients undergoing MRM, MRM + R and BCS were 30.34 Gy (47.54–14.88), 30.26 Gy (50–14.2) and 18.51 Gy (47.19–3.92), respectively (Fig. 2). Analysis of the patients in each group and the dose delivered to the IMC showed a greater coverage of IMC in the MRM group compared with BCS (p < 0.001) and MRM + R compared with BCS (p < 0.001). However, there was no significant difference (p = 0.9486) between MRM and MRM + R (Table 2, Table 3).
Fig. 2.
Boxplot: mean dose (Gy) per surgery group. Dark line = median dose. BCS: breast conserving surgery. MRM: modified radical mastectomy. MRM + R: modified radical mastectomy plus immediate reconstruction.
Table 2.
Comparisons of the average dose in IMC among the study subgroups.
| p | |
|---|---|
| Surgery | |
| BCSa vs. MRMb | <0.001 |
| BCSa vs. MRM + Rc | <0.001 |
| MRMb vs. MRM + Rc | 0.9486 |
| Radiotherapy | |
| with SCFd vs. without SCFd | 0.3766 |
| with boost vs. without boost | 0.889 |
| Disease stage | |
| T1–T2 vs. T3–T4 | 0.0096 |
| N0 vs. N+ | 0.0017 |
BCS: breast conserving surgery.
MRM: modified radical mastectomy.
MRM + R: modified radical mastectomy plus immediate reconstruction.
SCF: supraclavicular fossa.
Table 3.
Dose/volume analysis (average Dmean).
BCS: breast conserving surgery.
MRM: modified radical mastectomy.
MRM + R: modified radical mastectomy plus immediate reconstruction.
Most cases (79.5%) had at least a partial inclusion of the PTV IMC in the treatment volume. The median of Dmean was 9.97 Gy when it was out of the field (n = 23), while in the case of a partial overlap (n = 83) the average dose was 23.13 Gy. In patients with IMC completely within the radiation field, the Dmean was 41.52 Gy (n = 6).
Separating the patients according to stage groups of early disease (Tis, T1–T2 or N0) and advanced disease (T3–T4 or N+), Dmean was also higher in patients with stages T3–T4, when compared with patients staged as Tis, T1–T2 (29.3 Gy vs. 19.9 Gy, p = 0.0096). There is also a difference between the stage groups regarding lymph nodes metastases when comparing positive and negative lymph node disease (26.1 Gy vs. 17.8 Gy, p = 0.0017). There was no significant increase in the mean dose of IMC due to the addition of the FSC field in cases of modified radical mastectomy (31.54 Gy vs. 29.15 Gy, respectively, p = 0.3766). The addition of a boost dose to the cavity also did not increase the Dmean of IMC significantly in the BCS group (18.17 Gy with boost vs. 18.57 Gy without boost, p = 0.889) (Table 2).
4. Discussion
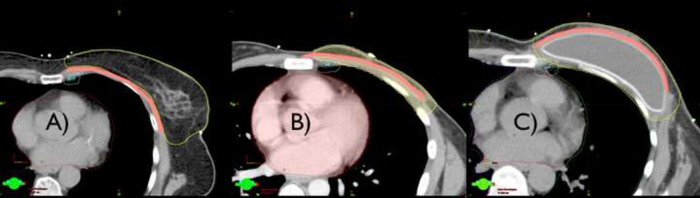
In this study, we observed significant differences in IMC coverage that can be credited to the anatomic changes resulting from the type of surgery used. Patients treated with MRM, with or without immediate reconstruction, had a Dmean 60% higher at the IMC as compared with those undergoing BCS (18.51 Gy × 30.30 Gy). However, in mastectomy patients immediate reconstruction did not affect the coverage of the IMC, compared to mastectomy without immediate reconstruction. Although counterintuitive, this fact could be explained by the placement of the breast prosthesis behind the pectoralis major muscle, deepening the posterior border of the CTV breast and chest wall, (even without its involvement) (Fig. 3). The proximity of the CTV region to the chest wall, and consequently to the IMC, in combination with the geometric distribution of the tangential fields of radiotherapy results in greater unintended IMC coverage.
Fig. 3.
Delineation of three cases of post-operative radiotherapy: (a) breast conserving surgery, (b) modified radical mastectomy, and (c) modified radical mastectomy plus immediate reconstruction. Structures: yellow: CTV breast, blue: IMC, white: PTV IMC, pink: pectoralis major muscle.
A previous similar study14 selected patients with a preponderance draining lymph node for IMC, detected in lymphoscintigraphy, to assess the unintentional coverage of the treatment with conventional breast tangential fields. The authors verified an at least partial coverage in 73%, and total coverage in 18% of the cases suggesting further that the unintentional inclusion of IMC was also inversely proportional to the thickness of parasternal tissue. In our study, we observed a similar rate of at least partial coverage in 76%, and total coverage in 4% of the cases.
The relevance of unintended irradiation of IMC can be highlighted by the historical context of surgical treatment and adjuvant breast cancer irradiation. Those in favor to the IMC irradiation usually refer to the post-mastectomy radiation therapy (PMRT) studies, where the inclusion of this region in the treatment fields was a common practice. The results of these studies are compiled in a meta-analysis that showed a long-term overall survival (OS) benefit.17 In addition, even after 20 years follow-up, the survival benefit was still significant, despite the observed excess of deaths unrelated to breast cancer (probably related to treatment with extended fields and obsolete radiation techniques). As regional irradiation usually included axilla, SCF and IMC, at PMRT studies, it is not possible to separate the individual contribution from each coverage site. The opponents of IMC irradiation, on the other hand, allude to studies comparing radical mastectomy with or without resection of IMC, in which no survival benefit was observed.18, 19
Another important issue regarding IMC irradiation is its toxicity profile, particularly concerning the heart, lung, and skin. Long-term heart and lung toxicity may diminish the OS benefit of adjuvant treatment, due to an excess of deaths related to therapy and not related to cancer. Skin toxicity,20 however, has no impact on survival but has an influence on cosmesis, especially at the areas of potential field overlapping. There is conflicting data in the literature regarding the adverse effects of IMC irradiation21, 22, 23 and several techniques were developed, such as the partially extended tangent fields10 and irradiation during inspiration,24 in the pursuit of a more suitable therapeutic profile, with higher IMC coverage and decreased irradiation of adjacent organs.
To our knowledge, currently, there are four important studies evaluating the potential benefits of IMC irradiation. The first of them, a recently published randomized prospective study, initially showed no survival benefit with the addition IMC irradiation. However, the study has not enough statistical power to detect differences smaller than 10%.7 The others, still unpublished, evaluate the inclusion of IMC and medial supraclavicular chain in the treatment fields (EORTC 22922/10925), assess the impact of regional irradiation (supraclavicular, axillary and IMC) on early invasive breast cancer (NCIC MA.20), and randomize patients to irradiate or not the IMN (KROG-0806).
In the scenario of the lack of irrefutable evidence to justify the elective irradiation of the IMC, the hypothesis that the unintended IMC irradiation might be enough to control subclinical disease in this region becomes attractive. Although the mean dose of approximately 30 Gy in IMC is below the dose range of 45–50 Gy, usually recommended for adjuvant treatment of breast cancer, the use of more effective chemotherapy regimens, nowadays, may eventually render unnecessary higher doses of radiation to obtain adequate control of subclinical disease.25 Similarly, in non small cell lung cancer (NSCLC) treatment, many authors suggest that unintentional irradiation of the clinically non-affected mediastinum generally provides satisfactory control of subclinical disease, with average doses lower than 40 Gy and with less local toxicity.26, 27 However, patients with malignant neoplasms of the lung have a high rate of local failure and reduced survival, which can underreport a nodal failure.
Despite the lack of clinical evidence, a mechanism likely to justify the benefit of low dose irradiation to the IMC would be the local anatomical and functional changes of the lymph nodes in this region, altering the lymphatic architecture and possibly hampering the regional spread of neoplastic cells.28 This effect, for example, would not diminish the action of chemotherapy in this region, since systemic treatment tends to be offered to the patients prior to radiotherapy.29
On the assumption that the IMC incidental irradiation has a therapeutic impact, an interesting note is that precisely the group that received the higher doses to the IMC comprises mastectomy patients, regardless of whether an immediate reconstruction was performed or not. These patients usually have more advanced disease, which might translate into a higher risk of relapse at the IMC,30 and would so benefit from this approach. On the other hand, patients with multiple positive lymph nodes and undergoing conservative surgery, although with significant risk of regional recurrence, would be receiving reduced unintended radiation doses in the IMC, requiring, therefore, a regional approach with more extensive irradiation.
A basic limitation for dosimetric evaluation in this study is that, currently, there is no specific tool to evaluate the quality of unintended irradiation. Another challenge is to analyze the dose in a small structure close to the treatment volume, especially with the respiratory movements, if no respiratory controlled treatment is used. Even the Dmean analysis can be unrealistic in a specific patient because, in fact, the movements are not geometrically symmetric.
Determining the clinical significance of the unintentional IMC coverage and its influence on disease control or survival, especially in conjunction with the use of chemotherapy, is beyond the scope of this study. A clinical study with a larger number of patients and that addresses this issue directly would help in understanding the real benefit of the unintended irradiation of IMC, and may even explain the absence of clinical benefit observed in previous studies that evaluated the intentional irradiation of this region.7
5. Conclusion
Irradiation with tangential fields is associated with high rates of coverage (80%), at least partially of the IMC, with moderate mean doses (30 Gy) in a specific group of patients. These high rates occur predominantly in patients undergoing MRM and MRM + R, compared to patients undergoing BCS. The hypothesis that the incidental irradiation of the IMC is beneficial in controlling localized disease should be tested in clinical studies, although our data suggests that the dose is insufficient to treat subclinical disease.
Conflict of interest
None declared.
Financial disclosure
None declared.
Footnotes
This study was presented in poster format at the congress of ESTRO 33 in Vienna, Austria (EP-1213 – Radiotherapy and Oncology Vol. 111, Supplement 1, April 2014 – ISSN 0167 8140).
References
- 1.Stibbe E.P. The internal mammary lymphatic glands. J Anat. 1918;LII:257–264. [PMC free article] [PubMed] [Google Scholar]
- 2.Kong A.L., Tereffe W., Hunt K.K. Impact of internal mammary lymph node drainage identified by preoperative lymphoscintigraphy on outcomes in patients with stage I to III breast cancer. Cancer. 2012;118:6287–6296. doi: 10.1002/cncr.27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson R.A., Woods R., Speers C. Does the intent to irradiate the internal mammary nodes impact survival in women with breast cancer? A population-based analysis in British Columbia. Int J Radiat Oncol Biol Phys. 2012;83(1):e35–e41. doi: 10.1016/j.ijrobp.2011.11.066. [DOI] [PubMed] [Google Scholar]
- 4.Freedman G.M., Fowble B.L., Nicolaou N. Should internal mammary lymph nodes in breast cancer be a target for the radiation oncologist? Int J Radiat Oncol Biol Phys. 2000;46(4):805–814. doi: 10.1016/s0360-3016(99)00481-2. [DOI] [PubMed] [Google Scholar]
- 5.Handley R.S. Carcinoma of the breast. Ann R Coll Surg Engl. 1975;57:59–66. [PMC free article] [PubMed] [Google Scholar]
- 6.Fowble B., Hanlon A., Freedman D. Internal mammary node irradiation neither decreases distant metastases nor improves survival in stage I and II breast cancer. Int J Radiat Oncol Biol Phys. 2000;47(4):883–894. doi: 10.1016/s0360-3016(00)00526-5. [DOI] [PubMed] [Google Scholar]
- 7.Hennequin C., Bossard N., Servagi-Vernat S. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2013;86(5):860–866. doi: 10.1016/j.ijrobp.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Chang J.S., Park W., Kim Y.B. Long-term survival outcomes following internal mammary node irradiation in stage II–III breast cancer: results of a large retrospective study with 12-year follow-up. Int J Radiat Oncol Biol Phys. 2013;86(5):867–872. doi: 10.1016/j.ijrobp.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Obedian E., Haffty B.G. Internal mammary nodal irradiation in conservatively-managed breast cancer patients: is there a benefit? Int J Radiat Oncol Biol Phys. 1999;44(5):997–1003. doi: 10.1016/s0360-3016(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 10.Marks L.B., Hebert M.E., Bentel G. To treat or not to treat the internal mammary nodes: a possible compromise. Int J Radiat Oncol Biol Phys. 1994;29(4):903–909. doi: 10.1016/0360-3016(94)90584-3. [DOI] [PubMed] [Google Scholar]
- 11.Ceilley E., Jagsi R., Goldberg S. Radiotherapy for invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys. 2005;61(2):365–373. doi: 10.1016/j.ijrobp.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 12.Hui Z., Li Y., Yu Z., Liao Z. Survey on use of postmastectomy radiotherapy for breast cancer in China. Int J Radiat Oncol Biol Phys. 2006;66(4):1135–1142. doi: 10.1016/j.ijrobp.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 13.Chen R.C., Lin N.U., Harris J.R. Internal mammary nodes in breast cancer: diagnosis and implications for patient management – a systematic review. J Clin Oncol. 2008;26(30):4981–4989. doi: 10.1200/JCO.2008.17.4862. [DOI] [PubMed] [Google Scholar]
- 14.Proulx G.M., Lee J., Stomper P.C. Internal mammary lymph node inclusion in standard tangent breast fields: effects of body habitus. Breast J. 2001;7(2):111–116. doi: 10.1046/j.1524-4741.2001.007002111.x. [DOI] [PubMed] [Google Scholar]
- 15.Hare G.B., Proulx G.M., Lamonica D.M. Internal mammary lymph node (IMN) coverage by standard radiation tangent fields in patients showing IMN drainage on lymphoscintigraphy – therapeutic implications. Am J Clin Oncol. 2004;27:274–278. doi: 10.1097/01.coc.0000092596.03967.80. [DOI] [PubMed] [Google Scholar]
- 16.Feuvret L., Noël G., Mazeron J.J. Conformity index: a review. Int J Radiat Oncol Biol Phys. 2006;64(2):333–342. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials – Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 18.Lacour J., Le M.G., Hill C. Is it useful to remove internal mammary nodes in operable breast cancer? Eur J Surg Oncol. 1987;13(4):309–314. [PubMed] [Google Scholar]
- 19.Veronesi U., Marubini E., Mariani L. The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30-year results of a randomised trial. Eur J Cancer. 1999;35(9):1320–1325. doi: 10.1016/s0959-8049(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 20.Kugnt T., Richter C., Enke H. Acute radiation reaction and local control in breast cancer patients treated with postmastectomy radiotherapy. Strahlenther Onkon. 1998;174(5):257–261. doi: 10.1007/BF03038718. [DOI] [PubMed] [Google Scholar]
- 21.Matzinger O., Heimsoth I., Poortmans P. Toxicity at three years with or without irradiation of the internal mammary and medial supraclavicular lymph node chain in stage I to III breast cancer (EORTC trial 22922/10925) Acta Oncol. 2010;49:24–34. doi: 10.3109/02841860903352959. [DOI] [PubMed] [Google Scholar]
- 22.Kaija H., Maunu P. Tangential breast irradiation with or without internal mammary chain irradiation: results of a randomized trial. Radiother Oncol. 1995;36(3):172–176. doi: 10.1016/0167-8140(95)01607-i. [DOI] [PubMed] [Google Scholar]
- 23.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 24.Hjelstuen M.H.B., Mjaaland I., Vikström J. Radiation during deep inspiration allows loco-regional treatment of left breast and axillary-, supraclavicular-, and internal mammary lymph nodes without compromising target coverage or dose restrictions to organ at risk. Acta Oncol. 2012;51:333–344. doi: 10.3109/0284186X.2011.618510. [DOI] [PubMed] [Google Scholar]
- 25.Bouganim N., Tsvetkova E., Clemons M. Evolution of sites of recurrence after early breast cancer over the last 20 years: implications for patient care and future research. Breast Cancer Res Treat. 2013;139(2):603–606. doi: 10.1007/s10549-013-2561-7. [DOI] [PubMed] [Google Scholar]
- 26.Rosenzweig K.E., Sim S.E., Mychalczak B. Elective nodal irradiation in the treatment of non-small-cell lung cancer with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50(3):681–685. doi: 10.1016/s0360-3016(01)01482-1. [DOI] [PubMed] [Google Scholar]
- 27.Chen M., Hayman J.A., Ten Haken R.K. Long-term results of high-dose conformal radiotherapy for patients with medically inoperable T1-3N0 non-small-cell lung cancer: is low incidence of regional failure due to incidental nodal irradiation? Int J Radiat Oncol Biol Phys. 2006;64(1):120–126. doi: 10.1016/j.ijrobp.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Avraham T., Yan A., Zampell J.C. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-β1-mediated tissue fibrosis. Am J Physiol Cell Physiol. 2010;299(3):C589–C605. doi: 10.1152/ajpcell.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recht A., Come S.E., Henderson I.C. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med. 1996;334(21):1356–1361. doi: 10.1056/NEJM199605233342102. [DOI] [PubMed] [Google Scholar]
- 30.Huang O., Wang L., Shen K. Breast cancer sub population with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008;107:379–387. doi: 10.1007/s10549-007-9561-4. [DOI] [PubMed] [Google Scholar]