Abstract
Aim
The aim of this study is to evaluate performance of ArcCHECK diode array detector for the volumetric modulated arc therapy (VMAT) patient specific quality assurance (QA). VMAT patient specific QA results were correlated with ion chamber measurement. Dose response of the ArcCHECK detector was studied.
Background
VMAT delivery technique improves the dose distribution. It is complex in nature and requires proper QA before its clinical implementation. ArcCHECK is a novel three dimensional dosimetry system.
Materials and methods
Twelve retrospective VMAT plans were calculated on ArcCHECK phantom. Point dose and dose map were measured simultaneously with ion chamber (IC-15) and ArcCHECK diode array detector, respectively. These measurements were compared with their respective TPS calculated values.
Results
The ion chamber measurements are in good agreement with TPS calculated doses. Mean difference between them is 0.50% with standard deviation of 0.51%. Concordance correlation coefficient (CCC) obtained for ion chamber measurements is 0.9996. These results demonstrate a strong correlation between the absolute dose predicted by our TPS and the measured dose. The CCC between ArcCHECK doses and TPS predictions on the CAX was found to be 0.9978. In gamma analysis of dose map, the mean passing rate was 98.53% for 3% dose difference and 3 mm distance to agreement.
Conclusions
The VMAT patient specific QA with an ion chamber and ArcCHECK phantom are consistent with the TPS calculated dose. Statistically good agreement was observed between ArcCHECK measured and TPS calculated. Hence, it can be used for routine VMAT QA.
Keywords: Quality assurance, VMAT, Diode array, ArcCHECK, Gamma
1. Background
Intensity modulation in radiation beam improves the treatment plan quality in terms of tumor control probability (TCP) and normal tissue complication probability (NTCP). Intensity modulation can be achieved by fixed gantry delivery as well as arc based delivery.1 Yu2 proposed intensity modulated arc therapy (VMAT) with dynamic MLC as an alternative to tomotherapy in 1995. Although the concept of VMAT was developed much earlier, it took more time for clinical implementation. This is because of limited scientific work, lack of appropriate and efficient planning algorithm, delivery technique and weak commercial interest. Several researchers developed different techniques to realize single arc VMAT.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Karl Otto developed progressive resolution optimization (PRO) for single VMAT. The clinical applications and comparisons between existing delivery techniques of VMAT were studied and published in various research works.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Stringent quality assurance (QA) is required for assessment of any new equipment and technique before its clinical implementation. VMAT delivery is complex in nature, it involved simultaneous changes in gantry speed, multileaf collimator speed and dose rate. VMAT patient specific QA is important to assess the coordination of these parameters and delivery accuracy. One of the novel equipment intended for patient specific QA is ArcCHECK phantom. This equipment is specially designed for VMAT delivery. More reliable, robust and simple method of patient specific QA is absolute point dose measurement using an ion chamber. Ion chambers carry the calibration traceable to the primary standard dosimetry laboratory (PSDL). The aim of this study is to evaluate performance of ArcCHECK diode array detector for the volumetric modulated arc therapy (VMAT) patient specific quality assurance (QA).
2. Materials and methods
2.1. ArcCHECK
The ArcCHECK, (Model 1220, Sun Nuclear, Melbourne, FL), is a 3-dimensional beam dosimetry QA system intended for the measurement of radiotherapy dose distributions that are delivered, as defined by a planning system, and compared to the dose distribution, as calculated by the planning system. It is a cylindrical water-equivalent phantom with a three-dimensional array of 1386 diode detectors, arranged in a spiral pattern, with 10 mm sensor spacing. The center of the phantom (15 cm diameter) is designed to accommodate various accessories such as a solid homogeneous core, a dosimetric core with ion chamber(s) or diode arrays, an imaging QA core, a core with heterogeneous materials for dose studies, etc. The ArcCHECK also features two inclinometers to measure the angle of rotation about the cylinder axis and to measure the tilt of the axis. A temperature sensor measures the ambient temperature of the detector area. Dose measurements from each sensor are updated every 50 ms; there is no time limit or dose limit for a measurement (1386 precision diode detectors [size 0.8 × 0.8 mm]) (Fig. 1).
Fig. 1.
ArcCHECK phantom with cavity plug and chamber insert.
2.2. Linear accelerator
The measurements were carried out on a Clinac iX linear accelerator (Varian Medical Systems, Palo Alto, USA), capable of generating 6 MV and 15 MV photon beams. Dose rates available for photon are 100 MU/min to 600 MU/min in steps of 100 MU/min for VMAT delivery the dose rate range 0–600 MU/min (continuous). It is equipped with Millennium 120 MLC with central 40 pairs of 5 mm and peripheral 20 pairs of 10 mm leaf width at the isocentre. By design round tip multi-leaf collimators are at the tertiary level.
2.3. Performance tests
2.3.1. Linearity and reproducibility of dose
Linearity of dose measurement of ArcCHECK was evaluated. Monitor units ranging from 1 MU to 500 MU were delivered to ArcCHECK for 10 cm × 10 cm jaw setting. A range of monitor unit (MU) was chosen to cover the clinical VMAT plans. Phantom response to 100 MU was normalized to calculate linearity of dose. Similar measurement was carried out with an ion chamber in water phantom for comparison. Reproducibility of dose delivered is checked for ArcCHECK. It is evaluated in terms of coefficient of variation (COV). Ten repeated dose measurements were observed for 100 MU and 10 cm × 10 cm jaw setting. Similar measurement was carried out with an ion chamber in water phantom for comparison.
2.3.2. Field size and dose rate dependence
Response of ArcCHECK for various field sizes was evaluated. Field sizes ranging from 3 cm × 3 cm to 25 cm × 25 cm were exposed to a dose of 100 MU, and respective dose measured by ArcCHECK recorded. All readings of ArcCHECK phantom were normalized to the response of 10 cm × 10 cm field size. Similar measurement was carried out with an ion chamber in water phantom for comparison. The dose response of ArcCHECK was measured with different dose rates ranging from 100 MU/min to 600 MU/min. This measurement shows the Dose rate dependence of ArcCHECK. Similar measurement was carried out with an ion chamber in water phantom for comparison.
2.4. VMAT Plan QA
2.4.1. Treatment planning
All the plans in this study were performed in Eclipse treatment planning system (TPS), version 10.0 (Varian Medical Systems, Palo Alto, USA) using 6 MV photon beam. A dose rate of 600 MU/min was opted for all the arcs used, but the final dose rate was decided by the optimization algorithm. All the plans were optimized by a progressive resolution optimizer 3 (PRO3, Second generation).28 In this algorithm full arc is optimized at 178 control points progressively in four phases. At every iteration level it optimizes multi-leaf collimator (MLC) position and monitor unit (MU) weight within limitations (MLC speed, gantry speed, dose rate, and mechanical limits) of the delivery unit.4 During optimization, dose calculation is performed with a simplified multi resolution dose calculation (MRDC) algorithm. In order to mitigate the leakage due to tongue and groove effect, collimator rotated to 30° from the nominal angle. Number of full length arcs used ranged from 1 to 2 and the maximum number of partial ones was 3. Final dose calculations carried out with Analytical Anisotropic Algorithm (AAA). The Dose grid resolution for dose calculation was set to 2 mm. All the plan goals set to have 100 percent prescription dose to 95 percent of the target volume (D95 = 100% Rx dose). Objectives to other normal structures are set to clinically acceptable tolerance dose. Verification plans were created on ArcCHECK phantom using patient plans.
2.4.2. Phantom setup
A specially designed cradle supports the cylindrical ArcCA specially designed cradle supports the cylindrical ArcCHECK phantom. This stand was made up of low atomic number material to avoid beam attenuation. Electronic circuit of the phantom was placed away from the primary beam and in the direction away from the gantry. An inclinometer served to identify and correct the rotation (roll) and tilt (pitch). Coronal and sagittal lasers are matched with their corresponding line on the phantom. Then setup was verified by reading same SSD (∼86.7 cm) on the phantom surface irrespective of the gantry angle.
2.4.3. Measurement
Calibrated CC 13S (IBA Dosimetry, Germany) ion chamber with Dose 1 (IBA Dosimetry, Germany) electrometer was used for absolute point dose measurements. Ion chamber was placed in the central core of the ArcCHECK at the isocenter of the linear accelerator. Diodes are kept at 2.9 cm below the surface of the ArcCHECK; this thickness of the buildup material is equivalent to 3.3 cm of water. Dose maps were acquired at the diode level (89.6 cm). It is measured in cumulative mode for all the fields of the plan. Dose map and absolute point dose are measured simultaneously. This eliminates any minor difference in setup. Ion chamber readings were corrected for temperature and pressure variation.
3. Results
The most robust, reliable and simple technique for patient specific quality assurance is point dose measurement of IMRT plans. Ion chambers are recommended for absolute dose measurement of high energy photon beam. Point dose measurements are usually performed at one or more points in the phantom and compared with the calculation for the same measurement points. Twelve different patient plans were used in this study. Six head and neck plans, one breast plan, two esophagus plans, one abdomen plan and two pelvis plans.
3.1. Performance tests
3.1.1. Linearity and reproducibility of dose
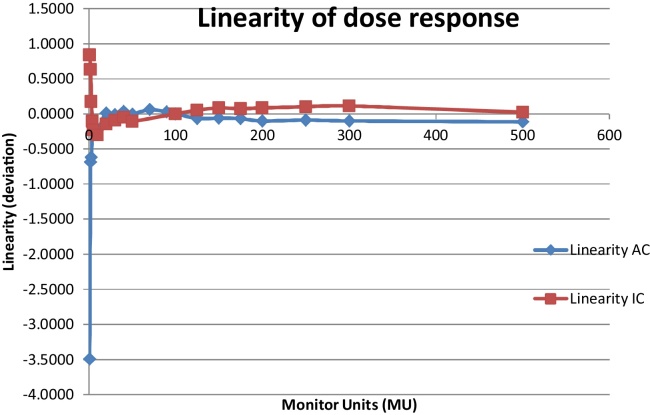
The response of the ArcCHECK as a function of dose delivered (MU) shows that linearity is better than 0.25% for monitor units ≥5 MU. In lower monitor units linearity response is within 0.25–0.68%, except for 1 MU (3.5%). The slope of the trendline along the Arccheck phantom response curve is 0.998 and R2 value is 1.000. The response observed with an ion chamber shows linearity better than 0.25% for monitor units ≥3 MU (Fig. 2). In lower monitor units linearity response is within 0.63–0.84%. The slope of the trendline along the ion chamber response curve is 1.000, R2 value is 1.000. Reproducibility observed for Arccheck phantom is 0.028% and for an ion chamber it is 0.038%.
Fig. 2.
Linearity of dose response for Ion chamber and ArcCHECK phantom.
3.1.2. Field size and dose rate dependence
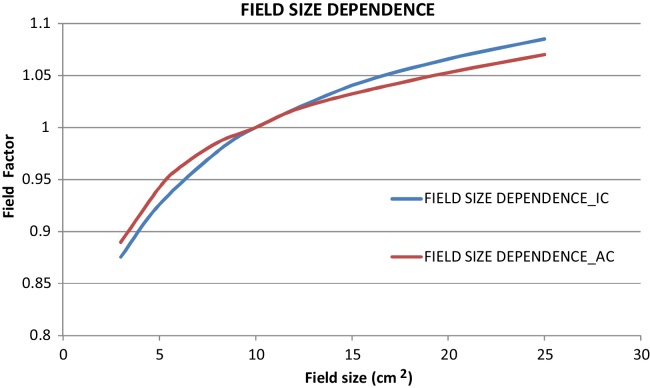
Field factors calculated for the respective measurements from ArcCHECK and an ion chamber. Fig. 3 shows the normalized field factor calculated for ArcCHECK and an ion chamber. For field sizes less than 10 cm × 10 cm, ArcCHECK shows higher response than an ion chamber and vice versa for larger field sizes. However, the deviation in the field factor is not more than 1.6% (SD 1.3%). Dose response of ArcCHECK phantom was identifies at different dose rates measured. These measurements were normalized to the response at 300 MU/min. The dose responses observed for dose rates less than 300 MU/min were less than the response at 300 MU/min and vice versa for the larger dose rates. Maximum deviation observed for 600 MU/min is 0.39%.
Fig. 3.
Field size dependent response of Ion chamber and ArcCHECK.
3.2. VMAT plan QA
3.2.1. Ion chamber results
The dose values at the measurement point of an ion chamber were calculated and collected from Eclipse TPS. Ion chamber measured doses and TPS calculated doses are given in Table 1.
Table 1.
Point dose measured and calculated at the isocenter/ion chamber.
| Patient | Site | Ion chamber (ArcCHECK) (cGy) | TPS (cGy) | Dose diff (cGy) | % Dose diff |
|---|---|---|---|---|---|
| Patient 1 | H & N | 158.63 | 159 | −0.37 | −0.23 |
| Patient 2 | ESOPHAGUS | 182.93 | 184.1 | −1.17 | −0.64 |
| Patient 3 | H & N | 130.37 | 129.7 | 0.67 | 0.52 |
| Patient 4 | H & N | 159.98 | 154.9 | 5.08 | 3.17 |
| Patient 5 | H & N | 110.31 | 109.6 | 0.71 | 0.64 |
| Patient 6 | ABDOMEN | 191.86 | 193.3 | −1.44 | −0.75 |
| Patient 7 | H & N | 145.98 | 145.4 | 0.58 | 0.39 |
| Patient 8 | H & N | 141.32 | 140.9 | 0.42 | 0.3 |
| Patient 9 | PELVIS | 198.87 | 199.6 | −0.73 | −0.37 |
| Patient 10 | BREAST | 158.21 | 159.2 | −0.99 | −0.63 |
| Patient 11 | PELVIS | 173.14 | 174.1 | −0.96 | −0.55 |
| Patient 12 | ESOPHAGUS | 169.66 | 170.4 | −0.74 | −0.44 |
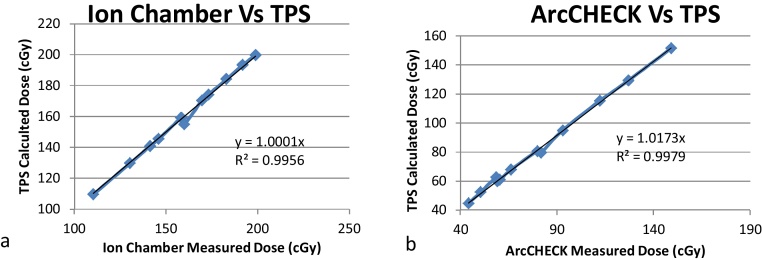
The point dose difference calculated between ArcCHECK measurement and TPS calculation was 0.50 ± 0.51%. This satisfies the indispensable idea of intensity modulated beam QA guidelines.30 Fig. 4a shows the linear relationship between measured and calculated doses and the slope of the trend line is equal to 1. Statistical analysis reveals a strong correlation between the ion chamber measured dose and the TPS predicted dose.
Fig. 4.
(a) Graphical plot of ion chamber measured dose and TPS calculated dose. (b) Graphical plot of ArcCheck dose and TPS calculated dose.
3.2.2. ArcCHECK results
Dose at a point (PAC) where ArcCHECK diode plane intersects with the central axis was compared between TPS calculation and ArcCHECK measurement. This enabled us to make a direct comparison of point dose analogous to ion chamber measurement. No diode was present on the point of measurement (CAX intersection), so mean dose of adjacent diodes was taken as the measured dose. TPS calculated and measured doses are shown in Table 2.
Table 2.
Point dose measured and calculated at the diode plane along the CAX.
| Patient | Site | ArcCHECK (Diode plane) (cGy) | TPS (cGy) | Dose diff (cGy) | % Dose diff |
|---|---|---|---|---|---|
| Patient 1 | H & N | 60.36 | 60.7 | −0.34 | −0.56 |
| Patient 2 | ESOPHAGUS | 79.74 | 80.5 | −0.76 | −0.95 |
| Patient 3 | H & N | 58.42 | 62.51 | −4.09 | −7 |
| Patient 4 | H & N | 50.43 | 52.2 | −1.77 | −3.51 |
| Patient 5 | H & N | 112.08 | 115.2 | −3.12 | −2.78 |
| Patient 6 | ABDOMEN | 92.86 | 94.86 | −2 | −2.15 |
| Patient 7 | H & N | 66.09 | 67.75 | −1.66 | −2.51 |
| Patient 8 | H & N | 44.13 | 44.66 | −0.53 | −1.2 |
| Patient 9 | PELVIS | 149.16 | 151.5 | −2.34 | −1.57 |
| Patient 10 | BREAST | 81.64 | 79.54 | 2.1 | 2.57 |
| Patient 11 | PELVIS | 126.93 | 129.2 | −2.27 | −1.79 |
| Patient 12 | ESOPHAGUS | 59.01 | 59.8 | −0.79 | −1.34 |
The dose difference between ArcCHECK measurement and TPS prediction on the CAX ranged from 0.56% to 3.51%, except for one patient (7.00%). The mean dose difference in the central axis dose is 2.11% and standard deviation is 2.2%. The 95% confidence interval is from 0.9932 to 0.9995. In gamma analysis of dose/fluence map the mean passing rate was 98.53% for 3% dose difference and 3 mm distance to agreement (Table 3).
Table 3.
Gamma analysis results.
| Patient | Site | Gamma (ArcCHECK) (DD 3%, DTA 3 mm) |
|---|---|---|
| Patient 1 | H & N | 99.1 |
| Patient 2 | ESOPHAGUS | 97.8 |
| Patient 3 | H & N | 98 |
| Patient 4 | H & N | 96.1 |
| Patient 5 | H & N | 99.9 |
| Patient 6 | ABDOMEN | 100 |
| Patient 7 | H & N | 98.9 |
| Patient 8 | H & N | 97.2 |
| Patient 9 | PELVIS | 99.3 |
| Patient 10 | BREAST | 99.9 |
| Patient 11 | PELVIS | 100 |
| Patient 12 | ESOPHAGUS | 95.8 |
4. Discussion
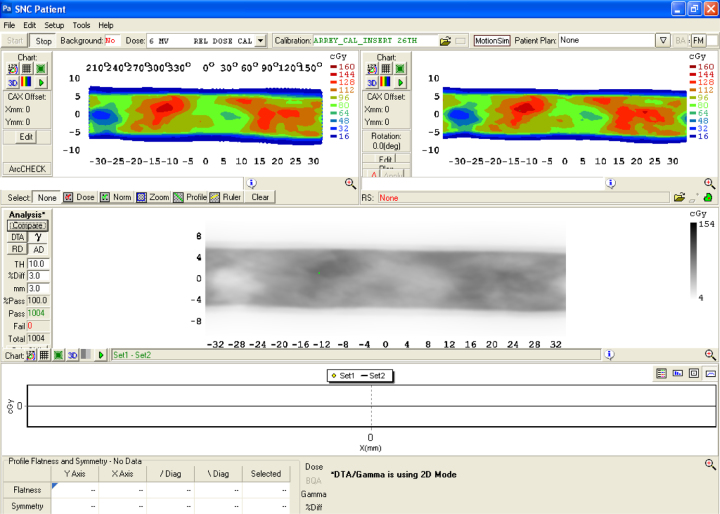
Quality assurance becomes an integral part of complex radiation therapy delivery techniques. Patient specific quality assurance identifies the existence of manual error, mechanical inaccuracy and computerized faults. ArcCHECK phantom is an independent quality assurance tool. The diode detector's response with respect to the gantry angle is corrected using Virtual inclinometer algorithm.31 Comparison was made of the device measured and TPS calculated dose for different patients studied. All the patients had field sizes <20 cm along the y-direction, so that the field fitted within the active area of the ArcCHECK phantom. Also the electronic circuits of ArcCHECK were protected against unwanted radiation damage. This phantom is very much suitable for hybrid plan verification15 where errors undetectable at 0° or any other fixed gantry angle can be detected. Couch attenuation is accounted in the planning process by including couch modeling. Basic requirement of any detector used for hybrid plan verification is that its response should be independent of beam orientation, energy and dose rate. SNC Patient6® (SunNuclear Systems) software applied required correction to the diode response (Background correction, Angular correction, Field size correction). The spiral nature of diode placement virtually increases the measurement resolution (Fig. 5).
Fig. 5.
ArcCHECK measured does map compared with TPS calculated dose map in SNC Patient software (Sun Nuclear, Melbourne, FL).
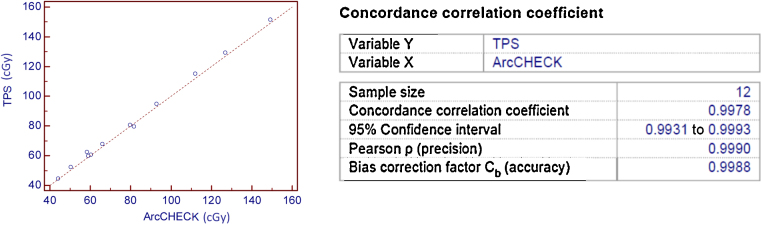
Tests relevant to the performance of the Arccheck phantom yielded a satisfactory outcome. Linearity of dose response of Arccheck phantom is very similar to that of Ion chamber. Monitor units of more than 5 MU show good linearity (within 0.25%). The deviation in linearity is more than 0.25% for lower MU, but all the clinical VMAT plans used in this study had MU in the range of 300–600 MU. Field size dependence test shows a closer agreement between the response of Arccheck phantom and Ion chamber. Absolute output of the linear accelerator was measured with default dose rate of 300 MU/min (Fig. 6). The default dose rate of the machine was used as reference dose rate to evaluate the dose rate dependence of Arccheck phantom. There are a number of QA devices and accessories available for VMAT patient specific QA. Many authors investigated the accuracy and implementation of these devices. Bedford et al.29 evaluated Delta4 phantom (Scandidos, Uppsala, Sweden) for VMAT delivery and observed dose difference within 2.5%. Also, they demonstrated that Delta 4 phantom resulted in higher gamma passing rate than film. The absolute dose measured at the central axis diode plane is within 2.11 ± 2.2% of the TPS calculated dose. Concordance correlation coefficient (CCC) calculated between the ion chamber measured dose and the TPS calculated dose is 0.9996. These results again demonstrate a strong correlation between the absolute dose predicted by our TPS and the measured dose. CCC contains precision and accuracy of the data used. The advantage of using CCC over the Pearson's correlation coefficient (r) (PCC) is that it indicates its components (accuracy and precision) in a single parameter (CCC = accuracy × precision). The calculated accuracy coefficient and precision coefficient of the TPS prediction were 0.9999 and 0.9997, respectively. It means that the precision of the TPS model and calculation was 0.9999, and its accuracy was 0.9997. This method validates the point dose calculation of the TPS software. Dose map and central axis diode dose were compared and evaluated with TPS predicted dose map and dose, respectively. Central axis diode dose measurement shows CCC of 0.9978.
Fig. 6.
TPS calculated dose potted against ArcCHECK measured dose and CCC calculated.
5. Conclusion
This study evaluated the performance of ArcCHECK phantom and it is found to be clinically acceptable. The VMAT patient specific QA measurements with ion chamber and ArcCHECK phantom are consistent with the TPS dose calculation. Statistically good agreement was observed between the ArcCHECK measured dose and the TPS calculated dose. Hence, it can be used for routine VMAT patient specific QA. Also, it is a valid tool for acceptance and commissioning of the VMAT delivery technique.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Mundt A.J., Roeske J.C. BC Decker Inc.; 2005. Intensity modulated radiation therapy: a clinical perspective; pp. 20–22. [Google Scholar]
- 2.Yu C.X. Intensity-modulated arc therapy with dynamic multileaf collimation: an alternative to tomotherapy. Phys Med Biol. 1995;40:1435–1449. doi: 10.1088/0031-9155/40/9/004. [DOI] [PubMed] [Google Scholar]
- 3.MacKenzie M.A., Robinson D.M. Intensity modulated arc deliveries approximated by a large number of fixed gantry position sliding window dynamic multileaf collimator fields. Med Phys. 2002;29:2359–2365. doi: 10.1118/1.1508110. [DOI] [PubMed] [Google Scholar]
- 4.Crooks S.M., Wu X., Takita C., Watzich M., Xing L. Aperture modulated arc therapy. Phys Med Biol. 2003;48:1033–1044. doi: 10.1088/0031-9155/48/10/307. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Z., Shepard D.M., Earl M.A., Zhang G.W., Yu C.X. An examination of the number of required apertures for step-and-shoot IMRT. Phys Med Biol. 2005;50:5653–5663. doi: 10.1088/0031-9155/50/23/017. [DOI] [PubMed] [Google Scholar]
- 6.Cameron C. Sweeping-window arc therapy: an implementation of rotational IMRT with automatic beam-weight calculation. Phys Med Biol. 2005;50:4317–4336. doi: 10.1088/0031-9155/50/18/006. [DOI] [PubMed] [Google Scholar]
- 7.Tang G., Earl M.A., Luan S., Naqvi S.A., Yu C.X. Converting multiple-arc intensity-modulated arc therapy into a single arc for efficient delivery. Int J Radiat Oncol Biol Phys. 2007;69:S673. [Google Scholar]
- 8.Ulrich S., Nill S., Oelfke U. Development of an optimization concept for arc-modulated cone beam therapy. Phys Med Biol. 2007;52:4099–4119. doi: 10.1088/0031-9155/52/14/006. [DOI] [PubMed] [Google Scholar]
- 9.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35(1):310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 10.Luan S., Wang C., Cao D., Chen D.Z., Shepard D.M., Yu C.X. Leaf-sequencing for intensity-modulated arc therapy using graph algorithms. Med Phys. 2008;35:61–69. doi: 10.1118/1.2818731. [DOI] [PubMed] [Google Scholar]
- 11.Wang C., Luan S., Tang G., Chen D.Z., Earl M.A., Yu C.X. Arc-modulated radiation therapy (AMRT): a single-arc form of intensity-modulated arc therapy. Phys Med Biol. 2008;53:6291–6303. doi: 10.1088/0031-9155/53/22/002. [DOI] [PubMed] [Google Scholar]
- 12.Bzdusek K., Friberger H., Eriksson K., Hardemark B., Robinson D., Kaus M. Development and evaluation of an efficient approach to volumetric arc therapy planning. Med Phys. 2009;36:2328–2339. doi: 10.1118/1.3132234. [DOI] [PubMed] [Google Scholar]
- 13.Bignardi M., Cozzi L., Fogliata A. Critical appraisal of volumetric modulated arc therapy in stereotactic body radiation therapy for metastases to abdominal lymph nodes. Int J Radiat Oncol Biol Phys. 2009;75(5):1570–1577. doi: 10.1016/j.ijrobp.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Beatriz E.A., Marco A., Naipy P., Alejandro I., Xiaodong W. Volumetric-modulated arc therapy with RapidArc®: an evaluation of treatment delivery efficiency. Rep Pract Oncol Radiother. 2013;18(6):383–386. doi: 10.1016/j.rpor.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang G., Earl M.A., luan S., Wang C., Mohiuddin M.M., Yu C.X. Comparing radiation treatments using intensity-modulated beams, multiple arcs, and single arcs. Int J Radiat Oncol Biol Phys. 2010;76(5):1554–1562. doi: 10.1016/j.ijrobp.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karthikeyan N., Ganesh K.M., Vikraman S. Influence of segment width on plan quality for volumetric modulated arc based stereotactic body radiotherapy. Rep Pract Oncol Radiother. 2014;19(5):287–295. doi: 10.1016/j.rpor.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cozzi L., Dinshaw K.A., Sshrivastava S.K. A treatment planning study comparing volumetric arc modulation with rapidarc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol. 2008;89:180–191. doi: 10.1016/j.radonc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran P., Milind K., Suja C., Silpa J., Satheesan B., Jim C. Volumetric modulated arc therapy for prostate cancer patients with hip prosthesis. Rep Pract Oncol Radiother. 2013;18(4):209–213. doi: 10.1016/j.rpor.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palma D., Vollans E., James K. Volumetric modulated arc therapy for delivery of prostate radiotherapy. Comparison with intensity modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 20.Duthoy W., De G.W., Vergote K. Clinical implementation of intensity modulated arc therapy (IMAT) for rectal cancer. Int J Radiat Oncol Biol Phys. 2004;60:794–806. doi: 10.1016/j.ijrobp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Syam K., Raghavendra H., Prabakar S., Sriram P., Nagarajan V. Treatment planning and dosimetric comparison study on two different volumetric modulated arc therapy delivery techniques. Rep Pract Oncol Radiother. 2013;18(2):87–94. doi: 10.1016/j.rpor.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao D., Holmes T.W., Afghan M.K., Shepard D.M. Comparison of plan quality provided by intensity-modulated arc therapy and helical tomotherapy. Int J Radiat Oncol Biol Phys. 2007;69:240–250. doi: 10.1016/j.ijrobp.2007.04.073. [DOI] [PubMed] [Google Scholar]
- 23.Fogliata A., Clivio A., Nicolini G., Vanetti E., Cozzi L. Intensity modulation with photons for benign intracranial tumours. A planning comparison of volumetric single arc, helical arc and fixed gantry techniques. Radiother Oncol. 2008;89:254–262. doi: 10.1016/j.radonc.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Matuszak M.M., Yan D., Grills I., Martinez A. Clinical applications of volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2010;77(2):608–616. doi: 10.1016/j.ijrobp.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Yu C.X., Li X.A., Ma L. Clinical implementation of intensity-modulated arc therapy. Int J Radiat Oncol Biol Phys. 2002;53(2):453–463. doi: 10.1016/s0360-3016(02)02777-3. [DOI] [PubMed] [Google Scholar]
- 26.Weber D.C., Peguret N., Dipasquale G., Cozzi L. Involved-node and involved-field volumetric modulated arc vs. fixed beam intensity-modulated radiotherapy for female patients with early-stage supra-diaphragmatic hodgkin lymphoma: a comparative planning study. Int J Radiat Oncol Biol Phys. 2009;75(5):1578–1586. doi: 10.1016/j.ijrobp.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Wong E., D'souza D.P., Chen J.Z. Intensity-modulated arc therapy for treatment of high-risk endometrial malignancies. Int J Radiat Oncol Biol Phys. 2005;61(3):830–841. doi: 10.1016/j.ijrobp.2004.06.253. [DOI] [PubMed] [Google Scholar]
- 28.Vanetti E., Nicolini G., Nord J. On the role of the optimization algorithm of RapidArc® volumetric modulated arc therapy on plan quality and efficiency. Med Phys. 2011;38(11):5844–5856. doi: 10.1118/1.3641866. [DOI] [PubMed] [Google Scholar]
- 29.Bedford J.L., Lee Y.K., Wai P., South C.P., Warrington A.P. Evaluation of the Delta4 phantom for IMRT and VMAT verification. Phys Med Biol. 2009;54:N167–N176. doi: 10.1088/0031-9155/54/9/N04. [DOI] [PubMed] [Google Scholar]
- 30.Ezzell G.A., Galvin J.M., Low D. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: report of the IMRT Subcommittee of the AAPM Radiation Therapy Committee. Med Phys. 2003;30(8):2089–2115. doi: 10.1118/1.1591194. [DOI] [PubMed] [Google Scholar]
- 31.Kozelka J., Robinson J., Nelms B., Zhang G., Savitskij D., Feygelman V. Optimizing the accuracy of a helical diode array dosimeter: a comprehensive calibration methodology coupled with a novel virtual inclinometer. Med Phys. 2011;38(9):5021–5032. doi: 10.1118/1.3622823. [DOI] [PubMed] [Google Scholar]