Abstract
Background
The incidence of childhood thyroid cancer is increasing in several populations; however, contributing factors have not been adequately discussed.
Objectives
Our aim was to identify trends of childhood thyroid cancer based on the Korea Central Cancer Registry (KCCR) database and to elucidate changes in detection methods of cancers using a single-center database.
Methods
Data from the KCCR and Statistics Korea between 1999 and 2012 were used to calculate the crude incidence of thyroid cancer in children. To analyze detection methods for cancers, pediatric patients (aged 0-19 years, n = 126) who underwent thyroid surgery for thyroid cancers at our institution were identified. Subjects were divided into two groups by detection method: (1) palpation group and (2) screening group.
Results
The crude incidence of childhood thyroid cancer increased from 0.5 per 100,000 in 1999 to 1.7 in 2012. The proportion of thyroid cancer among total cancers also increased from 4.4% in 1999 to 10.6% in 2012. Among 126 children from our institution, 91 cases (72%) were identified as palpable neck masses, and the remainder were discovered during imaging studies. The numbers in both groups gradually increased during the study period.
Conclusions
The incidence of childhood thyroid cancer has steadily increased in Korea. Regarding the detection methods of cancers, most tumors are detected by palpation rather than screening, although the rate of masses identified during screening has increased.
Key Words: Thyroid cancer, Childhood, Detection, Screening, Republic of Korea
Introduction
Childhood thyroid cancer is a rare malignancy, but its incidence has been gradually increasing according to several epidemiologic studies [1,2]. An increase in the incidence of childhood thyroid cancer (aged 0-14 years) was observed in Great Britain, with an approximately 1.5-fold increase in females and a 3-fold increase in males from 1976-1986 to 1997-2005 [1]. A study using the SEER database also reported that the annual percentage change (APC) in thyroid cancer incidence was 4.9 (95% CI: 3.2-6.6) in children aged 0-19 years between 2001 and 2009 [2].
In adults, the incidence of thyroid cancer has increased more rapidly than any other cancer in the last 20 years, and many studies have demonstrated factors contributing to the steep rise in the incidence of thyroid cancer [3,4,5]. Although improved detection with advanced imaging modalities is regarded as the main cause of the increasing incidence of small thyroid cancers [5], an increase in larger thyroid cancers indicates a rising occurrence of true thyroid cancer [3,4]. Environmental and multiple other factors have been suggested [6]; however, the exact causes leading to the increase in thyroid cancer incidence are still unknown.
Little is known about the factors responsible for the increase in childhood thyroid cancer due to its overall low incidence (6.8 cases per million in the USA) [2]. Vergamini et al. [7] reported that an increasing incidence of thyroid cancer of all sizes was identified in children and young adults using the SEER database, with the most prominent increase noted in small tumors (0.5-1.0 cm). However, the study population consisted mainly of young adults (approx. 85%, aged 20-29 years), who have a greater chance of undergoing thyroid screening exams (ultrasonography) than children, and their epidemiologic and biochemical characteristics are expected to be different from those in childhood thyroid cancer [8].
The Ministry of Health and Welfare initiated a nationwide hospital-based cancer registry called the Korea Central Cancer Registry (KCCR) in 1980 [9]. Since 1999, national population-based cancer incidence data have been generated systematically [9]. The KCCR data from 1999 to 2002 have been published as Cancer Incidence in Five Continents, vol. IX, which reflects the completeness and validity of the incidence data [10]. However, the KCCR data do not provide information on cancer staging or detection methods. To elucidate whether thyroid screening is a contributing factor for the detection of thyroid cancer in children, we used clinical data from our institution.
In the present study, we aim to present the incidence trends of childhood thyroid cancer based on the KCCR data and to identify changes in the routes of detection and size of thyroid cancer using our tertiary hospital database.
Materials and Methods
Data Source for Calculating the Crude Incidence of Childhood Thyroid Cancer
Until 1998, the KCCR collected cancer cases annually from more than 180 hospitals in Korea, and the database is believed to represent 80-90% of all cancer cases in Korea [9]. Since 1999, the registry has covered the entire Korean population [9]; the data are available at http://ncc.re.kr/.
Thyroid cancer is classified as ICD-O-3: C73.9 according to the International Classification of Diseases for Oncology, ed. 3 [11]. The crude incidence of childhood thyroid cancer (aged 0-19 years) was calculated based on the KCCR database.
Study Population at Samsung Medical Center
A total of 126 pediatric patients (aged 0-19 years) who had undergone thyroidectomy at Samsung Medical Center between 1995 and 2013 were eligible for this study. Age, gender, history of other malignancy, history of radiation or chemotherapy, cancer staging, thyroid cancer histology and route of detection of thyroid cancer were retrospectively reviewed. For anatomical staging, the 7th edition of the Tumor, Node, Metastasis/American Joint Cancer Committee (TNM/AJCC) system was used (available at https://cancerstaging.org).
The mean age at diagnosis was 16 ± 3 years (mean ± SD), and 110 (87%) patients were female. Five (4%) had a previous history of other malignancy; among them, 4 patients (3%) had received external radiation prior to the diagnosis of thyroid cancer. Papillary thyroid carcinoma (PTC) accounted for 91% (115/126) of all cases, followed by follicular thyroid carcinoma (6%, 8/126), poorly differentiated thyroid carcinoma (2%, 2/126) and medullary thyroid carcinoma (1%, 1/126). The median size of the thyroid tumors was 2.0 cm in diameter, and 33 patients (26%) had multifocal tumors. Distant metastasis at initial presentation was identified in 10 patients (8%), all of whom had lung metastasis.
Route of Detection
The route of detection for thyroid cancer was described as follows: (1) by palpation of a neck mass by family members or a physician, (2) by ultrasonography screening due to various reasons such as a family history of thyroid cancer, (3) by imaging studies for other diseases, including other malignancies or benign thyroid diseases, and (4) by surgical specimens from thyroid surgery for benign thyroid diseases. Patients with thyroid cancer that was discovered by a palpable neck mass were regarded as the ‘palpation group’, and others were considered as the ‘screening group’.
Statistical Analysis
To calculate the crude incidence of childhood thyroid cancer in Korea, data on the numbers of newly diagnosed thyroid cancers every year and population size were obtained from the KCCR database (http://ncc.re.kr/) and Statistics Korea (http://kosis.kr/eng/), respectively.
Statistical calculation was conducted using SPSS Statistics 18 (SPSS Inc., Chicago, Ill., USA). Descriptive statistical analysis was used for demographics (mean, SD, median, interquartile ranges). The independent t test and the χ2 test were used for comparison of variables between the palpation group and the screening group, as appropriate. For comparison between more than two groups, the one-way ANOVA test for parametric variables and the Kruskal-Wallis test for nonparametric variables were used. This study was approved by the Institutional Review Board at Samsung Medical Center.
Results
Increasing Incidence of Childhood Thyroid Cancer in Korea
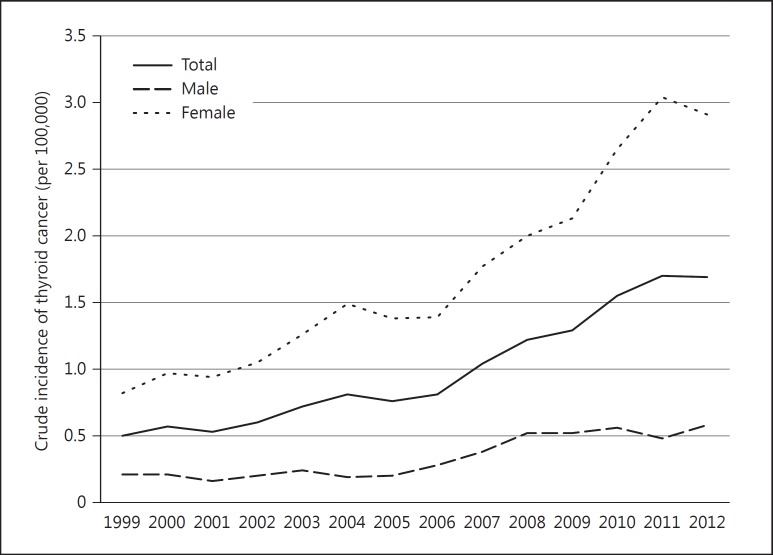
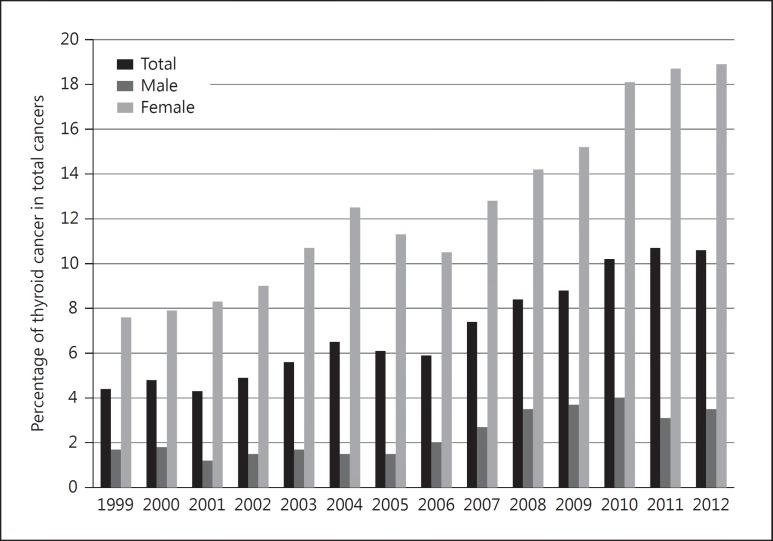
The crude incidence of childhood thyroid cancer in Korea (0-19 years) steadily increased from 1999 to 2012 based on the KCCR data. The incidence changed from 0.5 per 100,000 children in 1999 to 1.7 per 100,000 children in 2012, which was mainly driven by an increase in females (0.8 in 1999 and 2.9 in 2012) rather than in males (0.2 in 1999 and 0.6 in 2012). The proportion of thyroid cancer among all pediatric cancers also increased from 4.4% in 1999 to 10.6% in 2012. The trends in childhood thyroid cancer incidence and the proportions among overall cancers are presented in figures 1 and 2, respectively.
Fig. 1.
Crude incidence of childhood thyroid cancer (0-19 years) in Korea according to year of diagnosis (1999-2012).
Fig. 2.
Proportions of childhood thyroid cancer (0-19 years) in total cancers in Korea (1999-2012).
Detection of Thyroid Cancer in Children
Among 126 childhood thyroid cancers, tumors were diagnosed after the detection of a palpable neck mass by family members or a physician in 91 patients (72%, palpation group) and by thyroid screening in 20 patients (16%); 15 patients (12%) were diagnosed during a general checkup without any other signs or symptoms, and 5 patients (4%) underwent thyroid screening due to a family history of thyroid cancer. Thyroid cancer was incidentally detected during follow-up imaging studies for other diseases in 14 patients (11%): 3 (2%) were malignancies, and 11 (9%) were benign thyroid disease. In 1 patient (1%), thyroid cancer was incidentally found in a surgical specimen during thyroid surgery for intractable Graves' disease (table 1).
Table 1.
Routes of detection of childhood thyroid cancer (n = 126)
| Route of detection | n (%) |
|---|---|
| Palpation (palpable neck mass) | 91 (72) |
| Screening | 35 (28) |
| Ultrasonography screening | 20 (16) |
| During health checkup | 15 (12) |
| Family history of thyroid cancer | 5 (4) |
| During imaging follow-up for other diseases | 14 (11) |
| Other malignancies | 3 (2) |
| Other benign thyroid diseases1 | 11 (9) |
| Incidentally detected on pathologic report during surgery for Graves’ disease | 1 (1) |
Thyroid cancer that was not palpable and that was found during ultrasonography for other benign thyroid diseases was included in this category.
Comparison of Clinical Features according to the Route of Detection of Thyroid Cancer
Patients were divided into the palpation group (n = 91) and the screening group (n = 35). The median tumor size was significantly larger in the palpation group than in the screening group (2.8 vs. 0.9 cm, p < 0.001). In the palpation group, a higher proportion of patients showed advanced TNM stage: 66 patients (73%) had a T3 or T4 disease (vs. 34% in the screening group), and 38 patients (42%) had a lateral lymph node metastasis (vs. 11% in the screening group). The rate of distant metastasis at initial presentation was significantly higher than that in the screening group (11 vs. 0%). Other variables, including age at diagnosis, gender, previous history of radiation and histology of thyroid cancer, were comparable between the groups (table 2).
Table 2.
Comparison of clinical features according to the route of detection of thyroid cancer
| Variable | Palpation group (n = 91) | Screening group (n = 35) | p value |
|---|---|---|---|
| Age (mean ± SD), years | 16 ± 3 | 17 ±2 | 0.79 |
| Female, n (%) | 79 (87) | 31 (89) | 0.79 |
| History of radiation, n (%) | 3 (3) | 1 (3) | 0.90 |
| Histology, n (%) | – | – | 0.75 |
| Papillary | 82 (90) | 33 (94) | |
| Classic | 73 (80) | 29 (83) | |
| Follicular | 2 (2) | 1 (3) | |
| Diffuse sclerosing | 5 (6) | 3 (8) | |
| Cribriform-morular | 2 (2) | 0 | |
| Follicular | 7 (8) | 1 (3) | |
| Medullary | 0 | 1 (3) | |
| Poorly differentiated | 2 (2) | 0 | |
| Median mass size, cm | 2.8 | 0.9 | <0.001 |
| Mass size, n (%) | |||
| <0.5 cm | 0 | 5 (14) | |
| 0.5 – 0.9 cm | 4 (4) | 18 (52) | |
| 1.0 – 1.9 cm | 15 (17) | 11 (31) | |
| ≥2.0 cm | 72 (79) | 1 (3) | |
| T stage, n (%) | <0.001 | ||
| T1 | 12 (13) | 23 (66) | |
| T2 | 13 (14) | 0 | |
| T3 | 32 (35) | 6 (17) | |
| T4 | 34 (38) | 6 (17) | |
| N stage, n (%) | |||
| N0 | 23 (25) | 20 (57) | <0.001 |
| N1a | 30 (33) | 11 (32) | |
| N1b | 38 (42) | 4 (11) | |
| Extrathyroidal extension, n (%) | |||
| None | 29 (32) | 23 (66) | 0.001 |
| Minimal | 28 (31) | 6 (17) | |
| Gross | 34 (37) | 6 (17) | |
| Distant metastasis at presentation, n (%) | 10 (11) | 0 | 0.001 |
TNM stage was described according to the 7th edition of the TNM/AJCC system.
Comparison of Clinical Features according to the Year of Diagnosis of Thyroid Cancer
The number of thyroid cancer cases in childhood gradually increased during the study period. An increase in the number of cases in the palpation group was observed; however, the figures and proportions of cases in the screening group also increased. The median tumor size tended to be smaller in patients with recently diagnosed thyroid cancer, although this finding was not significant, whereas masses identified by palpation were similar in size across the study period. Other parameters, including age at diagnosis, gender, previous history of radiation and histology of thyroid cancer, were not different during the study period (table 3).
Table 3.
Comparison of clinical features according to year of diagnosis of thyroid cancer
| Variable | Year of diagnosis |
p value | |||
|---|---|---|---|---|---|
| 1995 – 1999 | 2000 – 2004 | 2005 – 2009 | 2010 – 2013 | ||
| Patients, n | 6 | 24 | 39 | 57 | – |
| Median age, years | 18 | 17 | 17 | 17 | 0.97 |
| Female, n (%) | 6 (100) | 22 (92) | 35 (90) | 47 (82) | 0.44 |
| History of radiation, n (%) | 0 | 1 (4) | 0 | 3 (5) | 0.50 |
| Histology, n (%) | 4 (67) | 23 (96) | 37 (95) | 51 (89) | 0.07 |
| Papillary | |||||
| Classic | 3 (50) | 23 (96) | 32 (82) | 44 (77) | |
| Follicular | 1 (17) | 0 | 2 (5) | 0 | |
| Diffuse sclerosing | 0 | 0 | 2 (5) | 6 (10) | |
| Cribriform-morular | 0 | 0 | 1 (3) | 1 (2) | |
| Follicular | 2 (33) | 1 (4) | 2 (5) | 3 (5) | |
| Medullary | 0 | 0 | 0 | 1 (2) | |
| Poorly differentiated | 0 | 0 | 0 | 2 (4) | |
| Mass found by palpation, n (%) | 6 (100) | 22 (92) | 27 (69) | 32 (56) | 0.03 |
| Median palpable mass size1, cm | 2.4 | 2.6 | 2.3 | 2.8 | 0.38 |
| Median mass size, cm | 2.4 | 2.6 | 1.5 | 2.1 | 0.042 |
Masses found by palpation were included for this calculation.
The Kruskal-Wallis test was used for non-parametric variables comparing more than two groups, and the median mass size was significantly different between the four groups. According to the Mann-Whitney U test and Bonferroni correction for post hoc analysis, the difference was significant between the two groups from 2000 – 2004 and 2005 – 2009.
Discussion
We describe an increasing incidence in childhood thyroid cancer between 1999 and 2012 using data from the KCCR database. To analyze the patterns of thyroid cancer detection, we used the database from a single tertiary center, which identified that the majority of cancers were initially discovered by palpation, and the number of masses found during both screening and palpation gradually increased, although the detection of palpable tumors increased to a lesser degree.
An increasing incidence of childhood thyroid cancer has been observed in several epidemiologic studies (table 4). A British group reported that the age-standardized incidence rates in northern England increased from 0.2 per million male children aged 0-14 years in 1976-1986 to 0.6 in 1997-2005, and from 0.3 per million female children aged 0-14 years to 0.5 [1]. In the USA, the incidence rate ranged from 0.4 to 0.7 per 100,000 children (0-19 years), and a gradually increasing tendency with an APC of 1.1% per year was observed between 1973 and 2007, in spite of its fluctuating pattern [12]. Another study using the SEER database reported a 4.9 APC of thyroid cancer (0-19 years) in 2001-2009, whereas the rate in overall pediatric cancers did not change in the same period (APC = 0.0) [2]. Comparing these two studies using the SEER database, a rapid increase of childhood thyroid cancers is observed in recent years [2,12]. Our results are comparable, but present a higher incidence with previous data from Western countries, which demonstrate increasing trends and rates of childhood thyroid cancer among all cancers. However, most prior studies [1,2,12], except one by Vergamini et al. [7], did not analyze cancer incidence according to tumor size. Thus, the influence of screening studies on the detection of childhood thyroid cancer is difficult to estimate in epidemiologic studies, including the KCCR.
Table 4.
Comparison of changes in incidence of childhood thyroid cancer
| Nation | Age, years | Year(s) | Incidence (per 100,000) | Year(s) | Incidence (per 100,000) | APC |
|---|---|---|---|---|---|---|
| South Korea (KCCR) | ||||||
| Total | 0 – 19 | 1999 | 0.5 | 2012 | 1.7 | 10.3 |
| Male | 0 – 19 | 1999 | 0.2 | 2012 | 0.6 | 10.2 |
| Female | 0 – 19 | 1999 | 0.8 | 2012 | 2.9 | 10.8 |
| USA (SEER) [2] | ||||||
| Total | 0 – 19 | 2001 – 2009 | 0.7 | – | – | 4.9 |
| Male | 0 – 19 | 2001 – 2009 | 0.3 | – | – | 4.7 |
| Female | 0 – 19 | 2001 – 2009 | 1.1 | – | – | 4.9 |
| USA (SEER) [12] | ||||||
| Total | 0 – 19 | 1973 | 0.4 | 2007 | 0.7 | 1.1 |
| Great Britain [1] | ||||||
| Male | 0 – 14 | 1976 – 1986 | 0.2 (per million) | 1997 – 2005 | 0.6 (per million) | N/A |
| Female | 0 – 14 | 1976 – 1986 | 0.3 (per million) | 1997 – 2005 | 0.5 (per million) | N/A |
N/A = Not available.
To examine trends in mass detection and thyroid screening, we used the data from our institution, information that was not available in epidemiologic studies. Among 126 children with thyroid cancer, approximately 72% were initially detected by palpation. Although the percentage of palpable masses decreased in recently diagnosed childhood cancers, the absolute numbers constantly increased across the study period. It is worth mentioning that the proportion of cases detected by imaging studies increased, and these tumors were significantly smaller than the palpable masses. A recent study conducted by Vergamini et al. [7] showed that thyroid cancers ≥2.0 cm in size constituted approximately 60% of all cases of childhood thyroid cancer (0-19 years) based on the SEER database (1984-2010). An increasing incidence of large (≥2.0 cm, APC 2.96, 95% CI: 2.34-3.59) and small tumors (0.5-0.9 cm, APC 8.45, 95% CI: 7.09-9.82) was identified in females (0-29 years), which indicates that improved diagnosis with sensitive imaging modalities does not fully explain an increasing trend of thyroid tumors [7]. Although a prior study included young adults and did not present subanalysis results of the APCs according to tumor size in children (0-19 years), a significant increase in total thyroid cancers was observed in the age groups of 10-14 and 15-19 years [7]. Our findings conform to the results of the SEER database and provide additional information, including the route of mass detection. Although we cannot generalize these results for Korean pediatrics because our data were drawn from a single-center population, it seems that palpation is still the main route of thyroid mass detection, even in recently diagnosed thyroid cancers, which is different from the manner common in adults [13,14].
In our data, histologic changes in childhood thyroid cancers were observed in each period, although these were not significant. As with the previous literature [15,16], PTC accounted for more than 90% throughout the study period, except the early era (1995-1999), perhaps due to the small number of patients. It is notable that the rate of rare variants of PTC, such as the diffuse sclerosing variant of PTC (DSV-PTC), gradually increased during the study period (10% in 2010-2013). Among 8 cases of DSV-PTC, 5 (63%) were in the palpation group, and 3 (37%) were in the screening group: 1 case of the screening group was detected in 2005-2009, and the other 2 cases were found in 2010-2013. One case had received previous irradiation treatment for acute leukemia. As DSV-PTC is related to young ages and irradiation, this rare subtype of thyroid cancers has been studied in atomic bomb accident areas. A recent study conducted in Belarus reported that DSV was 7.7% of PTC in prescreening cases and 1.2% in US screening cases [17]. These results are comparable with our data in that most DSV-PTC are detected by clinical symptoms or signs. Other studies also reported a similar prevalence (10-13%) of DSV-PTC in children with thyroid cancers in Belarus [18,19]. However, pathologic changes in childhood thyroid cancers have not been reported in the literature. To figure out whether the pathology change is a true phenomenon, further studies with a longer follow-up duration are needed.
Several genetic susceptibilities and environmental factors have been studied to further elucidate the etiology of thyroid cancer. Regarding genetic susceptibility, it has been suggested that the genetic factors contributing to thyroid cancer development are considerable compared with those of other cancers [20]. Mousavi et al. [21] reported that East and Southeast Asians, including South Koreans, were one of the most susceptible populations for developing thyroid cancer according to the Swedish Family-Cancer Database. Familial nonmedullary thyroid cancer (FNMTC), which accounts for approximately 5% of all thyroid cancers, is diagnosed when two or more first-degree relatives have differentiated thyroid cancer without other familial syndromes [22]. Park et al. [23] reported a higher prevalence of FNMTC (9.6%) in Korea, a considerably high figure that is comparable with the prevalence in Italy (11.3%) [24] and the USA (8.8%) [25]. The rates of FNMTC have not changed in Korea between 1962 and 2010 [23]. With regard to environmental factors, ionizing radiation is an established cause of thyroid cancer. The thyroid gland is very sensitive to radiation in childhood owing to its small size, and younger ages are more susceptible to thyroid cancer related to irradiation [26]. In particular, therapeutic radiation for other pediatric malignancies, such as leukemia, lymphoma and neuroblastoma, is associated with the development of secondary thyroid cancer [26]. In our population, 4 children (3.2%) were exposed to external radiation before the diagnosis of thyroid cancer. Considering that only 3% of childhood thyroid cancers are diagnosed among survivors of other previous cancers, a history of medical irradiation seems to constitute a small portion of the factors contributing to the occurrence of thyroid cancer.
Recent changes in the trends of childhood thyroid cancer are not completely explained by the factors described above. Additional explanations are posited below, although these issues were not addressed in the present study. Irradiation is a well-known risk factor for thyroid cancer, and some authors have suggested that the risk of thyroid cancer is increasing due to exposure to medical radiation such as computed tomography scans or diagnostic X-ray [27,28], although others disagree with this suggestion [29]. The association between obesity and thyroid cancer has also been reported, and recent data with pooled analysis suggest that a higher body mass index is a risk factor for thyroid cancer in both genders [30]. As in Western countries, the prevalence of obesity in children has rapidly increased in Korea, and this might be a possible factor contributing to the trends of thyroid cancer. High iodine nutritional status is considered as a risk factor for thyroid cancer due to BRAF mutation [31], and Korea is known to be a high iodine intake country [32,33]. According to data from the Korea National Health and Nutrition Examination Survey, the consumption of food with a high iodine content has increased steadily over the past few decades in Korea (available online at http:kosis.kr/), and these changes in dietary habits could affect the increase in thyroid cancer.
The palpation group presented aggressive features of thyroid cancers compared with the screening group: advanced TNM stage and distant metastasis at the initial diagnosis. As both groups showed no difference in gender, age, histology of thyroid cancers and irradiation history, thyroid ultrasonography screening may be beneficial to detect childhood thyroid cancers in an early stage. Gupta et al. [34] also reported that childhood thyroid cancers incidentally discovered by radiologic studies tended to be small and showed a lower rate of metastasis. However, recently published guidelines by the American Thyroid Association recommend physical examination for children at high risk for thyroid neoplasia because of insufficient data to clarify the screening effect of thyroid ultrasonography [35]. This issue is beyond our scope and should be addressed for the improvement of the quality and longevity of life.
We report a 3-fold increasing incidence of childhood thyroid cancer in Korea between 1999 and 2012 according to the KCCR database, and the majority of cancers are initially detected by palpation rather than thyroid screening in the population from our institution, although the proportion of masses found during screening studies is increasing. To clarify the trends in childhood thyroid cancer and its risk factors, further investigations on a larger scale with long-term follow-up data are required.
Disclosure Statement
The authors declare that there are no conflicts of interest.
Acknowledgements
This study was supported by grants from the Korean Foundation for Cancer Research (CB-2011-03-02) and Samsung Medical Center Clinical Research (CRO1121021 and SMO113145).
References
- 1.McNally RJ, Blakey K, James PW, Gomez Pozo B, Basta NO, Hale J. Increasing incidence of thyroid cancer in Great Britain, 1976-2005: age-period-cohort analysis. Eur J Epidemiol. 2012;27:615–622. doi: 10.1007/s10654-012-9710-x. [DOI] [PubMed] [Google Scholar]
- 2.Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001-2009. Pediatrics. 2014;134:e945–e955. doi: 10.1542/peds.2013-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg. 2010;200:454–461. doi: 10.1016/j.amjsurg.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandeya N, McLeod DS, Balasubramaniam K, Baade PD, Youl PH, Bain CJ, Allison R, Jordan SJ. Increasing thyroid cancer incidence in Queensland, Australia 1982-2008 – true increase or overdiagnosis? Clin Endocrinol (Oxf) 2015, Epub ahead of print. [DOI] [PubMed]
- 5.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 6.Vigneri R, Malandrino P, Vigneri P. The changing epidemiology of thyroid cancer: why is incidence increasing? Curr Opin Oncol. 2015;27:1–7. doi: 10.1097/CCO.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 7.Vergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr. 2014;164:1481–1485. doi: 10.1016/j.jpeds.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 8.Rivkees SA, Mazzaferri EL, Verburg FA, Reiners C, Luster M, Breuer CK, Dinauer CA, Udelsman R. The treatment of differentiated thyroid cancer in children: emphasis on surgical approach and radioactive iodine therapy. Endocr Rev. 2011;32:798–826. doi: 10.1210/er.2011-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, Noh HI, Lee JK, Pisani P, Park JG. Nationwide cancer incidence in Korea, 1999∼2001; first result using the national cancer incidence database. Cancer Res Treat. 2005;37:325–331. doi: 10.4143/crt.2005.37.6.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curado M, Edward BHS. Cancer Incidence in Five Continents. Lyon: IARC; 2007. [Google Scholar]
- 11.Fritz AG. International Classification of Diseases for Oncology: ICD-O. ed 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 12.Holmes L, Jr, Hossain J, Opara F. Pediatric thyroid carcinoma incidence and temporal trends in the USA (1973-2007): race or shifting diagnostic paradigm? ISRN Oncol. 2012;2012:906197. doi: 10.5402/2012/906197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Shin SW. Overdiagnosis and screening for thyroid cancer in Korea. Lancet. 2014;384:1848. doi: 10.1016/S0140-6736(14)62242-X. [DOI] [PubMed] [Google Scholar]
- 14.Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer ‘epidemic’ – screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 15.Demidchik YE, Demidchik EP, Reiners C, Biko J, Mine M, Saenko VA, Yamashita S. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. 2006;243:525–532. doi: 10.1097/01.sla.0000205977.74806.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta A, Ly S, Castroneves LA, Frates MC, Benson CB, Feldman HA, Wassner AJ, Smith JR, Marqusee E, Alexander EK, Barletta J, Doubilet PM, Peters HE, Webb S, Modi BP, Paltiel HJ, Kozakewich H, Cibas ES, Moore FD, Jr, Shamberger RC, Larsen PR, Huang SA. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab. 2013;98:3238–3245. doi: 10.1210/jc.2013-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zablotska LB, Nadyrov EA, Rozhko AV, Gong Z, Polyanskaya ON, McConnell RJ, O'Kane P, Brenner AV, Little MP, Ostroumova E, Bouville A, Drozdovitch V, Minenko V, Demidchik Y, Nerovnya A, Yauseyenka V, Savasteeva I, Nikonovich S, Mabuchi K, Hatch M. Analysis of thyroid malignant pathologic findings identified during 3 rounds of screening (1997-2008) of a cohort of children and adolescents from Belarus exposed to radioiodines after the Chernobyl accident. Cancer. 2015;121:457–466. doi: 10.1002/cncr.29073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fridman MV, Savva NN, Krasko OV, Zborovskaya AA, Mankovskaya SV, Schmid KW, Demidchik YE. Clinical and pathologic features of ‘sporadic’ papillary thyroid carcinoma registered in the years 2005 to 2008 in children and adolescents of Belarus. Thyroid. 2012;22:1016–1024. doi: 10.1089/thy.2011.0005. [DOI] [PubMed] [Google Scholar]
- 19.Nikiforov Y, Gnepp DR. Pediatric thyroid cancer after the Chernobyl disaster. Pathomorphologic study of 84 cases (1991-1992) from the Republic of Belarus. Cancer. 1994;74:748–766. doi: 10.1002/1097-0142(19940715)74:2<748::aid-cncr2820740231>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int J Cancer. 2002;99:260–266. doi: 10.1002/ijc.10332. [DOI] [PubMed] [Google Scholar]
- 21.Mousavi SM, Brandt A, Sundquist J, Hemminki K. Risks of papillary and follicular thyroid cancer among immigrants to Sweden. Int J Cancer. 2011;129:2248–2255. doi: 10.1002/ijc.25867. [DOI] [PubMed] [Google Scholar]
- 22.Ito Y, Kakudo K, Hirokawa M, Fukushima M, Yabuta T, Tomoda C, Inoue H, Kihara M, Higashiyama T, Uruno T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery. 2009;145:100–105. doi: 10.1016/j.surg.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Park YJ, Ahn HY, Choi HS, Kim KW, Park DJ, Cho BY. The long-term outcomes of the second generation of familial nonmedullary thyroid carcinoma are more aggressive than sporadic cases. Thyroid. 2012;22:356–362. doi: 10.1089/thy.2011.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capezzone M, Marchisotta S, Cantara S, Busonero G, Brilli L, Pazaitou-Panayiotou K, Carli AF, Caruso G, Toti P, Capitani S, Pammolli A, Pacini F. Familial non-medullary thyroid carcinoma displays the features of clinical anticipation suggestive of a distinct biological entity. Endocr Relat Cancer. 2008;15:1075–1081. doi: 10.1677/ERC-08-0080. [DOI] [PubMed] [Google Scholar]
- 25.Moses W, Weng J, Kebebew E. Prevalence, clinicopathologic features, and somatic genetic mutation profile in familial versus sporadic nonmedullary thyroid cancer. Thyroid. 2011;21:367–371. doi: 10.1089/thy.2010.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigurdson AJ, Ronckers CM, Mertens AC, Stovall M, Smith SA, Liu Y, Berkow RL, Hammond S, Neglia JP, Meadows AT, Sklar CA, Robison LL, Inskip PD. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 27.Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI, Feigelson HS, Roblin D, Flynn MJ, Vanneman N, Smith-Bindman R. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700–707. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Memon A, Godward S, Williams D, Siddique I, Al-Saleh K. Dental X-rays and the risk of thyroid cancer: a case-control study. Acta Oncol. 2010;49:447–453. doi: 10.3109/02841861003705778. [DOI] [PubMed] [Google Scholar]
- 29.Hammer GP, Seidenbusch MC, Regulla DF, Spix C, Zeeb H, Schneider K, Blettner M. Childhood cancer risk from conventional radiographic examinations for selected referral criteria: results from a large cohort study. AJR Am J Roentgenol. 2011;197:217–223. doi: 10.2214/AJR.10.4979. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Port M, Landi S, Gemignani F, Cipollini M, Elisei R, Goudeva L, Muller JA, Nerlich K, Pellegrini G, Reiners C, Romei C, Schwab R, Abend M, Sturgis EM. Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case-control studies. Thyroid. 2014;24:966–974. doi: 10.1089/thy.2013.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, Zhang Y, Shan Z, Teng W, Xing M. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612–1617. doi: 10.1210/jc.2008-2390. [DOI] [PubMed] [Google Scholar]
- 32.Cho YY, Kim HJ, Oh SY, Choi SJ, Lee SY, Joung JY, Jeong DJ, Sohn SY, Chung JH, Roh CR, Kim SW. Iodine status in healthy pregnant women in Korea: a first report. Eur J Nutr 2015, Epub ahead of print. [DOI] [PubMed]
- 33.Kim JY, Moon SJ, Kim KR, Sohn CY, Oh JJ. Dietary iodine intake and urinary iodine excretion in normal Korean adults. Yonsei Med J. 1998;39:355–362. doi: 10.3349/ymj.1998.39.4.355. [DOI] [PubMed] [Google Scholar]
- 34.Gupta A, Ly S, Castroneves LA, Frates MC, Benson CB, Feldman HA, Wassner AJ, Smith JR, Marqusee E, Alexander EK, Barletta J, Muyide F, Doubilet PM, Peters HE, Webb S, Modi BP, Paltiel HJ, Martins Y, Burmeister K, Kozakewich H, Hollowell M, Cibas ES, Moore FD, Jr, Shamberger RC, Larsen PR, Huang SA. How are childhood thyroid nodules discovered: opportunities for improving early detection. J Pediatr. 2014;164:658–660. doi: 10.1016/j.jpeds.2013.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25:716–759. doi: 10.1089/thy.2014.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]