Abstract
Objective
Graves' ophthalmopathy (GO) is an inflammatory disease in the orbital region. The first-line medical treatment is glucocorticoids. An important potential side effect of glucocorticoid treatment is suppression of the hypothalamic-pituitary-adrenal (HPA) axis with impairment of endogenous cortisol production, implicating symptoms of adrenocortical insufficiency, especially in the period after cessation of therapy with possible risks in cases of intercurrent illness. The aim of this study was to evaluate HPA axis function before and after methylprednisolone pulse treatment of GO.
Study Design
HPA axis function was evaluated by measurements of plasma ACTH and an ACTH stimulation test with plasma cortisol measurements at 0 and 30 min after an intravenous bolus of synthetic ACTH (Synacthen® 250 µg). This was done in 12 patients with GO before and at cessation of methylprednisolone pulse treatment (500 mg i.v. per week for 6 weeks followed by 250 mg i.v. per week for an additional 6 weeks).
Results
All patients included fulfilled the criteria of intact HPA axis function before and at cessation of methylprednisolone pulse treatment. Data are given as medians (with ranges). Before glucocorticoid treatment basal plasma cortisol was 290 nM (196-579) and 786 nM (612-1,050) after ACTH stimulation. At cessation of therapy the corresponding values were 309 nM (88-718) and 852 nM (524-1,011), respectively. Thus, all patients passed a 30-min stimulated plasma cortisol of 500 nM. Before treatment plasma ACTH was 4.2 pmol/l (4-16) and at cessation of therapy the corresponding value was 4.8 pmol/l (2-9; p = 0.27).
Conclusion
Transient suppression of the HPA axis with secondary adrenocortical insufficiency does not seem to be a common phenomenon after intravenous methylprednisolone pulse therapy for GO. Therefore, routine precautions are not necessary. However, our results do not exclude that transient secondary adrenocortical insufficiency might occur occasionally.
Key Words: Adrenocortical insufficiency, Graves’ ophthalmopathy, Methylprednisolone, Glucocorticoids
Introduction
Graves' disease is an autoimmune disease of the thyroid gland leading to hyperthyroidism. An extrathyroidal manifestation of Graves' disease is Graves' ophthalmopathy (GO): an autoimmune, inflammatory disease in the orbital region leading to peri- and intraorbital edema, eye muscle palsies and optic nerve neuropathy. Thereby, GO can result in prominent cosmetic inconveniences, visual disturbances such as double vision, visual impairment and eventually blindness. Glucocorticoid, administered as intravenous pulse therapy, is the first-line treatment of patients with moderate-to-severe GO [1] and the superior dose schedule seems to be a once weekly infusion of methylprednisolone [1,2,3].
An important potential side effect of glucocorticoid treatment is the suppression of the hypothalamic-pituitary-adrenal (HPA) axis with impairment of endogenous cortisol production, implicating symptoms of adrenocortical insufficiency, especially in the period after cessation of therapy with possible risks in cases of intercurrent illness [4]. Suppression of the HPA axis has been reported as a side effect of many different glucocorticoid treatment schedules for various inflammatory diseases [5,6].
We hypothesized that transient secondary adrenocortical insufficiency was a common side effect of methylprednisolone pulse therapy. The purpose of this paper was to evaluate HPA axis function before and after methylprednisolone pulse treatment of GO, employing an ACTH (Synacthen®) stimulation test and plasma ACTH measurements.
Materials and Methods
Twelve consecutive patients treated for GO at the Department of Internal Medicine and Endocrinology at Copenhagen University Hospital at Herlev were studied from August 2013 to December 2014. There were 3 men and 9 women. The median age was 57 years (34-71). All patients were euthyroid at the time of treatment. In the time period of the study we included, as a safety routine, measurements of ACTH and an ACTH stimulation test before and at cessation of treatment (see below). The results were collected in January 2015. The patients were diagnosed with hyperthyroidism and GO with a clinical activity score ≥4 [7]. None of the patients had previously been treated with glucocorticoids. None of the women received estrogen therapy.
Data are given as medians (with ranges). Nonparametric statistics were used for the statistical analysis and the analyses were performed using IBM SPSS statistics 21.
The glucocorticoid treatment for GO comprised a once weekly infusion of methylprednisolone: 500 mg over 6 h for the first 6 weeks and 250 mg over 3 h for the next 6 weeks [1,2]. HPA axis function was evaluated by measurements (08.00-09.00 h) of plasma ACTH and an ACTH stimulation test with plasma cortisol measurements at 0 and 30 min after an intravenous bolus of synthetic ACTH (Synacthen® 250 µg). These measurements were performed immediately before administration of the first and the final (12th) pulse infusion. ACTH was measured by an immunoradiometric assay (BRAHMS Diagnostica GmbH, Berlin, Germany) with qualities as described previously [8]. Cortisol was measured by autoanalytical methods. All patients were measured with the same assay, either Liason (10 patients; August 2013 to August 2014) or Cobas (2 patients; August to December 2014). At a cortisol concentration in the range of 500-600 nM, the Liason method gives results about 5% lower and the Cobas method about 15% higher compared to analysis by mass spectrometry (unpubl. data). This would apply to cortisol cutoff values for adrenocortical insufficiency of 475 nM for the Liason assay and 575 nM for the Cobas assay.
Results
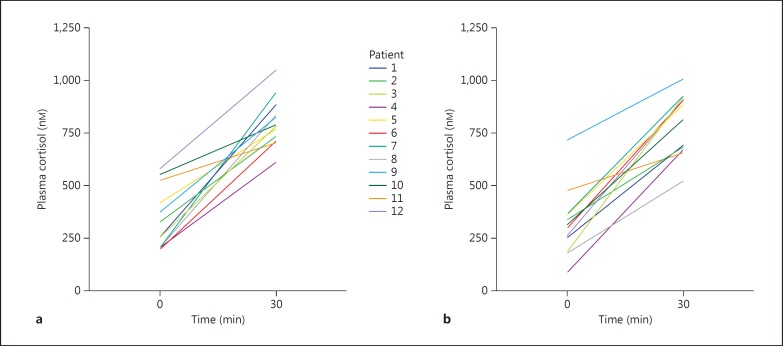
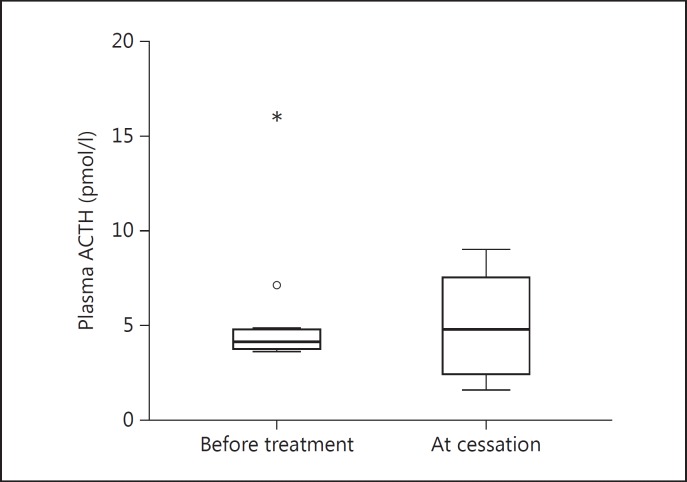
Results of the plasma cortisol measurements are shown in figure 1. Before glucocorticoid treatment basal plasma cortisol was 290 nM (196-579) and 786 nM (612-1,050) after ACTH stimulation. At cessation of therapy the corresponding values were 309 nM (88-718) and 852 nM (524-1,011). The increment was 463 nM (179-738) before treatment and 523 nM (177-743) after treatment. None of the differences before versus after the glucocorticoid treatment were significant (p = 0.70-0.99). All patients passed a 30-min ACTH-stimulated plasma cortisol of 500 nM. Plasma ACTH was 4.2 pmol/l (4-16)before treatment and 4.8 pmol/l (2-9; p = 0.27) at cessation of therapy, as shown in figure 2.
Fig. 1.
Plasma cortisol at 0 and 30 min after ACTH stimulation prior to (a) and at cessation (b) of glucocorticoid treatment (color in online version only).
Fig. 2.
Plasma ACTH levels prior to and at cessation of glucocorticoid treatment of GO. ° = SPSS-calculated upper normal limit; * = outlier.
Discussion
Suppression of HPA axis function with adrenocortical insufficiency is a common side effect of glucocorticoid treatment but it is somewhat unpredictable [9,10,11]. There are no obvious correlations between the total dose of oral glucocorticoid or the duration of treatment and adrenocortical insufficiency [12]. This may to some extent be due to polymorphisms of the glucocorticoid receptor [13]. Furthermore, daily versus intermittent administration could be of importance. Dinsen et al. [4] reviewed this topic and concluded that oral glucocorticoids could induce adrenocortical insufficiency in up to 46-100% of patients. Even inhaled glucocorticoid treatment can lead to adrenocortical insufficiency [14,15,16]. Only few results of adrenocortical function in relation to intermittent glucocorticoid pulse therapy have been reported [17,18,19].
In the present study of 12 patients receiving a 12-week intravenous pulse treatment with methylprednisolone no cases of adrenocortical insufficiency were observed. Cortisol levels were unchanged and all patients passed a limit of 500 nM after ACTH stimulation. At cessation of glucocorticoid treatment, the lowest ACTH-stimulated cortisol concentration was 524 nM (measured by the Liason method) and in the remaining 11 patients it was between 656 and 1,011 nM. Thus, all patients passed the presumed cutoff levels given in Materials and Methods. Furthermore, neither a significant suppression of ACTH nor a compensatory ACTH increase to recover from transient adrenocortical insufficiency was observed [20]. Thus, the HPA axis function seems to be essentially unchanged in relation to the now widely used methylprednisolone pulse treatment schedule for GO. Albeit this study was limited by the number of patients, the conclusion is supported by a recent report published during the preparation of the present paper [17]. An ACTH stimulation test was performed at the termination of treatment for GO with the same methylprednisolone schedule and all of the 32 patients had a sufficient cortisol response; however, ACTH was not measured.
A low-dose ACTH stimulation test might be more sensitive in detecting mild secondary adrenocortical insufficiency. However, two different meta-analyses do not agree on this point [21,22] and the specificity of the standard dose test seems to be higher [23].
Taken together, the results so far indicate that the employed treatment schedule for GO is safe as regards secondary adrenocortical insufficiency. This may be somewhat surprising since the total dose of methylprednisolone from start to conclusion was 4,500 mg, corresponding to a mean daily dose of about 60 mg, which probably illustrates that intermittent weekly glucocorticoid administration implicates far less perturbation of the HPA axis compared to daily doses. On the other hand, concerning the clinical anti-inflammatory effect on GO, the pulse therapy schedule has been demonstrated as superior to a daily oral dose schedule [2]. Thus, effect and side effect are not necessarily linked together. It is tempting to speculate whether such a favorable therapeutic profile would justify a more widespread use of glucocorticoid pulse therapy in various other diseases.
Conclusion
Transient suppression of the HPA axis with secondary adrenocortical insufficiency does not seem to be a common phenomenon after intravenous methylprednisolone pulse therapy for GO. Therefore, routine precautions are not necessary. However, our results do not exclude that transient secondary adrenocortical insufficiency might occasionally occur in a very small number of patients.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158:273–285. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 2.Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell'Unto E, Rocchi R, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves' ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001;86:3562–3567. doi: 10.1210/jcem.86.8.7737. [DOI] [PubMed] [Google Scholar]
- 3.Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves' orbitopathy. J Clin Endocrinol Metab. 2012;97:4454–4463. doi: 10.1210/jc.2012-2389. [DOI] [PubMed] [Google Scholar]
- 4.Dinsen S, Baslund B, Klose M, Rasmussen AK, Friis-Hansen L, Hilsted L, et al. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. Eur J Intern Med. 2013;24:714–720. doi: 10.1016/j.ejim.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Zietz B, Reber T, Oertel M, Gluck T, Scholmerich J, Straub RH. Altered function of the hypothalamic stress axes in patients with moderately active systemic lupus erythematosus. II. Dissociation between androstenedione, cortisol, or dehydroepiandrosterone and interleukin 6 or tumor necrosis factor. J Rheumatol. 2000;27:911–918. [PubMed] [Google Scholar]
- 6.Habib G, Jabbour A, Artul S, Hakim G. Intra-articular methylprednisolone acetate injection at the knee joint and the hypothalamic-pituitary-adrenal axis: a randomized controlled study. Clin Rheumatol. 2014;33:99–103. doi: 10.1007/s10067-013-2374-4. [DOI] [PubMed] [Google Scholar]
- 7.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf) 1997;47:9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 8.Klose M, Kofoed-Enevoldsen A, Kristensen LO. Single determination of plasma ACTH using an immunoradiometric assay with high detectability differentiates between ACTH-dependent and -independent Cushing's syndrome. Scand J Clin Lab Invest. 2002;62:33–37. doi: 10.1080/003655102753517181. [DOI] [PubMed] [Google Scholar]
- 9.Han HS, Shim YK, Kim JE, Jeon HJ, Lim SN, Oh TK, et al. A pilot study of adrenal suppression after dexamethasone therapy as an antiemetic in cancer patients. Support Care Cancer. 2012;20:1565–1572. doi: 10.1007/s00520-011-1248-z. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel RJ, Vigersky RA, Oliff AI, Echelberger CK, Bruton J, Poplack DG. Adrenal suppression after short-term corticosteroid therapy. Lancet. 1979;i:630–633. doi: 10.1016/s0140-6736(79)91077-8. [DOI] [PubMed] [Google Scholar]
- 11.Schlaghecke R, Kornely E, Santen RT, Ridderskamp P. The effect of long-term glucocorticoid therapy on pituitary-adrenal responses to exogenous corticotropin-releasing hormone. N Engl J Med. 1992;326:226–230. doi: 10.1056/NEJM199201233260403. [DOI] [PubMed] [Google Scholar]
- 12.LaRochelle GE, Jr, LaRochelle AG, Ratner RE, Borenstein DG. Recovery of the hypothalamic-pituitary-adrenal (HPA) axis in patients with rheumatic diseases receiving low-dose prednisone. Am J Med. 1993;95:258–264. doi: 10.1016/0002-9343(93)90277-v. [DOI] [PubMed] [Google Scholar]
- 13.Huizenga NA, Koper JW, De Lange P, Pols HA, Stolk RP, Burger H, et al. A polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab. 1998;83:144–151. doi: 10.1210/jcem.83.1.4490. [DOI] [PubMed] [Google Scholar]
- 14.Molimard M, Girodet PO, Pollet C, Fourrier-Reglat A, Daveluy A, Haramburu F, et al. Inhaled corticosteroids and adrenal insufficiency: prevalence and clinical presentation. Drug Saf. 2008;31:769–774. doi: 10.2165/00002018-200831090-00005. [DOI] [PubMed] [Google Scholar]
- 15.Mortimer KJ, Tata LJ, Smith CJ, West J, Harrison TW, Tattersfield AE, et al. Oral and inhaled corticosteroids and adrenal insufficiency: a case-control study. Thorax. 2006;61:405–408. doi: 10.1136/thx.2005.052456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zollner EW, Lombard CJ, Galal U, Hough FS, Irusen EM, Weinberg E. Hypothalamic-pituitary-adrenal axis suppression in asthmatic school children. Pediatrics. 2012;130:e1512–e1519. doi: 10.1542/peds.2012-1147. [DOI] [PubMed] [Google Scholar]
- 17.Giotaki Z, Fountas A, Tsirouki T, Bargiota A, Tigas S, Tsatsoulis A. Adrenal reserve following treatment of Graves' orbitopathy with intravenous glucocorticoids. Thyroid. 2015;25:462–463. doi: 10.1089/thy.2014.0533. [DOI] [PubMed] [Google Scholar]
- 18.Levic Z, Micic D, Nikolic J, Stojisavljevic N, Sokic D, Jankovic S, et al. Short-term high dose steroid therapy does not affect the hypothalamic-pituitary-adrenal axis in relapsing multiple sclerosis patients. Clinical assessment by the insulin tolerance test. J Endocrinol Invest. 1996;19:30–34. doi: 10.1007/BF03347855. [DOI] [PubMed] [Google Scholar]
- 19.Levitt M, Sharma RN, Faiman C. Normal metyrapone response after 1 month of high-dose methylprednisolone in cancer patients: a phase I study. Cancer Treat Rep. 1979;63:1327–1330. [PubMed] [Google Scholar]
- 20.Klose M, Jorgensen K, Kristensen LO. Characteristics of recovery of adrenocortical function after treatment for Cushing's syndrome due to pituitary or adrenal adenomas. Clin Endocrinol (Oxf) 2004;61:394–399. doi: 10.1111/j.1365-2265.2004.02111.x. [DOI] [PubMed] [Google Scholar]
- 21.Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139:194–204. doi: 10.7326/0003-4819-139-3-200308050-00009. [DOI] [PubMed] [Google Scholar]
- 22.Kazlauskaite R, Evans AT, Villabona CV, Abdu TA, Ambrosi B, Atkinson AB, et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a meta-analysis. J Clin Endocrinol Metab. 2008;93:4245–4253. doi: 10.1210/jc.2008-0710. [DOI] [PubMed] [Google Scholar]
- 23.Suliman AM, Smith TP, Labib M, Fiad TM, McKenna TJ. The low-dose ACTH test does not provide a useful assessment of the hypothalamic-pituitary-adrenal axis in secondary adrenal insufficiency. Clin Endocrinol (Oxf) 2002;56:533–539. doi: 10.1046/j.1365-2265.2002.01509.x. [DOI] [PubMed] [Google Scholar]