Abstract
Background
Several studies have suggested that selenium may influence the natural history of autoimmune thyroiditis (AIT). Recently, IFNγ-inducible chemokines (CXCL-9, −10 and −11) were shown to be elevated in AIT patients.
Objective
This prospective, randomized, controlled study was conducted to evaluate the effect of two doses of selenomethionine (Semet; 80 or 160 µg/day) versus placebo in euthyroid women with AIT, in terms of reduction of anti-thyroid antibodies, CXCL-9, −10 and −11 and improvement of thyroid echogenicity, over 12 months.
Patients and Methods
Sixty patients, aged 21-65 years, were equally randomized into 3 groups: placebo, 80 µg/day of Semet (80-Semet) or 160 µg/day of Semet (160-Semet).
Results
Anti-thyroperoxidase antibody (TPOAb) levels remained unaffected by Semet supplementation; anti-thyroglobulin antibody levels showed a significant reduction in the 160-Semet and the placebo group at 12 months. No significant change in thyroid echogenicity, thyroid volume and quality of life was observed within and between the groups. Subclinical hypothyroidism was diagnosed in 2 patients of the placebo group versus 1 patient in each Semet group. Serum CXCL-9 and −10 were significantly reduced in both Semet groups at 6 and 12 months, while they remained unchanged or increased in the placebo group. CXCL-11, TNFα and IFNγ showed a transient decrease at 6 months in both Semet groups but returned nearly to the basal levels at 12 months.
Conclusions
Semet supplementation had no positive effect on thyroid echogenicity or TPOAb in our patients. However, we observed a Semet-dependent downregulation of the IFNγ-inducible chemokines, especially CXCL-9 and −10, which may serve as helpful biomarkers in future selenium supplementation trials.
Key Words: Selenomethionine, Chemokines, Thyroiditis
Introduction
Autoimmune thyroiditis (AIT) is a chronic inflammation of the thyroid gland, which affects 10% of females and 2% of the male population [1]. An inverse correlation between serum selenium concentration and the incidence of AIT has been observed, particularly in areas with selenium deficiency [2], which affects the oxidative balance of the thyroid cells [3].
Some studies have reported that selenium supplementation, either as selenomethionine (Semet) or as sodium selenite, can significantly reduce anti-thyroperoxidase antibody (TPOAb) levels [1,4,5,6,7,8] and ameliorate [1,6] or prevent worsening of thyroid echogenicity [8]. These results were not confirmed by two other studies [9,10], and notably, no study has observed a positive effect of selenium supplementation on normalization of TSH or levothyroxine (LT4) dose reduction in patients treated for hypothyroidism. The majority of the studies have used 200 µg of selenium per day and two studies have administered 100 or 80 µg of the element per day [5,8].
From the available data published to date, no consistent health effects of selenium supplementation in AIT can be deduced, and there are no biomarkers identified that are suitable to identify which patients may profit most from an adjuvant selenium supplementation [11].
A subgroup of chemokines, CXCL-9, −10 and −11, have been reported to be elevated in the serum of patients with AIT, especially those who are hypothyroid [12,13]. These proinflammatory cytokines are secreted upon IFNγ stimulation, potentially by both lymphocytes and thyroid follicular cells, and they may recruit activated Th1 lymphocytes to the inflammation site, thus maintaining and amplifying the autoimmune process in the thyroid [12,14,15]. Selenium has been shown to exert immunomodulatory effects by, for example, inhibiting the release of IFNγ and TNFα by lymphocytes [16,17].
The aim of this prospective, randomized, controlled study was to evaluate, over 12 months, the effect of two different doses of Semet (80 or 160 µg/day) versus placebo in euthyroid women with AIT, in terms of modulation of established (reduction of anti-thyroid antibodies and improvement of thyroid echogenicity) and novel (decrease of CXCL-9, −10 and −11) markers of AIT. Besides serum selenium concentration, chemokine regulators like TNFα and INFγ, as well as thyroid function and volume and quality of life (QoL), were also monitored and analyzed.
Patients and Methods
Patients
Sixty female patients, aged 21-65 years (mean ± standard deviation, SD = 43 ± 11 years), with a known diagnosis of AIT and a normal thyroid function and who had not received LT4 replacement therapy, were consecutively selected from our outpatient clinic. AIT was defined by the presence of elevated TPOAb and/or anti-thyroglobulin autoantibody (TgAb) serum levels (≥100 U/ml) and a characteristic thyroid ultrasound pattern (scattered or widespread hypoechogenicity). The study was approved by the local ethics committee and all patients gave their written informed consent before participating.
Patients were randomly divided into 3 groups (20 patients per group) according to the treatment modality: 80 µg/day of Semet (80-Semet), 160 µg/day of Semet (160-Semet) or placebo, in a single oral administration daily for a total study period of 12 months.
Methods
Thyroid hormones, TPOAb/TgAb, urinary iodine excretion (UIE), thyroid volume and echogenicity (gray-scale method), and QoL (SF-36 questionnaire), were evaluated basally and every 3 months. Serum CXCL-9, −10 and −11, TNFα, IFNγ, selenoprotein P (SePP), selenium, and plasma glutathione peroxidase (GPx3) were determined every 6 months. All the measurements were performed in a blinded fashion with respect to patient characteristics.
Serum TSH, free triiodothyronine (fT3), free thyroxine (fT4), TPOAb, and TgAb were measured by immunometric assays (chemiluminescence; Immulite 2000; Diagnostic Products Corporation, Los Angeles, Calif., USA). UIE was measured using a colorimetric method (Auto-Analyzer 3; Bran+Luebbe, Gallarate, Italy). Serum CXCL-9, −10 and −11 were measured by a quantitative sandwich immunoassay (R&D Systems, Minneapolis, Minn., USA) and IFNγ and TNFα by ELISA (R&D Systems, Milan, Italy) following the manufacturer's instructions.
Serum SePP was measured by a sandwich assay as described earlier [18]. GPx3 activity was determined by the coupled enzymatic assay with t-butyl-hydroperoxide as substrate [19]. Serum selenium was measured by an atomic fluorescence spectrometer (AFS TITAN 8220; Beijing Titan Instruments, Co., Ltd).
Thyroid Ultrasound
Thyroid ultrasound was performed with a color Doppler apparatus (AU 590 Asynchronous; Esaote Biomedica, Florence, Italy) and a 7.5 -MHz linear transducer. For each patient thyroid volume was calculated by the ellipsoid formula (width × depth × length × 0.524) [20].
Echogenicity was assessed by converting high-resolution captured images of each thyroid lobe into a spectrum of gray-scale pixels, ranging from 0 (corresponding to black) to 255 (corresponding to white) using the public domain software (ImageJ); a mean value for each spectrum was then obtained.
Statistics
The sample size was determined by increasing the number of subjects by 50%, calculated by the Student t test for paired data, in order to achieve a probability of type-I error equal to 0.05, a study power not lower than 80% and SD of paired differences not higher than 120% of the corresponding mean. A sample size of at least 20 patients per group was then calculated [21]. The Anderson-Darling test was used to estimate the normality of each variable considered in the study.
Basal levels of study parameters (age, TSH, fT3, fT4, serum selenium, thyroid echogenicity) were defined as means ± SD or as medians with interquartile ranges (BMI, TPOAb, TgAb, UIE, CXCL-9, −10, and −11, TNFα, IFNγ).
Intragroup differences were analyzed using the Wilcoxon signed-rank test if the distribution of parameters was not Gaussian (TPOAb, TgAb, UIE, CXCL-9, −10, and −11, TNFα, IFNγ) and the t test for paired data if the parameters had a normal distribution (TSH, fT3, fT4, thyroid echogenicity defined by a mean gray-scale pixel value, serum selenium concentration). Differences among the groups were analyzed using the Kruskal-Wallis test or one-way ANOVA test for continuous parameters and the χ2 test for nominal parameters. Correlation between parameters was calculated using Spearman's rank correlation coefficient.
Statistical analysis was performed using StatView software for Windows version 5.0.1 (SAS Institute, Cary, N.C., USA). A p value <0.05 was always assumed for the statistical significance of sample differences.
Results
Patient Baseline Characteristics
The 3 groups did not differ in terms of clinical and biochemical parameters (table 1). Patients were all euthyroid without LT4 replacement. A total of 54/60 patients (90%) showed positivity (defined by serum values ≥100 UI/ml) for TPOAb and 29/60 (48.3%) were negative for TgAb (defined by levels <20 UI/ml). The median UIE (91.5 μg/l) of the study population was consistent with the UIE of the Italian general population [22]. The mean serum selenium concentration was 81.8 ± 14.6 μg/l (median = 83; range = 32-112) and was consistent with the values reported in Europeans [23]. Thyroid echogenicity, measured as mean gray-scale pixel value (89.8 ± 16.9), was consistent with the levels reported in patients with AIT [8].
Table 1.
Baseline characteristics of the patients
| Clinical and biochemical parameters | Patients treated with placebo (n = 20) | Patients treated with 80-Semet (n = 20) | Patients treated with 160-Semet (n = 20) | p |
|---|---|---|---|---|
| Age, years | 43.0 ± 11.2 | 48.8 ± 14 | 46.9 ± 7.6 | n.s. |
| BMI | 22.4 (5.3) | 23.6 (8.3) | 22.5 (4.4) | n.s. |
| Smokers, n (%) | 8/20 (40) | 3/20 (15) | 4/20 (20) | n.s. |
| TSH, μU/ml | 2.23 ± 0.95 | 2.43 ± 0.9 | 2.62 ± 0.91 | n.s. |
| fT3, pg/ml | 3.18 ± 0.28 | 3.1 ± 0.31 | 3.11 ± 0.35 | n.s. |
| fT4, pg/ml | 8.31 ± 1.23 | 8.17 ± 1.1 | 8.05 ± 1.13 | n.s. |
| TPOAb, U/ml | 408.5 (557) | 409.5 (718.5) | 186 (436) | n.s. |
| TgAb, U/ml | 40.5 (312) | 0 (53.65) | 31.6 (216) | n.s. |
| UIE, μg/l | 105.5 (127) | 90.5 (84.5) | 78 (99) | n.s. |
| Selenium serum concentration, μg/l | 82.1 ± 13.8 | 82.7 ± 11.6 | 80.5 ± 18.2 | n.s. |
| Thyroid volume, ml | 11.7 (3.4)a | 10.4 (4.4) | 9.0 (4.2)a | 0.014a |
| Thyroid echogenicity, gsp | 90.86 ± 13.09 | 89.96 ± 20.43 | 92.43 ± 14.9 | n.s. |
Values are presented as means ± SD for normal parameters or as medians (IQR) for non-Gaussian parameters. Intergroup differences were tested with the Kruskal-Wallis test or one-way ANOVA test for continuous parameters and the χ2 test for nominal parameters. gsp = Grey-scale pixels. p < 0.05: statistically significant.
The 3 groups showed similar characteristics except for the thyroid volume, which was smaller in the 160-Semet group compared to the placebo group.
Anti-Thyroid Antibodies
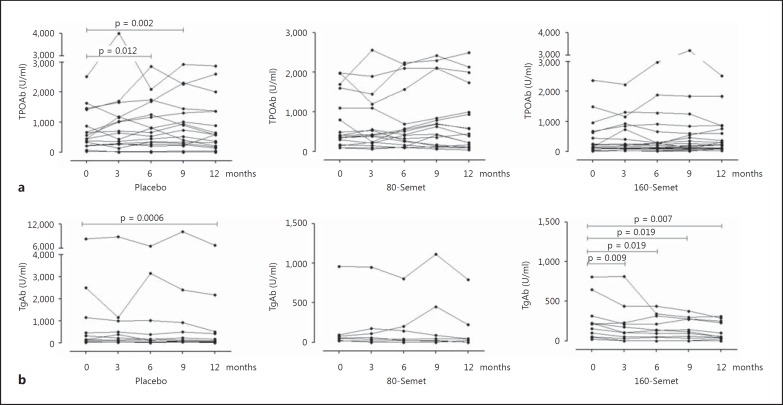
TPOAb levels showed a significant increase in the placebo group at 6 and 9 months, from a basal median of 408.5 to 594.5 UI/ml (p = 0.012) and to 518 UI/ml (p = 0.0018), respectively, and then remained stable, while they were unchanged compared to baseline in the 2 Semet groups during the follow-up (fig. 1a).
Fig. 1.
Serum levels of TPOAb (a) and TgAb (b) in patients randomized to Semet (80 or 160 µg) or placebo. Intragroup differences were tested with Wilcoxon signed-rank test and intergroup differences with Kruskal-Wallis test. p < 0.05: statistically significant. a No significant increase of TPOAb was observed in the 2 Semet groups. b TgAb showed a significant and stable reduction in the 160-Semet group. There was no significant difference in terms of TPOAb and TgAb levels between the groups at any time point.
TgAb levels were significantly reduced in the 160-Semet group, reaching the lowest values at 12 months, from a basal median of 212 to 54.1 U/ml (p = 0.007). However, TgAb levels also showed a significant decrease in the placebo group at 12 months, from a basal median of 143.5 to 87.8 U/ml (p = 0.0006). TgAb values remained unchanged in the 80-Semet group. Patients with undetectable values were excluded from the analysis (fig. 1b). For both TPOAb and TgAb there was no statistical difference between the groups at any time point.
Thyroid Echogenicity and Volume
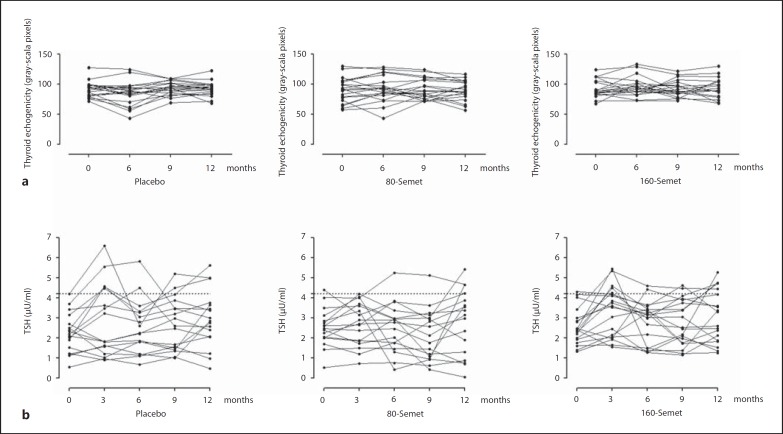
There was no change in thyroid echogenicity evaluated by the gray-scale method within and between the groups (fig. 2). Similarly, thyroid volume remained unchanged within the groups but was statistically different at baseline and at 6 months between the placebo and the 160-Semet group, where it was slightly smaller (online suppl. table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000439589).
Fig. 2.
Thyroid echogenicity (a) and thyroid function (b) in patients randomized to Semet (80 or 160 µg) or placebo. Intragroup differences were tested with paired t test and intergroup differences with one-way ANOVA test. p < 0.05: statistically significant. a Echogenicity defined as mean gray-scale pixels, in a spectrum ranging from 0 (corresponding to black) to 255 (corresponding to white). No significant difference was observed within and between the groups. b Two patients developed subclinical hypothyroidism in the placebo group compared to 1 patient in each Semet group.
Thyroid Function
Subclinical hypothyroidism, defined as TSH levels higher than 4.2 µU/ml in two consecutive measurements, was observed in 2 patients of the placebo group and 2 patients treated with Semet, 1 for each dose (fig. 2).
Serum Selenium
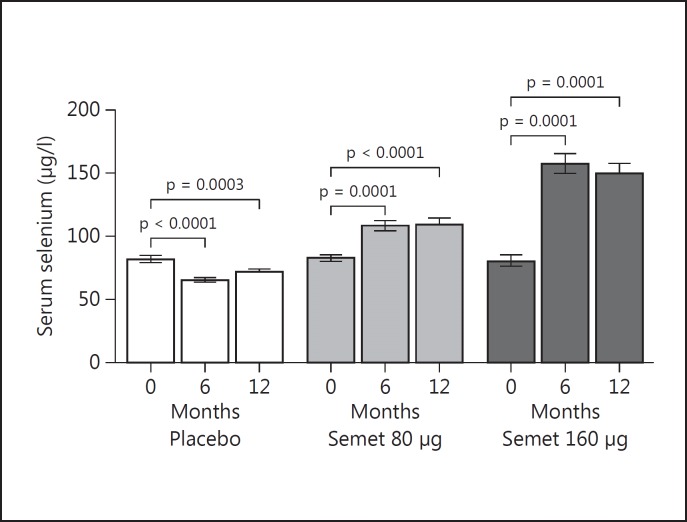
Serum selenium levels increased significantly in the 2 Semet groups in a dose-dependent fashion, from a basal median of 84 to 112 µg/l at 6 months in the 80-Semet group and from a basal median of 80 to 156 µg/l at 6 months in the 160-Semet group, without a further increase at 12 months (fig. 3).
Fig. 3.
Serum selenium levels in patients randomized to Semet (80 or 160 µg) or placebo. Intragroup differences were tested with paired t test. p < 0.05: statistically significant. Serum selenium levels increased significantly at 6 months in a dose-dependent fashion in the 2 Semet groups and there was no further increase at 12 months, remaining in a safe range of concentration.
Selenoprotein P and Glutathione Peroxidase
SePP and GPx3 concentrations were analyzed in the 80-Semet group only, as the samples in the placebo and 160-Semet group had undergone multiple rounds of freezing and thawing. No significant differences were observed in GPx3 activities (online suppl. fig. 1a) and SePP concentrations (online suppl. fig. 1b) after 6 or 12 months of supplementation, indicating a moderately replete selenium status of the participants already at study entry.
Chemokines and Cytokines
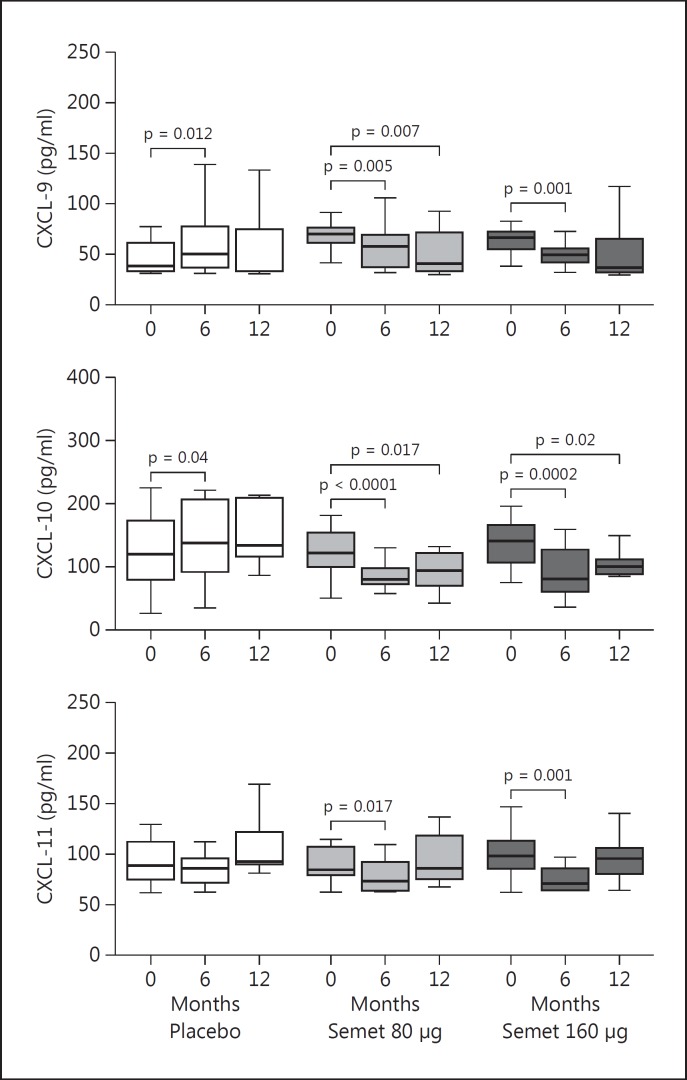
Serum CXCL-9 levels were significantly reduced in the 80-Semet group after 12 months of supplementation (from a basal median of 70 to 40.9 pg/ml, p = 0.007), while in the 160-Semet group they showed a significant decrease already at 6 months (from a basal median of 66.4 to 49.0 pg/ml, p = 0.001) and then remained stable. Serum CXCL-10 values were significantly reduced after 12 months of supplementation in the 80-Semet group (from a basal median of 122.5 to 93.8 pg/ml, p = 0.017) and in the 160-Semet group (from a basal median of 141.7 to 99.6 pg/ml, p = 0.002). Serum CXCL-11 levels were significantly reduced after 6 months of supplementation in the 80-Semet group (from a basal median of 84.4 to 73.7 pg/ml, p = 0.017) and in the 160-Semet group (from a basal median of 99.2 to 71.9 pg/ml, p = 0.001); however, after 12 months CXCL-11 had returned to the basal values (fig. 4).
Fig. 4.
IFNγ-inducible chemokine levels in patients randomized to Semet (80 or 160 µg) or placebo. Intragroup differences were tested with Wilcoxon signed-rank test. p < 0.05: statistically significant. Serum CXCL-9 and −10 levels showed a significant and stable decrease in the 2 Semet groups compared to placebo.
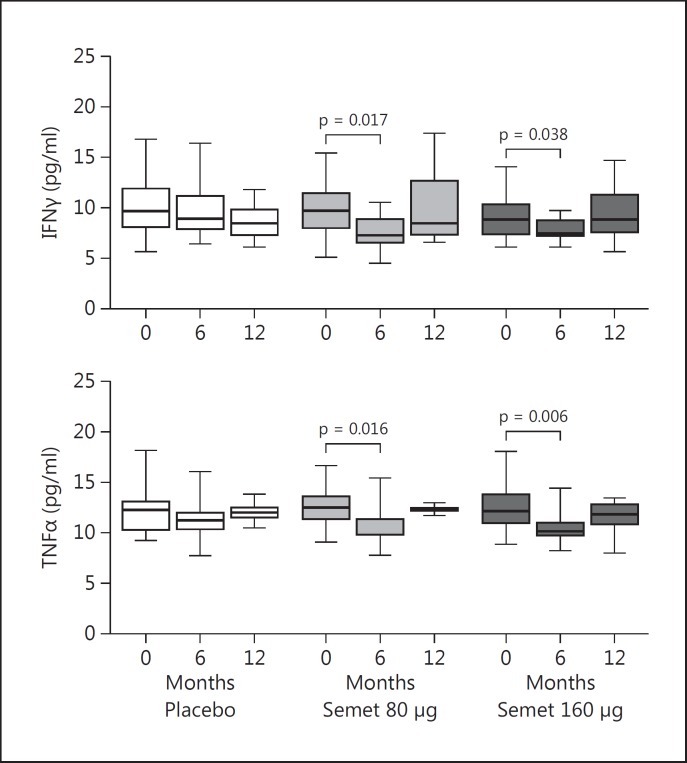
Serum IFNγ was reduced after 6 months of supplementation in the 80-Semet group (from a basal median of 9.7 to 7.3 pg/ml, p = 0.017) and in the 160-Semet group (from a basal median of 8.9 to 7.8 pg/ml, p = 0.055) but after 12 months, IFNγ increased again, almost returning to the basal values (fig. 5). Serum TNFα showed a significant decrease after 6 months of supplementation in the 80-Semet group (from a basal median of 12.4 to 9.9 pg/ml, p = 0.016) and in the 160-Semet group (from a basal median of 12.1 to 10.1 pg/ml, p = 0.006); however, after 12 months TNFα had returned back to almost basal levels.
Fig. 5.
Regulatory cytokine levels in patients randomized to Semet (80 or 160 µg) or placebo. Intragroup differences were tested with Wilcoxon signed-rank test. p < 0.05: statistically significant. Serum IFNγ and TNFα levels showed a transient decrease at 6 months in the 2 Semet groups compared to placebo.
Quality of Life
QoL as assessed by the SF-36 questionnaire was not significantly affected by Semet, as determined at baseline and after 6 and 12 months of supplementation within and between the groups (online suppl. table 1).
Discussion
Our study was the first to compare two forms of Semet supplementation, a rather physiological (80 μg/day) and a supraphysiological (160 μg/day) dose, versus placebo, and to evaluate the effect of Semet on the IFNγ-inducible chemokines CXCL-9, −10 and −11 in euthyroid women with AIT, who had not received LT4 replacement therapy, for a total study period of 12 months.
In the 80- and 160-Semet groups we did not observe any change in the TPOAb levels compared to baseline. However, TPOAb increased significantly in the placebo group, suggesting a potential protective effect of selenium on disease progression in the Semet treatment groups. Our data are consistent with two previous studies [9,10]. Conversely, other authors [1,4,5,6,7] reported a significant reduction in TPOAb values as early as 3 months after supplementation.
With regard to the TgAb levels, our data are consistent with those of Turker and Karapolat [5], who observed a decrease in the TgAb values in the group of patients treated with 200 µg/day of Semet after 3 months. However, in our study, a reduction of TgAb was also observed at 12 months in the placebo group; therefore this effect of Semet remains controversial.
Regarding echogenicity, we did not observe any change in the Semet treatment groups compared to baseline. On the contrary, one study, using 80 µg/day of selenite [8], showed a prevention of echogenicity worsening after 6 months of supplementation and another study, using 200 µg/day of selenite, reported a normalization of thyroid ultrasound pattern in 25% of patients [1]. However, in the latter case the authors used a qualitative method for this analysis and therefore it is difficult to make any comparison with our study.
The discrepant results in the literature, in terms of selenium effect on anti-thyroid antibodies and thyroid echogenicity, are a matter of intense discussion [11,24,25]. Several hypotheses and parameters have been advocated to explain the contradictory findings: a different selenium intake, LT4 administration (known to have itself an immuno-modulatory effect [26]) and TPOAb basal levels and their method of measurement.
An inverse relationship between serum selenium concentration and thyroid volume is known from a large epidemiological study [2] but we did not find this correlation likely due to the few patients with goiter (13%) and the short duration of the trial. Moreover, in our study selenium supplementation apparently had no effect on the QoL of the patients, which is in agreement with a recent study [9].
Regarding thyroid function, we observed few cases of hypothyroidism: 2 in the placebo group compared to 1 in each Semet group. The incidence of hypothyroidism in patients with AIT is low, about 4% per year. Our study, as well as other similar studies, had a duration of just 1 year and our sample size was relatively small. These limitations probably explain why we did not observe a significant difference in the occurrence of hypothyroidism between the placebo and the Semet groups.
A strength of our study was the analysis of a group of chemokines (CXCL-9, −10 and −11) actively involved in the pathogenesis of AIT. Our results indicate a specific, significant and stable reduction of two of them (CXCL-9 and −10) by Semet supplementation, while they remained unchanged or even increased in the placebo group. These findings suggest a positive immunomodulatory effect of Semet (partly mediated by the downmodulation of some regulatory cytokines), strengthening the rationale for selenium supplementation in patients with AIT even though positive health benefits were not (yet) detectable in our study. Moreover, these chemokines may serve as meaningful biomarkers of the success of selenium supplementation during study initiation and monitoring and may be helpful in the stratification of those AIT patients who are likely to benefit from selenium supplementation.
Finally, it needs to be mentioned that no side effects or significant changes in blood glucose levels were observed in any of the treatment groups over the course of this selenium supplementation study.
Conclusions
In our study, Semet supplementation with 80 or 160 μg was ineffective in positively reducing TPOAb levels in euthyroid women with AIT, nor did it positively affect thyroid echogenicity. However, with the chemokines CXCL-9 and −10, we succeeded in identifying two novel selenium-dependent biomarkers in AIT, which may prove of advantage in future trials for monitoring selenium supplementation success and for the identification of patients who may positively respond to adjuvant selenium supplementation. These hypotheses need to be tested in larger trials involving also patients with a more diverse range of selenium and iodine status and disease severity, respectively.
Disclosure Statement
No competing financial interests exist.
Supplementary Material
Supplementary Table
Supplementary Fig
Acknowledgment
This work was kindly supported by Ibsa Farmaceutici Italia.
References
- 1.Gärtner R, Gasnier BC, Dietrich JW, Krebs B, Angstwurm MW. Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations. J Clin Endocrinol Metab. 2002;87:1687–1691. doi: 10.1210/jcem.87.4.8421. [DOI] [PubMed] [Google Scholar]
- 2.Derumeaux H, Valeix P, Castetbon K, Bensimon M, Boutron-Ruault MC, Arnaud J, Hercberg S. Association of selenium with thyroid volume and echostructure in 35- to 60-year-old French adults. Eur J Endocrinol. 2003;148:309–315. doi: 10.1530/eje.0.1480309. [DOI] [PubMed] [Google Scholar]
- 3.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 4.Duntas LH, Mantzou E, Koutras DA. Effects of a six-month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur J Endocrinol. 2003;148:389–393. doi: 10.1530/eje.0.1480389. [DOI] [PubMed] [Google Scholar]
- 5.Turker O, Karapolat I. Selenium treatment in autoimmune thyroiditis. Thyroid. 2006;16:1326. [PubMed] [Google Scholar]
- 6.Gärtner R, Gasnier BC. Selenium in the treatment of autoimmune thyroiditis. Biofactors. 2003;19:165–170. doi: 10.1002/biof.5520190309. [DOI] [PubMed] [Google Scholar]
- 7.Mazokopakis EE, Papadakis JA, Papadomanolaki MG, Batistakis AG, Giannakopoulos TG, Protopapadakis EE, Ganotakis ES. Effects of 12 months treatment with L-selenomethionine on serum anti-TPO levels in patients with Hashimoto's thyroiditis. Thyroid. 2007;17:609–612. doi: 10.1089/thy.2007.0040. [DOI] [PubMed] [Google Scholar]
- 8.Nacamulli D, Mian C, Petricca D, Lazzarotto F, Barollo S, Pozza D, Masiero S, Faggian D, Plebani M, Girelli ME, Mantero F, Betterle C. Influence of physiological dietary selenium supplementation on the natural course of autoimmune thyroiditis. Clin Endocrinol (Oxf) 2010;73:535–539. doi: 10.1111/j.1365-2265.2009.03758.x. [DOI] [PubMed] [Google Scholar]
- 9.Eskes SA, Endert E, Fliers E, Birnie E, Hollenbach B, Schomburg L, Köhrle J, Wiersinga WM. Selenite supplementation in euthyroid subjects with thyroid peroxidase antibodies. Clin Endocrinol (Oxf) 2014;80:444–451. doi: 10.1111/cen.12284. [DOI] [PubMed] [Google Scholar]
- 10.Karanikas G, Schuetz M, Kontur S, Duan H, Kommata S, Schoen R, Antoni A, Kletter K, Dudczak R, Willheim M. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid. 2008;18:7–12. doi: 10.1089/thy.2007.0127. [DOI] [PubMed] [Google Scholar]
- 11.Schomburg L. Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol. 2011;8:160–171. doi: 10.1038/nrendo.2011.174. [DOI] [PubMed] [Google Scholar]
- 12.Antonelli A, Rotondi M, Fallahi P, Romagnani P, Ferrari SM, Buonamano A, Ferrannini E, Serio M. High levels of circulating CXC chemokine ligand 10 are associated with chronic autoimmune thyroiditis and hypothyroidism. J Clin Endocrinol Metab. 2011;89:5496–5499. doi: 10.1210/jc.2004-0977. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli A, Ferrari SM, Frascerra S, Di Domenicantonio A, Nicolini A, Ferrari P, Ferrannini E, Fallahi P. Increase of circulating CXCL9 and CXCL11 associated with euthyroid or subclinically hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab. 2011;96:1859–1863. doi: 10.1210/jc.2010-2905. [DOI] [PubMed] [Google Scholar]
- 14.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-López MA, Sancho D, Sánchez-Madrid F, Marazuela M. Thyrocytes from autoimmune thyroid disorders produce the chemokines IP-10 and Mig and attract CXCR3+ lymphocytes. J Clin Endocrinol Metab. 2001;86:5008–5016. doi: 10.1210/jcem.86.10.7953. [DOI] [PubMed] [Google Scholar]
- 16.Krysiak R, Okopien B. The effect of levothyroxine and selenomethionine on lymphocyte and monocyte cytokine release in women with Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2011;96:2206–2215. doi: 10.1210/jc.2010-2986. [DOI] [PubMed] [Google Scholar]
- 17.Kim IY, Stadtman TC. Inhibition of NF-κB DNA binding and nitric oxide induction in human T cells and lung adenocarcinoma cells by selenite treatment. Proc Natl Acad Sci USA. 1997;94:12904–12907. doi: 10.1073/pnas.94.24.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollenbach B, Morgenthaler NG, Struck J, Alonso C, Bergmann A, Kohrle J, Schomburg L. New assay for the measurement of selenoprotein P as a sepsis biomarker from serum. J Trace Elem Med Biol. 2008;22:24–32. doi: 10.1016/j.jtemb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Schomburg L, Schweizer U, Holtmann B, Flohé L, Sendtner M, Köhrle J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J. 2003;370:397–402. doi: 10.1042/BJ20021853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunn J, Block U, Ruf G, Bos I, Kunze WP, Scriba PC. Volumetric analysis of thyroid lobes by real-time ultrasound. Dtsch Med Wochenschr. 1981;106:1338–1340. doi: 10.1055/s-2008-1070506. [DOI] [PubMed] [Google Scholar]
- 21.Siegal S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw Hill; 1988. [Google Scholar]
- 22.International Council for the Control of Iodine Deficiency Disorders (ICCIDD) 2014 National Iodine Status. http://www.iccidd.org/ (accessed November 2014).
- 23.NatCen Social Research; MRC Human Nutrition Research. University College London Medical School National Diet and Nutrition Survey Years 1-4, 2008/09-2011/12 (computer file), ed 6. Colchester, UK Data Archive (distributor), 2014. http://dx.doi.org/10.5255/UKDA-SN-6533-5.
- 24.Duntas LH, Benvenga S. Selenium: an element for life. Endocrine. 2015;48:756–775. doi: 10.1007/s12020-014-0477-6. [DOI] [PubMed] [Google Scholar]
- 25.Van Zuuren EJ, Albusta AY, Fedorowicz Z, Carter B, Pijl H. Selenium supplementation for Hashimoto's thyroiditis. Cochrane Database Syst Rev. 2013;6:CD010223. doi: 10.1002/14651858.CD010223.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittag J, Behrends T, Hoefig CS, Vennström B, Schomburg L. Thyroid hormones regulate selenoprotein expression and selenium status in mice. PLoS One. 2010;5:e12931. doi: 10.1371/journal.pone.0012931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table
Supplementary Fig