Abstract
Pseudomyxoma peritonei is an extremely rare disease, characterised by mucinous ascites and implants, diffusely involving the peritoneal surfaces. Recent pathological and genetic advances indicate that they mostly originate from an appendiceal adenoma or adenocarcinoma. The successful treatment of peritoneal carcinomatosis requires a comprehensive management plan including cytoreductive surgery, intraoperative intraperitoneal heated chemotherapy and postoperative systemic chemotherapy. It is extremely rare to have intrathoracic spread of the disease at initial presentation. Some of the possible hypotheses of neoplastic cells spreading into the pleural cavity include congenital or acquired pleura-peritoneal communications, invasion of lymphovascular space and, rarely, through direct invasion of the diaphragm.
Background
Pseudomyxoma peritonei is an extremely rare disease, characterised by a ‘gelatinous abdomen’ or mucinous ascites from intra-abdominal neoplastic mucin secreting cells proliferating on the peritoneal surface.1 The annual incidence has been reported to be two per million individuals, the majority being secondary to mucinous adenocarcinoma of the appendix. Ovarian malignancy is another important source in women. Primary appendiceal malignancy is found in less than 2% of surgically removed appendices and accounts for less than 1% of intestinal malignancies.1 Although carcinoid tumour is the commonest appendicular malignancy, mucinous adenocarcinoma is unique as it causes cystic dilatation of the appendix due to accumulation of copious amounts of gelatinous material. This usually leads to appendicular rupture, resulting in dissemination throughout the peritoneal cavity in the form of gelatinous deposits.2 3 The mucus producing cells continue to proliferate on the peritoneal surface and mucinous fluid gradually fills the peritoneal cavity, resulting in the characteristic ‘jelly belly’. The patient usually remains asymptomatic for a long time before the diagnosis is performed.4 The most common clinical presentation includes increase in abdominal girth with occasional protrusion of umbilical and inguinal hernias.3 It can present as intestinal obstruction from bowel adhesion in the late stage of the disease. A rarer presentation of this rare disease includes intrathoracic extension of mucinous material at the time of presentation and a few case reports have been published so far.
Case presentation
A 35-year-old Caucasian man with no significant medical history presented to our outpatient office, with vague diffuse abdominal pain for the last 4 months, which localised more to the right lower abdomen over the past 3 weeks. The pain was a continuous 5/10, and unrelated to food and bowel movement. The patient also noticed increase in abdominal girth, which he attributed to dietary habit and lack of exercise. He denied any history of nausea, vomiting, change in bowel habit, urinary symptoms, weight loss, loss of appetite, melena and umbilical and inguinal herniation. A review of systems was negative for chest pain, cough, dyspnoea, fever and leg swelling. On physical examination, vital signs were within normal limits. The abdomen was soft, bowel sound was present, there was no guarding or rigidity, tenderness was noted on deep palpation in the right lower and upper abdomen, flanks were full, there was no hepatosplenomegaly and absent lymphadenopathy. Rectal examination and the rest of the physical examination were completely benign.
Investigations
Initial labs including complete blood count with differentials, complete metabolic panel, liver enzymes, amylase, lipase and urinalysis were within normal limits. Stool occult blood tested negative. Abdominal CT with contrast showed diffuse soft tissue, present throughout the abdomen and pelvis, involving the peritoneum (figures 1 and 2). The soft tissue pushed the bowel away from the abdominal wall, filling up the right paracolic gutter (figure 3). The mucinous soft tissue had density similar to fat. The classical ‘scalloping sign’ representing indentations on the capsular margins of the liver from the extrinsic pressure of the intraperitoneal mucinous implants was also seen (figure 3). There was also involvement of the lower portion of the thorax, including pleura and pericardium (figure 4).
Figure 1.
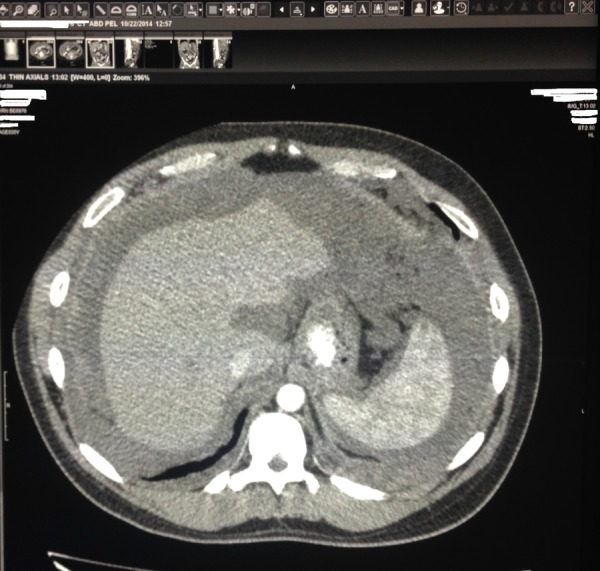
CT of the abdomen cross-sectional view: mucinous material of density similar to fat present throughout the abdomen and pelvis, involving the peritoneum.
Figure 2.
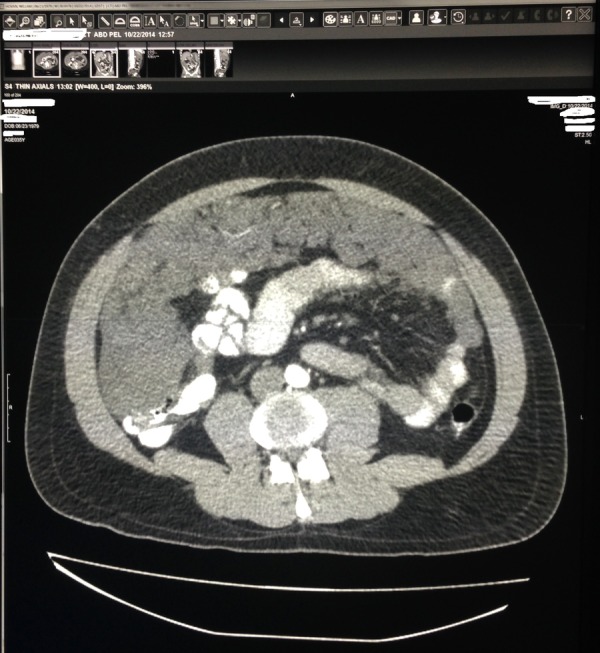
CT of the abdomen cross sectional view: mucinous material of density similar to fat present throughout the abdomen and pelvis, pushing intestines and mesentery to the centre.
Figure 3.
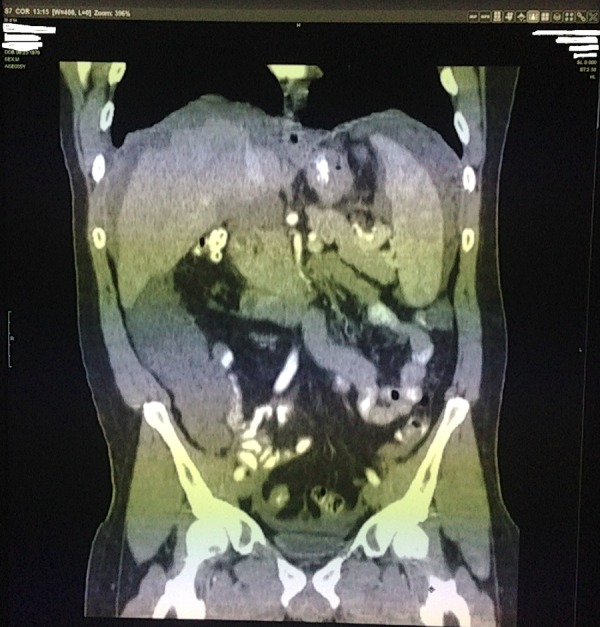
CT of the abdomen coronal view: soft tissue pushed the bowel away from the abdominal wall, filling up the right paracolic gutter. ‘Scalloping sign’ representing indentations on the capsular margins of the liver from the extrinsic pressure of the intraperitoneal mucinous implants.
Figure 4.
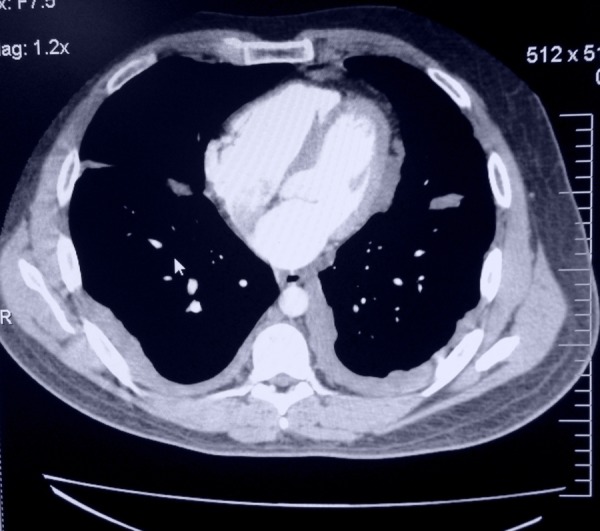
CT of the thorax cross-sectional view: Pseudomyxoma peritonei involving lower portion of the thorax including pleura and pericardium.
Treatment
The most likely radiological diagnosis was pseudomyxoma peritonei with rare spread into the thorax. Echocardiography was performed, which showed a restrictive pattern of ventricular filling and pericardial thickening suggestive of metastases. CT-guided biopsy of the abdominal mass was consistent with pseudomyxoma peritonei. However, no definite source was established. The patient and his family consented to proceed with laparoscopic surgery to identify the potential source and spread of the tumour in the abdomen. Extensive pseudomyxoma tumour and ‘omental caking’ were identified on the peritoneal surface during laparoscopic surgery. The appendix was found to be ruptured and surrounded by gelatinous material. Two surgeons agreed to remove the majority of the tumour mass fearing possible bowel adhesions and obstructions in the operating room. The family gave necessary consent for debulking surgery. Accordingly, open laparotomy was performed and a total of 8 lbs of pseudomyxoma tumour was removed from the abdomen. Two peritoneal drains were left behind. Histology analysis of the mucinous tissue and appendix confirmed diagnosis of pseudomyxoma peritonei from low-grade mucinous adenocarcinoma. The patient had an uneventful postoperative recovery and was started on a Folfox chemotherapy regimen. He was referred to a tertiary cancer centre for further management, where he underwent repeat laparotomy for further debulking and pleural biopsy, followed by intraperitoneal and intrapleural heated chemotherapy.
Discussion
Lack of large randomised control trials and multicentric outcome based studies has been a major challenge for this rare disease. Systemic chemotherapy has poor penetration on peritoneal mucus and some studies have proposed intraperitoneal hyperthermic chemoperfusion (HIPEC) following surgical cytoreduction of tumour burden. The rationale of HIPEC is to increase penetration of chemotherapeutic agents in the mucinous tumour, achieving therapeutic drug concentration while minimising systemic toxicity due to presence of a plasma peritoneal barrier.5 The most common forms of intrathoracic spread of pseudomyxoma peritonei include pleural effusion and pulmonary parenchymal metastasis.6 Pleural spread of pseudomyxoma peritonei mostly occurs following diaphragmatic injury during cytoreductive or debulking surgeries. Intrathoracic disease extension is extremely rare in the initial presentation. In a recent study, 23 (5.4%) out of 426 patients with pseudomyxoma peritonei had pleural extension, of which only four had intrathoracic extension before surgery.7 Intraoperative hyperthermic intrathoracic chemotherapy (HITHOC) along with cytoreductive surgery has been tried in patients of PMP involving pleura.7 8 Potential complications of HIPEC and HITHOC include systemic hyperthermia resulting in exacerbation of cardiac disease in patients with coronary artery disease, pulmonary oedema and acute lung injury.8 Pleural extension of the disease carries an extremely poor prognosis.7 Some of the possible hypotheses of neoplastic cells spreading into the pleural cavity include congenital or acquired pleura–peritoneal communications, invasion of lymphovascular space and, rarely, through direct invasion of the diaphragm.6
Learning points.
Pseudomyxoma peritonei is an extremely rare disease characterised by a ‘gelatinous abdomen’ or mucinous ascites.
The mucus producing cells continue to proliferate on the peritoneal surface, resulting in the characteristic ‘jelly belly’.
A rarer presentation of this rare disease includes intrathoracic extension of mucinous material at presentation.
The successful treatment of peritoneal carcinomatosis includes proper patient selection, complete resection of all visible disease, intraoperative intraperitoneal-heated chemotherapy and postoperative systemic chemotherapy.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Carr NJ. Current concepts in pseudomyxoma peritonei. Ann Pathol 2014;34:9–13. 10.1016/j.annpat.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 2.Panarelli NC, Yantiss RK. Mucinous neoplasms of the appendix and peritoneum. Arch Pathol Lab Med 2011;135:126–8. 10.5858/arpa.2011-0034-RA [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH, Ronnett BM, Archer A et al. Pseudomyxoma peritonei syndrome. Adv Surg 1996;30:233–80. [PubMed] [Google Scholar]
- 4.Cichon P, Drucis K, Kakol M et al. Pseudomyxoma peritonei spread into the right inguinal hernia sac a case report. Pol Merkur Lekarski 2013;35:217–20. [PubMed] [Google Scholar]
- 5.Vaira M, Cioppa T, DE Marco G et al. Management of pseudomyxoma peritonei by cytoreduction+HIPEC (hyperthermic intraperitoneal chemotherapy): results analysis of a twelve year experience. In Vivo 2009;23:639–44. [PubMed] [Google Scholar]
- 6.Geisinger KR, Levine EA, Shen P et al. Pleuropulmonary involvement in pseudomyxoma peritonei: morphologic assessment and literature review. Am J Clin Pathol 2007;127:135–43. 10.1309/601K2L2T7CR5U7G1 [DOI] [PubMed] [Google Scholar]
- 7.Pestieau SR, Esquivel J, Sugarbaker PH. Pleural extension of mucinous tumor in patients with pseudomyxoma peritonei syndrome. Ann Surg Oncol 2000;7:199–203. 10.1007/BF02523654 [DOI] [PubMed] [Google Scholar]
- 8.Dang A, Mansfield P, Ilsin B et al. Intraoperative hyperthermic intrathoracic chemotherapy for pleural extension of pseudomyxoma peritonei. J Cardiothorac Vasc Anesth 2007;21:265–8. 10.1053/j.jvca.2006.04.022 [DOI] [PubMed] [Google Scholar]


