Abstract
Bronchial anthracofibrosis (BAF), an emerging pulmonary disease due to long-standing exposure to biomass fuel smoke, is predominantly seen in females from developing nations. BAF is known to be associated with tuberculosis, pneumonia, chronic obstructive pulmonary disease and lung cancer, but the association of BAF with interstitial lung disease (ILD) is rare and yet to be highlighted. A 72-year-old woman with a 30-year history of exposure to biomass fuel smoke presented with dry cough and exertional dyspnoea. Imaging demonstrated interlobular, intralobular and peribronchovascular interstitial thickening and honeycombing adjoining the subpleural regions, suggestive of the usual interstitial pneumonia pattern. A restrictive pattern with diffusion defect was noted. Fibrebronchoscopy revealed a bluish-black anthracotic pigmentation with a narrowed and distorted left upper lobe, and apical segment of left lower lobe bronchus, confirming BAF. A diagnosis of BAF with ILD was made. To the best of our knowledge, this is the first detailed description of this association.
Background
In 1813, Pearson coined the term ‘anthracosis’ to describe bluish-black discolouration of the bronchial mucosa due to inhalation of soot. This was predominantly seen in coal workers, cigarette smokers and city dwellers.1 Narrowing of the middle lobe due to perforated tuberculous lymph nodes was described in eight female patients by Abraham Cohen,2 in 1951. Six of these patients had anthracotic pigmentation in the right middle lobe. This would appear to be the first ever portrayal of a clinical entity that is now labelled as ‘bronchial anthracofibrosis’, a term coined by Chung et al,3 from Korea. This description highlighted the occurrence of bronchoscopically visible anthracotic pigmentation associated with narrowing and/or obliteration of the bronchi. This disease entity was characterised when the authors documented this phenomenon in 28 elderly patients of whom 20 were females with significant exposure to wood smoke. In three-fourths of these patients, the middle lobe was involved and active tuberculosis was seen in 61% of them. Although it was initially postulated that BAF was caused by tuberculosis, there is now a mounting body of evidence to suggest that long-standing exposure to biomass fuel smoke was the most important factor in the occurrence of BAF.4
It is thought that half the world's population is dependent on biomass fuel for their daily existence.5 Long-standing exposure to biomass fuel smoke in poorly ventilated conditions is a common occurrence in developing countries. In India, it has been estimated that 76% of the rural population is dependent on biomass fuel for cooking.6
With increasing awareness of BAF as a distinct clinical entity, characteristic clinical, radiological and bronchoscopic findings have been described. A strong association with certain diseases such as tuberculosis, pneumonia, chronic obstructive pulmonary disease (COPD) and lung cancer is increasingly being recognised.4 7 However, its association with interstitial lung disease (ILD) is yet to be highlighted as only two studies8 9 mention the occurrence of BAF with ILD, in four patients. The rarity of such a description in the literature prompted us to report a case of a 72-year-old woman who presented with ILD and was also confirmed to have BAF on bronchoscopy. To the best of our knowledge, this is the first detailed description of the concomitant occurrence of both these clinical conditions.
Case presentation
A 72-year-old HIV-negative woman from a rural background was referred to our Institute for evaluation of cough and breathlessness of 15 years duration. The cough was predominantly dry and insidious in onset, but persistent and progressive. History of exertional dyspnoea was also present for the past 3 years. Although she was never a smoker, the patient had a significant history of 5–6 h/day exposure to biomass fuel smoke for 30 years while cooking in a poorly ventilated area. She had quit cooking on wood fire 20 years prior to presentation. Six years prior, based on her clinical and radiological profile, she had received antituberculous treatment for 9 months with no significant relief.
General physical examination revealed an elderly woman with no acute respiratory distress. There was no pallor, cyanosis, clubbing or lymphadenopathy. She was afebrile with a respiratory count of 18/min. Diaphragmatic excursion was comparable on both sides, with normal auscultatory findings. Oxygen saturation on room air was 96%.
Investigations
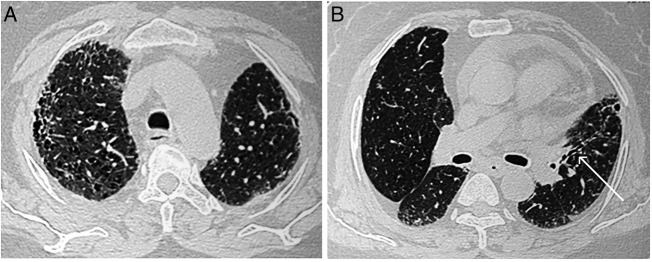
Total leucocyte count was 12 000 cells per mm3 with neutrophils constituting 76.5%. ECG, urine analysis, liver and renal functions were within normal limits. On presentation, chest radiograph revealed bilateral lower zone reticulonodular opacities with cardiomegaly (figure 1). High-resolution CT (HRCT) of the thorax demonstrated the presence of interlobular, intralobular and peribronchovascular interstitial thickening along with multiple hyperlucent cystic airspaces suggestive of honeycombing adjoining the subpleural regions with pericardial effusion. These findings on HRCT were suggestive of the usual interstitial pneumonia (UIP) pattern10 (figure 2A). In addition, multifocal narrowing of the left upper lobe bronchus was visible on HRCT, a feature characteristically seen in BAF (figure 2B). Two-dimensional echocardiography revealed normal chamber size and normal left ventricle systolic function (EF-58%) with grade I diastolic dysfunction and confirmed the presence of mild pericardial effusion. The peak and mean pulmonary artery pressures were within normal limits. Sputum stains and cultures for Mycobacterium tuberculosis and other aerobic organisms were negative. Antinuclear antibody, rheumatoid factor and anticyclic citrullinated peptide antibodies were not detected in serum.
Figure 1.
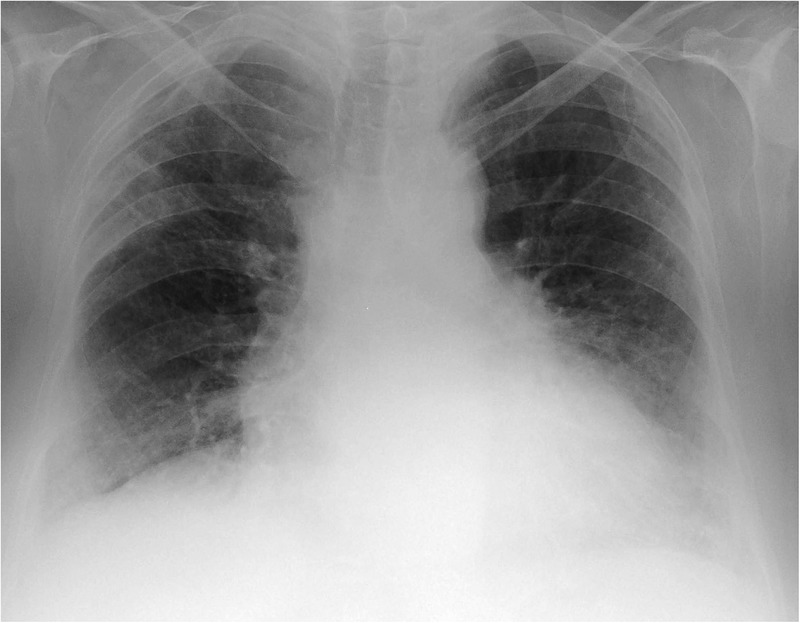
Chest X-ray showing bilateral lower zone reticulonodular opacities with cardiomegaly.
Figure 2.
(A) High-resolution CT (HRCT) of the thorax (lung window) showing presence of interlobular, intralobular and peribronchovascular interstitial thickening along with multiple hyperlucent cystic airspaces suggestive of honeycombing adjoining the subpleural regions along with pericardial effusion. (B) HRCT of the thorax (lung window) showing multifocal narrowing of the left upper lobe bronchus (white arrow).
Spirometry was normal with a forced vital capacity (FVC) of 1.58 L (86% of predicted), forced expiratory volume in 1 s (FEV1) of 1.22 L (82% of predicted) and FEV1/FVC ratio of 0.78 with no significant postbronchodilator response. However, a complete pulmonary function test (PFT) revealed a residual volume of 0.59 L (32% of predicted) and total lung capacity of 2.17 L (59% of predicted), suggestive of moderate restriction in lung functions. Diffusion capacity for carbon monoxide was 8.07 (52% of predicted). On 6 min walk test, the patient desaturated from 96% to 90% while covering a distance of 200 m. Based on imaging, PFT findings and the walk test, a diagnosis of ILD was considered and bronchoscopy planned for further evaluation.
Fibreoptic bronchoscopy (FOB) visualised dark bluish-black hyperpigmentation of the mucosa, suggestive of anthracotic patches with a narrowed and distorted but patent left upper lobe, and apical segment of left lower lobe bronchus (figure 3). The right bronchial tree was normal and patent. Bronchial/transbronchial biopsy could not be obtained as the patient desaturated during the procedure. Stains and cultures of the bronchial aspirate were negative for M. tuberculosis as well as for any other aerobic organism. The aspirate was also negative for Gene-Xpert.
Figure 3.
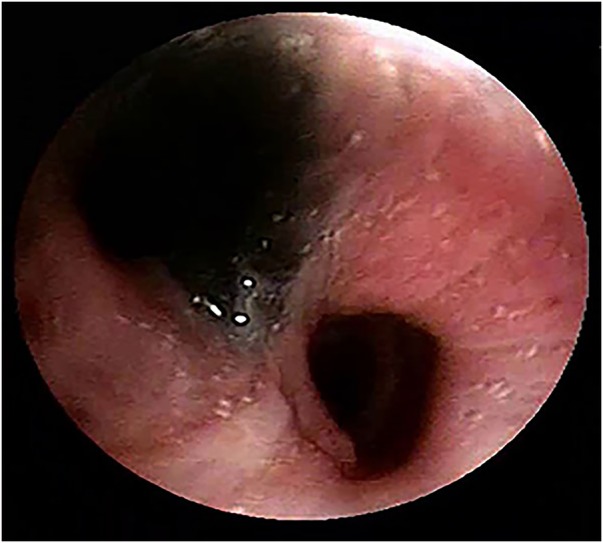
Fibreoptic bronchoscopic image showing anthracotic pigmentation of bronchial mucosa with narrowing and distortion of the left upper lobe bronchus.
Diagnosis
A diagnosis of BAF with ILD was made. The diagnosis of BAF was based on4 (1) long-standing history of biomass fuel smoke exposure, (2) presence of multifocal narrowing of left upper lobe bronchus on HRCT and (3) confirmed bronchoscopically by visualisation of (1) bluish-black mucosal hyperpigmentation, along with (2) narrowed and distorted left upper lobe and apical segment of left lower lobe bronchus. The diagnosis of ILD was based on (1) a restrictive pattern with reduced lung volumes and diffusion defect on complete PFT, (2) desaturation on 6 min walk test and (3) a UIP pattern on HRCT, as evidenced by the presence of interlobular, intralobular and peribronchovascular interstitial thickening along with multiple hyperlucent cystic airspaces suggestive of honeycombing adjoining the subpleural regions.
Treatment
The patient was initiated on oral corticosteroids in the form of deflazacort 0.6 mg/kg body weight, with which the patient experienced symptomatic improvement.
Outcome and follow-up
The patient was counselled regarding treatment of ILD and was apprised of the possible adverse consequences of the treatment involved. She then went back to her native place for continuation of therapy and was lost to follow-up.
Discussion
There is increasing recognition of BAF as a distinct clinical entity. This phenomenon was first documented in India in a 65-year-old woman who presented with a middle lobe syndrome.11 She had a long-standing history of exposure to wood smoke and, on FOB, anthracotic pigmentation of the middle lobe bronchus along with narrowing was visualised. Bronchial aspirate cultured M. tuberculosis. These patients are predominantly women from rural backgrounds who have spent most of their time cooking in poorly-ventilated kitchens. The diagnosis of BAF is confirmed only on FOB when bluish-black mucosal hyperpigmentation is visible along with narrowing and/or distortion of the associated bronchus. When pigmentation occurs alone, without anatomical distortion of the bronchi, this entity is known as bronchial anthracosis.
A study from Canada documented that BAF was more likely to develop in immigrants from the Indian subcontinent (50%) as compared to those from other Asian countries (3.7%).12 This was often associated with pulmonary tuberculosis.
BAF is known to be associated with a number of clinical conditions including tuberculosis, pneumonia, COPD and malignancy.4 7 Tuberculosis, once considered to be the aetiological agent of BAF, is now thought to be an association and is reported in 30% of patients with BAF.7 13 Although rare, the association of BAF and ILD has found mention in the literature. A search of the PubMed and other databases found mention of only four patients of BAF along with ILD, which were documented in two different case series.8 9 In a study from Korea,8 of the 114 patients with BAF, the authors mentioned that one had an associated UIP pattern, but no further information was available. While evaluating the radiological features of 27 patients of BAF from Turkey,9 the authors documented the presence of an ‘interstitial pattern’ on chest CT in three patients, but the detailed description of ILD on imaging was not mentioned.
On imaging, the UIP pattern is associated with interstitial or reticulonodular infiltrates. The classical description also includes areas of honeycombing, predominantly with a basal and subpleural distribution.10 Although a UIP pattern on HRCT has been classically described for idiopathic pulmonary fibrosis (IPF), it has also been documented in ILD associated with rheumatoid arthritis, chronic hypersensitivity pneumonitis, collagen vascular disease, drug toxicity, asbestosis, familial IPF and Hermansky-Pudlak syndrome.14 As mentioned above, our patient had a similar imaging appearance on HRCT suggestive of a UIP pattern as well. This was supported by a restrictive pattern along with diffusion defect on complete PFT and desaturation on 6 min walk test. Multifocal narrowing of the left upper lobe bronchus on HRCT is a characteristic feature of BAF, and pointed towards the diagnosis. We were unable to obtain a transbronchial biopsy as the patient desaturated during bronchoscopy, however, anthracotic pigmentation along with narrowing and distortion of the left upper lobe bronchus and apical segment of left lower lobe bronchus confirmed the presence of full-fledged BAF.
BAF, being a bronchoscopic diagnosis, presents the need to develop techniques that are non-invasive so that early diagnosis can be achieved. Definite treatment for BAF is yet to emerge.4 7 However, BAF is known to be associated with tuberculosis, pneumonia, COPD and malignancy, and it is imperative to treat these specific conditions to reduce morbidity.7 Oral corticosteroids have been evaluated in 14 patients with non-tuberculous BAF with clinical improvement observed in nine.15
The association of BAF and ILD is a distinct clinical rarity. It is still unclear whether this association is a cause and effect relationship or simply a chance occurrence. As awareness has increased, BAF is now being frequently recognised among elderly women with a history of prolonged biomass fuel smoke exposure in developing nations. BAF once again highlights the grave consequences of protracted biomass fuel smoke exposure.
Learning points.
Bronchial anthracofibrosis (BAF) should strongly be suspected in elderly women having significant exposure to biomass fuel smoke.
This is confirmed bronchoscopically on visualisation of anthracotic pigmentation along with narrowing and/or distortion of the associated bronchi.
This clinical entity is often associated with tuberculosis, pneumonia, chronic obstructive pulmonary disease and lung cancer.
BAF associated with interstitial lung disease has rarely been documented in the literature. The usual interstitial pneumonia pattern seen in our patient has been mentioned in one earlier patient.
It is still unclear whether this association has a cause and effect relationship or if it is entirely coincidental.
Footnotes
Contributors: SK, VP and AS collected the clinical data and reviewed the literature. SK, VP and AS drafted the manuscript and were responsible for the clinical work up of the patient. AS worked on the concept, and is responsible for the genuineness of the data and is also the guarantor of the paper. All the authors have read and approved the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Klotz O. Pulmonary anthracosis–a community disease. Am J Public Health (NY) 1914;4:887–916. 10.2105/AJPH.4.10.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen AG. Atelectasis of the right middle lobe resulting from perforation of tuberculous lymph nodes into bronchi in adults. Ann Intern Med 1951;35:820–35. 10.7326/0003-4819-35-4-820 [DOI] [PubMed] [Google Scholar]
- 3.Chung MP, Lee KS, Han J et al. Bronchial stenosis due to anthracofibrosis. Chest 1998;113:344–50. 10.1378/chest.113.2.344 [DOI] [PubMed] [Google Scholar]
- 4.Shah A. Bronchial anthracofibrosis: a perilous consequence of exposure to biomass fuel smoke [editorial]. Indian J Chest Dis Allied Sci 2015;57:151–3. [PubMed] [Google Scholar]
- 5.World Health Organization. World health statistics. Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- 6.Ministry of Statistics and Programme Implementation, Government of India. Energy sources of Indian households for cooking and lighting, NSS 66th round, July 2009- June 2010. Report No.: 542 New Delhi: National Sample Survey Office, 2012. [Google Scholar]
- 7.Gupta A, Shah A. Bronchial anthracofibrosis: an emerging pulmonary disease due to biomass fuel exposure. Int J Tuberc Lung Dis 2011;15:602–12. 10.5588/ijtld.10.0308 [DOI] [PubMed] [Google Scholar]
- 8.Lee HS, Maeng JH, Park PG et al. [Clinical features of simple bronchial anthracofibrosis which is not associated with tuberculosis] [Article in Korean]. Tuberc Respir Dis 2002;53:510–18. [Google Scholar]
- 9.Torun T, Gungor G, Ozmen I et al. Bronchial anthracostenosis in patients exposed to biomass smoke. Turkish Respir J 2007;8:48–51. [Google Scholar]
- 10.Hansell DM, Bankier AA, MacMahon H et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697–722. 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 11.Kala J, Sahay S, Shah A. Bronchial anthracofibrosis and tuberculosis presenting as a middle lobe syndrome. Prim Care Respir J 2008;17:51–5. 10.3132/pcrj.2008.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang J, Puttagunta L, Green F et al. Bronchial anthracofibrosis and tuberculosis in immigrants to Canada from the Indian subcontinent. Int J Tuberc Lung Dis 2010;14:231–7. [PubMed] [Google Scholar]
- 13.Mirsadraee MH, Asnashari AK, Attaran DM. Tuberculosis in patients with anthracosis of lung underlying mechanism or superimposed disease. Iran Red Crescent Med J 2011;13:670–3. 10.5812/kowsar.20741804.2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wuyts WA, Cavazza A, Rossi G et al. Differential diagnosis of usual interstitial pneumonia: when is it truly idiopathic? Eur Respir Rev 2014;23:308–19. 10.1183/09059180.00004914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang SJ, Lee SY, Kim SC et al. Clinical and radiological characteristics of non-tuberculous bronchial anthracofibrosis. Tuberc Respir Dis 2007;63:139–44. 10.4046/trd.2007.63.2.139 [DOI] [Google Scholar]