Abstract
A 2-year-old girl presented to hospital, with reduced consciousness and fever. She had a 4-week history of fever treated with two courses of amoxicillin for tonsillitis diagnosed in primary care. Neuroimaging revealed multiple cerebral abscesses and subdural empyema. Pus aspirated from the intracranial collections grew Fusobacterium necrophorum and meropenem was started. Following neurosurgery, the patient continued to be agitated with fluctuating fever. She underwent close monitoring with regular neuroimaging. To control the progression of intracranial infection, she underwent three separate neurosurgical procedures following which she made a good recovery. This case demonstrates how an organism rarely associated with childhood illnesses presented atypically and progressed into a complex potentially fatal intracranial infection requiring a high degree of neurosurgical intervention. Awareness of this organism is important. The combination of source control together with appropriate antibiotic use was crucial in controlling the infection.
Background
This case highlights the possible changing epidemiology of Fusobacterium necrophorum. There is increasing evidence to suggest an impact on the paediatric population, with potentially devastating consequences.
This case report raises awareness of an uncommonly considered, yet potentially fatal, pathogen.
Case presentation
A 2-year-old girl presented acutely to hospital, with reduced consciousness and fever. In the preceding 24 h, she had become intermittently unresponsive with vacant episodes and pronounced neck stiffness. Her parents described her as normally being fit and well, however, for 4 weeks prior to admission, she had not been herself, having had a reduced appetite and intermittent fever. She had become increasingly disinterested in play and was generally miserable. During this time, she was treated for tonsillitis and received two courses of amoxicillin from her general practitioner. Of note, there was no history of vomiting, headaches or otitis media. She had no other significant medical history and was not taking any regular medication.
On the admission examination, the patient was feverish, photophobic and drowsy, responding to voice in a confused manner. She was reluctant to move her neck, holding it in an extended arched position to the right. There was neither a rash nor any clinical evidence of otitis media or mastoiditis. She had no focal neurological signs and the rest of her systemic examination was unremarkable.
At this stage, the clinical impression was of an acute confusional state secondary to meningoencephalitis. Thus she was admitted to the ward, for close monitoring, and started on ceftriaxone, amoxicillin, acyclovir and dexamethasone.
Investigations
Laboratory investigations revealed haemoglobin 79 g/L, white cell count 16.1×109/L, platelets 33×109/L and C reactive protein (CRP) 144 mg/L. Admission CT of the head showed hypodense changes in the posterior left temporal lobe with central ring enhancing lesions. Follow-up MRI revealed left temporal lobe multiloculated abscesses with left lateral and sigmoid sinus thrombosis, and a small subdural empyema. Additionally, the left middle ear cavity and mastoid air cells were opacified with no evidence of effacement of the bony septa between the mastoid air cells, and no subperiosteal abscess collection.
Treatment
Following imaging, the patient was referred to a neurosurgical specialist centre. Ear, nose and throat surgeons assessed the patient and agreed that there were no clinical signs of otitis media and no acute mastoiditis. The patient underwent craniotomy and aspiration of the temporal lobe abscess together with a left myringotomy with grommet insertion. Frank pus was seen in the middle ear. Pus aspirated from the cerebral abscess grew F. necrophorum. Swabs from the left ear also grew F. necrophorum with Turicella otitidis and Coagulase-negative staphylococci (CNS) isolated (light growths), however, blood cultures were negative. Following the culture reports, the antibiotics were changed to high-dose meropenem, with the plan to continue intravenous antibiotics for 6 weeks following source control, as advised by the microbiologist.
The thrombocytopaenia was corrected with a platelet transfusion prior to surgical intervention and this slowly resolved as the patient improved. Owing to the left lateral and sigmoid sinus thrombosis, low-molecular-weight (LMW) heparin was started and anti-Xa levels monitored. Postoperatively, the patient clinically improved, but her mobility was limited by discomfort.
Within a week postoperatively, the patient developed fever of 39°C. Meropenem was continued and close monitoring of the intracranial infection was undertaken with weekly neuroimaging. During the next 2 weeks, the patient continued to have low-grade fever and became more agitated, with reduced appetite. There was no neurological deficit on examination. Repeat neuroimaging revealed significant deterioration with increasing abscess size causing mass effect (figure 1A). Bilateral extradural empyemas were evident and the ventricular size had increased, suggesting ventriculitis and hydrocephalus. There was increased signal in the cerebellar hemispheres and cerebrospinal fluid spaces, suggestive of meningitis (figure 1B,C). Additionally, the left transverse venous sinus thrombus extended to the jugular foramen (figure 2). The middle ear opacification improved. The patient underwent drainage and re-washout of the intracranial abscess and empyema twice more within 3 weeks of her initial surgery. On both occasions, the pus collected from the abscess yielded no growth and blood cultures remained negative.
Figure 1.
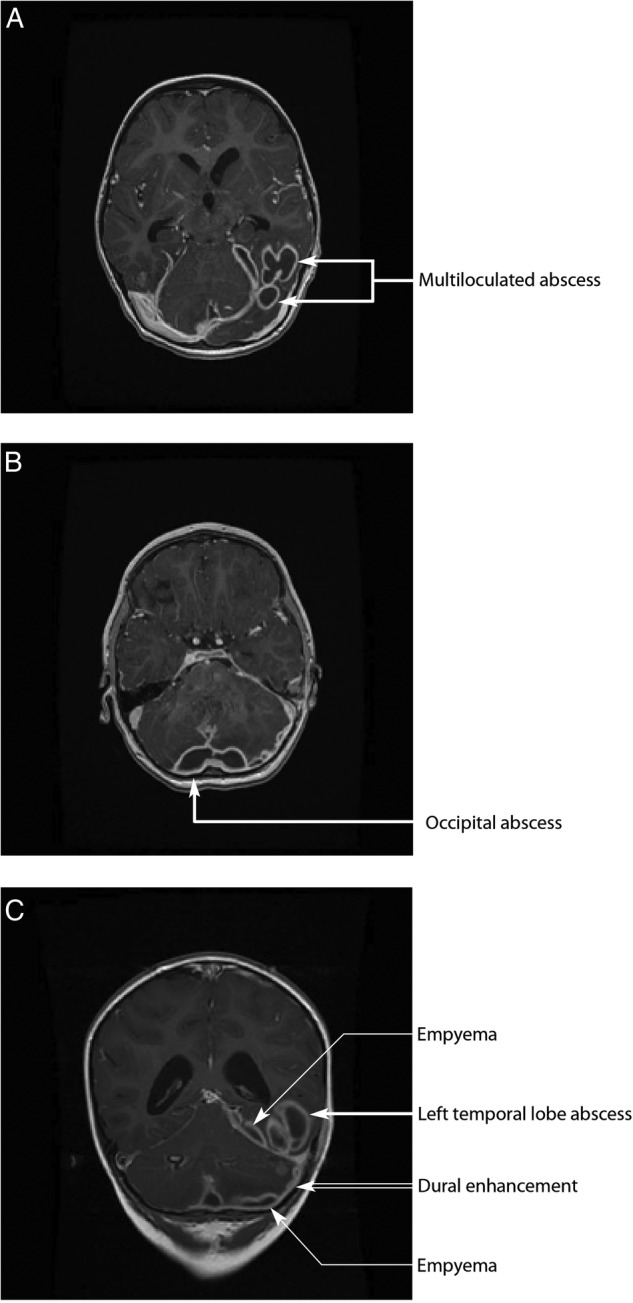
(A and B) (axial views) and (C) (coronal view). T1-weighted gadolinium-enhanced MRI, taken 2 weeks after initial surgery and before the second surgical drainage and washout. Images demonstrate multiloculated abscess in the left posterior temporooccipital lobes with oedema and mass effect on the left occipital horn. Empyema and dural enhancement are also seen overlying the left temporal lobe and left cerebellum hemisphere.
Figure 2.
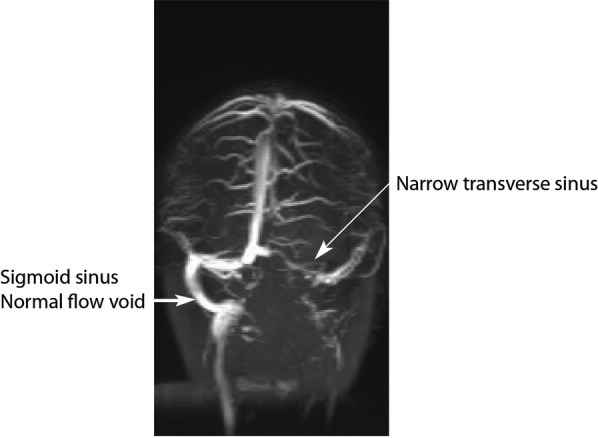
MR venogram demonstrates a narrow transverse sinus and absent flow void in the left sigmoid sinus.
Outcome and follow-up
Following the third neurosurgical operation, the patient improved clinically, becoming less agitated and more mobile. She remained afebrile with no focal neurological signs and her inflammatory markers improved (CRP<1 mg/L). Further extensive investigation did not reveal any immunocompromise. Antibiotics were continued for 6 weeks following her last surgery and were changed from meropenem to ceftriaxone, for ease of administration, in keeping with culture sensitivities. Neuroimaging continued every 2–4 weeks while on antibiotics. Follow-up CT of the head showed improvement with resolution of the hydrocephalus and reduced abscess size.
The patient was discharged from hospital on LMW heparin, having completed a total of 9 weeks of intravenous antibiotics. Since discharge, she has remained well and is developmentally appropriate for her age. The heparin was stopped after 6 months and the patient continues to undergo paediatric follow-up to monitor growth and development.
Discussion
F. necrophorum is a Gram negative, non-sporulating, obligate anaerobe. It is historically recognised as the causative agent of the systemic infection ‘Lemierre's disease’ (postanginal sepsis)—a condition that largely affects previously healthy young adults in the 16–23-year age group.1
F. necrophorum is known to have an important role in acute tonsillitis and peritonsillar abscesses, but may also have a wider pathogenic role. It has recently gained prominence as a potential cause of persistent sore throat syndrome (PSTS), with several authors concluding that it is present in as many cases of PSTS as is Group A Streptococcus,2–4 but more notably that perhaps it should be considered when treating pharyngitis in adolescents and young adults.5
A growing body of evidence also exists to suggest a recent shift in the presentation of F. necrophorum infection towards the otogenic variant primarily seen in paediatric patients.6 7 In this setting, infections (either otitis media or mastoiditis) are associated with intracranial complications, such as meningitis, cerebral abscess and sinus thrombosis. Further dissemination can occur. Indeed, this case fits well with the picture reported by French and Israeli clinicians whereby no history of recurrent otitis media was seen, a common clinical scenario in paediatric patients.7 8 Recent work from a Dutch group also describes a case series of five patients with such infections resulting in a spectrum of clinical sequelae, including one death.6
Interestingly, although this otogenic origin was first described in one of the original case reports,9 F. necrophorum is still not routinely considered as a potential pathogen in this clinical scenario, probably due to its low incidence. However, a review of isolates received at the UK Anaerobe Reference Unit (UKARU) over the past three decades shows an increase in the average number of yearly referrals of F. necrophorum, which may be indicative of increased incidence (unpublished data).
Other organisms isolated from this case were not considered pathogenic. T. otitidis is a coryneform bacteria and common commensal of the ear, with an unclear role (if any) in middle ear pathology. CNS is a skin commensal. Neither organism was isolated from the primary abscess, that yielded the heavy growth of F. necrophorum. Mixed culture is not uncommon in this setting.
Successful treatment of such infections relies on early recognition and identification of the causative pathogen, which then informs appropriate antimicrobial therapy coupled with source control.
Paediatricians need to remain vigilant to this uncommon but potentially fatal infection.
Patient's parents’ perspective.
Our daughter and only child was incredibly healthy from birth to a month before her admission to the hospital in December 2013.
Over the 4 weeks prior to her admission to accident and emergency (A&E) department, we took her to her local general practitioner (GP) on four separate occasions. Given her age, she was reluctant to allow the doctor to examine her throat. She was prescribed antibiotics as a precaution. Over the 4 weeks, she would have periods of sleepiness and high temperatures but we were always able to bring her temperature down with calpol and removal of her clothes.
The day before her admission to hospital, we took her to the GP and were told that they were not going to send her to the hospital for ‘unnecessary intrusive’ blood tests.
On the afternoon of Tuesday 10th December, she first showed signs of meningitis, staring at a fixed position and becoming upset at being moved. I, unfortunately, have experience within my family as to the devastating effects of meningitis and its warning signals, and therefore contacted National Health Service (NHS) Direct immediately to discuss our concerns. On speaking to NHS Direct, we were advised to take her immediately to A&E. From the moment we arrived at the hospital she was treated with the utmost care. We were shown her MRI scan and obviously were gravely concerned as to her chances of survival. We were rushed to the tertiary neurosurgical centre and all the members of the neurological team were amazing in treating her and providing us with detailed information as to her condition, treatment and prognosis.
Since being discharged from the hospital, she has shown incredible improvement and has returned to being a very healthy and happy little girl. We will be eternally grateful to all the staff who looked after her during the most traumatic experience of our lives.
Learning points.
Appropriate culture and identification of Fusobacterium necrophorum is important in complicated otogenic infections.
Source control is vital, with origins of infection being continually reviewed and managed, to improve patient morbidity and reduce mortality.
Neuroimaging remains a crucial tool for monitoring intracranial infections.
Acknowledgments
Mr Imran Bhatti (Consultant Neurosurgeon), Mr Paul Leach (Consultant Neurosurgeon), Dr Johann te Water Naude (Consultant Paediatric Neurologist), Mr Stuart Miles Quine (Consultant ENT surgeon), Dr Margaret Hourihan (Consultant Neuroradiologist), Dr Andrea Liu (Consultant Neuroradiologist), Dr Martin Edwards (Consultant Paediatrician), Public Health Wales Microbiology Department, Media Resources Centre Cardiff and Vale University Health Board.
Footnotes
Contributors: NH managed the case report, gathered information and liaised with the patient's parents for consent for article submission. NH also liaised with other contributors to ensure the report's appropriate, intellectual content. NH contributed to the report's design and planning, also wrote the case report and drafted and revised it. NH approved the final version to be submitted. TM identified the case as an idea for the article, conducted literature search, wrote the case report's discussion and drafted and revised it. TM revised the article critically for intellectual content and approved final version to be submitted. RD identified the case as an idea for the article, managed the case report, contributed to the design and writing of the case report and discussion. RD conducted literature search, revised the article critically for intellectual content and aided with interpretation of data. FG clinically managed the patient, revised the article critically for intellectual content and aided with interpretation of data. FG contributed to the writing of and edited the case report for submission, approved the final version to be submitted. RD and NH were the guarantors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Brazier JS. Human infections with Fusobacterium necrophorum. Anaerobe 2006;12:165–72. 10.1016/j.anaerobe.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Batty A, Wren MW. Prevalence of Fusobacterium necrophorum and other upper respiratory tract pathogens isolated from throat swabs. Br J Biomed Sci 2005;62:66–70. [DOI] [PubMed] [Google Scholar]
- 3.Amess JA, O'Neill W, Giollariabhaigh CN et al. . A six-month audit of the isolation of Fusobacterium necrophorum from patients with sore throat in a district general hospital. Br J Biomed Sci 2007;64:63–5. [DOI] [PubMed] [Google Scholar]
- 4.Price SL, Hardy S, Gale P et al. . Prevalence of Fusobacterium necrophorum in persistent sore throat samples Br J Biomed Sci 2002;68:209–10. [DOI] [PubMed] [Google Scholar]
- 5.Centor RM. Expand the pharyngitis paradigm for adolescents and young adults. Ann Intern Med 2009;151:812–15. 10.7326/0003-4819-151-11-200912010-00011 [DOI] [PubMed] [Google Scholar]
- 6.Creemers-Schild D, Gronthoud F, Spanjaard L et al. . Fusobacterium necrophorum, an emerging pathogen of otogenic and paranasal infections? New Microbe New Infect 2014;2:52–7. 10.1002/nmi2.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarden-Bilavsky H, Raveh E, Livni G et al. . Fusobacterium necrophorum mastoiditis in children—emerging pathogen in an old disease. Int J Pediatr Otorhinolaryngol 2013;77:92–6. 10.1016/j.ijporl.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 8.Le Monnier A, Jamet A, Carbonnelle E et al. . Fusobacterium necrophorum middle ear infections in children and related complications: report of 25 cases and literature review. Pediatr Infect Dis J 2008;27:613–17. 10.1097/INF.0b013e318169035e [DOI] [PubMed] [Google Scholar]
- 9.Veillon A, Zuber A. Sur quelques microbes strictement anaérobies et leur role en pathologie. Arch Méd Exp 1898;10:517–45. [Google Scholar]


