Abstract
Objective
The Affordable Care Act requires most health plans to cover the federal Recommended Uniform Screening Panel of newborn screening (NBS) tests with no cost sharing. However, state NBS programs vary widely in both the number of mandated tests and their funding mechanisms, including a combination of state laboratory fees, third-party billing, and other federal and state funding. We assessed the potential impact of the Affordable Care Act coverage mandate on states' NBS funding.
Method
We performed an extensive review of the refereed literature, federal and state agency reports, relevant organizations' websites, and applicable state laws and regulations; interviewed 28 state and federal officials from August to December 2014; and then assessed the interview findings manually.
Results
Although a majority of states had well-established systems for including laboratory-based NBS tests in bundled charges for newborn care, billing practices for critical congenital heart disease and newborn hearing tests were less uniform. Most commonly, birthing facilities either prepaid the costs of laboratory-based tests when acquiring the filter paper kits, or the facilities paid for the tests when the kits were submitted. Some states had separate arrangements for billing Medicaid, and smaller facilities sometimes contracted with hearing test vendors that billed families separately.
Conclusion
Although the Affordable Care Act coverage mandate may offset some state NBS funding for the screenings themselves, federal support is still required to assure access to the full range of NBS program services. Limiting reimbursement to the costs of screening tests alone would undermine the common practice of using screening charges to fund follow-up services counseling, and medical food or formula, particularly for low-income families.
The Patient Protection and Affordable Care Act (hereinafter, Affordable Care Act) requires most health plans to cover the federal Recommended Uniform Screening Panel (RUSP) of 31 core and 26 secondary newborn screening (NBS) tests with no coinsurance or copayments.1,2 This expansion of NBS coverage affects about 1.3 million of the 4 million annual U.S. births, in addition to the estimated 2 million births covered by Medicaid.3 The proportion of workers with employer-sponsored health benefits who are still in grandfathered plans outside the NBS coverage mandates4 will fall over time, giving almost all U.S. families coverage for their infants' RUSP-approved screenings. Estimates of the Affordable Care Act's fiscal impact are based in part on the premise that coverage mandates will reduce state and federal expenditures. However, for NBS, the tradeoff between public and commercial insurance funding is complicated by states' heterogeneous approaches to program support.
A few states have statutory mandates covering the full RUSP, but most rely on expert advisory committees, which recommend changes to state health officers.2 State adoption of RUSP recommendations follows paths that reflect NBS programs' five decades of evolution. Factors influencing program development include the interaction of scientific and technological advances, clinical treatment effectiveness, advocacy movements, and policy makers' openness to new initiatives.5,6 The number of tests has also increased in the past decade as relatively low-cost tandem mass spectrometry and microarray testing became available.2 The tests most commonly missing from fully implemented statutory requirements are those that require new instrumentation and procedures, such as the two most recent additions to the RUSP panel, severe combined immunodeficiency (SCID) and critical congenital heart disease (CCHD), along with newborn hearing testing.7,8 While SCID testing is like the heel stick panel in that it takes place at a clinical laboratory using the bloodspot sample, CCHD and newborn hearing testing are typically provided at birthing facilities.9–11 All three have seen substantial recent state legislative action moving toward inclusion in the screening panels, despite lingering funding concerns.12
An important corollary to the incomplete adoption of the full RUSP is that the Affordable Care Act entitles parents to coverage for tests that are not included in some states' standard fees. Current state laws denying coverage for unauthorized tests are superseded by section 2371 of the Affordable Care Act.13 The third-party payer, not the parents, is responsible for covering non-mandated RUSP tests.
METHODS
Study design
We conducted an extensive survey of peer-reviewed and other relevant literature, as well as online reports of the Association of Public Health Laboratories' Newborn Screening Technical Assistance and Evaluation Program7 and the National Newborn Screening and Global Resource Center.8 The latter source includes references to each state's statutes and regulations, which were also consulted. We undertook additional legal research using the Lexis® search engine. We then conducted semi-structured discussions from August to December 2014 with 28 state and federal officials, including representatives of the Centers for Disease Control and Prevention (CDC), the Health Resources and Services Administration (HRSA), and 15 state programs. State program officials were approached when (1) online information was incomplete or ambiguous or (2) federal officials identified the state program leader as having expertise in systems and funding areas; the majority of these discussions were conducted by telephone. All respondents were assured of anonymity, and responses were recorded and analyzed manually for both factual detail and qualitative themes.
RESULTS
Overall, informants demonstrated expertise about their own programs, but few respondents had comprehensive information on NBS funding, primarily because state programs are often fragmented. State agency staff members were largely unaware of hospital billing practices, and officials responsible for laboratory-based screenings had scant information in common with those responsible for hearing programs. Hearing and blood spot test findings were typically reported in different systems; informants expressed frustration that their states could not create a unified record.
Financial models for state NBS programs differed widely. At the time of our study, fees for the states' authorized panels of tests, in the 47 states that charged them, varied from $15.00 in Florida to $152.62 in Oklahoma. Whether charges were state assessments or fee-for-service billings, they were generally included in birthing facility charges for newborn care; this practice was confirmed in conversations with representatives of two major national insurance carriers. Both the insurance carrier staff and state program leadership noted that additional charges may have been assessed for hearing and CCHD screenings that were provided by nonhospital personnel. Most states provided public funding for NBS if no other source was available.14–16
Cost of screening
Several variables contributed to a lack of uniform cost structure across states. States with fewer than 40,000 births per year typically sent their metabolic testing out of state, but the number of births among states with higher test volumes varied. Given similar staffing and instrumentation, the marginal cost per test was lower for a state with a higher number of births. Second, in addition to the individual tests themselves, the instrumentation, training, quality assurance, and other operational costs required support. For example, state program directors estimated instrumentation for the SCID platform at $200,000, most of which had to be disbursed before receiving any offsetting revenue from the new tests. Third, in several states, screening programs had to be self-sustaining financially, so fees were increased to recoup higher costs. However, some states' executive or legislative branches opposed fee increases as de facto tax hikes; this factor was cited in one of the three no-fee states. Public officials who pledged not to raise taxes may reject pleas for fees that support up-to-date testing regimens.
Some birthing facilities outsourced hearing screening, which led to the generation of additional claims. The published state fee often did not include tests performed at the hospital (e.g., CCHD and hearing). As with all screenings, newborn metabolic screening tests generate false-positive findings;17 as such, positive results must be retested to determine diagnostic accuracy. Eight states required two screening rounds for all newborns.
State NBS fees do not reflect costs, and additional research has been undertaken to assist states in setting and justifying fees. In 2001, both the Government Accountability Office and the March of Dimes conducted surveys of state officials regarding costs of laboratory-based NBS. The Government Accountability Office found a mean per-infant cost of $29.44; 74% was for laboratory testing, while the balance covered shipping, administrative costs, and reporting functions.18 The March of Dimes data did not cover all current screenings, but some findings are still instructive.19 The wide range of costs—from $0.00 to $150.00—was attributed to economies of scale, variation in testing system or method, and instrumentation and staffing—factors that persist today.
Concerns about cost and cost-effectiveness in the United States and other Organisation for Economic Co-operation and Development countries have led to more focused assessments of cost-finding methods and related policies.20–24 On a smaller scale, a 2012 Wisconsin legislative staff assessment of fee components in neighboring states estimated testing costs at $44.00–$58.00, including program administration but not instrumentation acquisition, counseling, treatment, or other services.25 In Vermont, the cost of each test was estimated at $33.30, again limited to the screening itself.26 However, estimates from our interviews with other program directors for this study suggest that the average cost for initial screening alone was closer to $80.00 once instrumentation, training, and related costs were amortized across the total number of tests performed.
Services funded by the NBS fee
We found three broad categories of NBS fee allocation: (1) states that limit fees to screening cost and bill additional costs to the entities covering infants with positive diagnoses, (2) states that include post-screening services in fees charged for all newborns, and (3) states that charge no fees, thereby spreading NBS program costs across an even broader funding base.27,28 The American College of Medical Genetics advocated the comprehensive approach in 2006, describing an NBS program as “a coordinated and comprehensive system consisting of education, screening, follow-up, diagnosis, treatment and management, and program evaluation.”2 Provisions addressing comprehensive funding29 or limiting charge computation to tests alone30 are often explicit in state statutes or regulations.
From a policy perspective, the more restrictive approach allocates funding responsibility on an individual basis: because only screenings are performed for all newborns, subsequent confirmatory tests and other services are billed to third-party payers. Conversely, states that charge no fee for NBS spread the cost of the entire program across the full population, not just the cohort of newborns who are screened, thereby treating NBS and related services as public goods for which the state as a whole is responsible.
Other funding sources
The 50-state 2006 survey27 and a 2007 update28 found that funding sources most commonly included (1) fees collected from health-care providers, who pass them on to third-party payers and, in some cases, to parents (90% of respondents); (2) federal pass-through sources including Title V block grant and HRSA funding (61% of respondents); (3) state-general fund appropriations (33% of respondents); and (4) direct Medicaid payments beyond routine newborn care (24% of respondents).
A few states have tapped specific funding streams. In Kansas, program costs were covered through an -assessment on health insurance revenue.31 South Carolina funded newborn hearing screening from the 1998 Master Settlement Agreement with tobacco companies.32
Coverage mandates
State legislatures have enacted service and provider coverage mandates (Table), often responding to managed care organizations' denials of coverage. Calling these state coverage provisions “mandates” is somewhat misleading because they only apply to the small segment of the state's insurance market under state regulatory authority. Under §10104(e)(3) of the Affordable Care Act, the cost of state mandates that took effect after December 31, 2011, and were included in Marketplace offerings, can only be included in health plan premiums if the coverage is also mandated by the Affordable Care Act. New state-mandated benefits not required by the Affordable Care Act are the state's responsibility. For example, Hawaii tests for more thalassemias—conditions that are more prevalent among Asian Americans than among those of European descent—than other states.33 The cost of such additions to state NBS panels that took effect before the 2011 cutoff date are authorized for inclusion in Marketplace premiums.
Table.
State insurance mandates for newborn hearing tests and metabolic screening in the United States as of 2010a
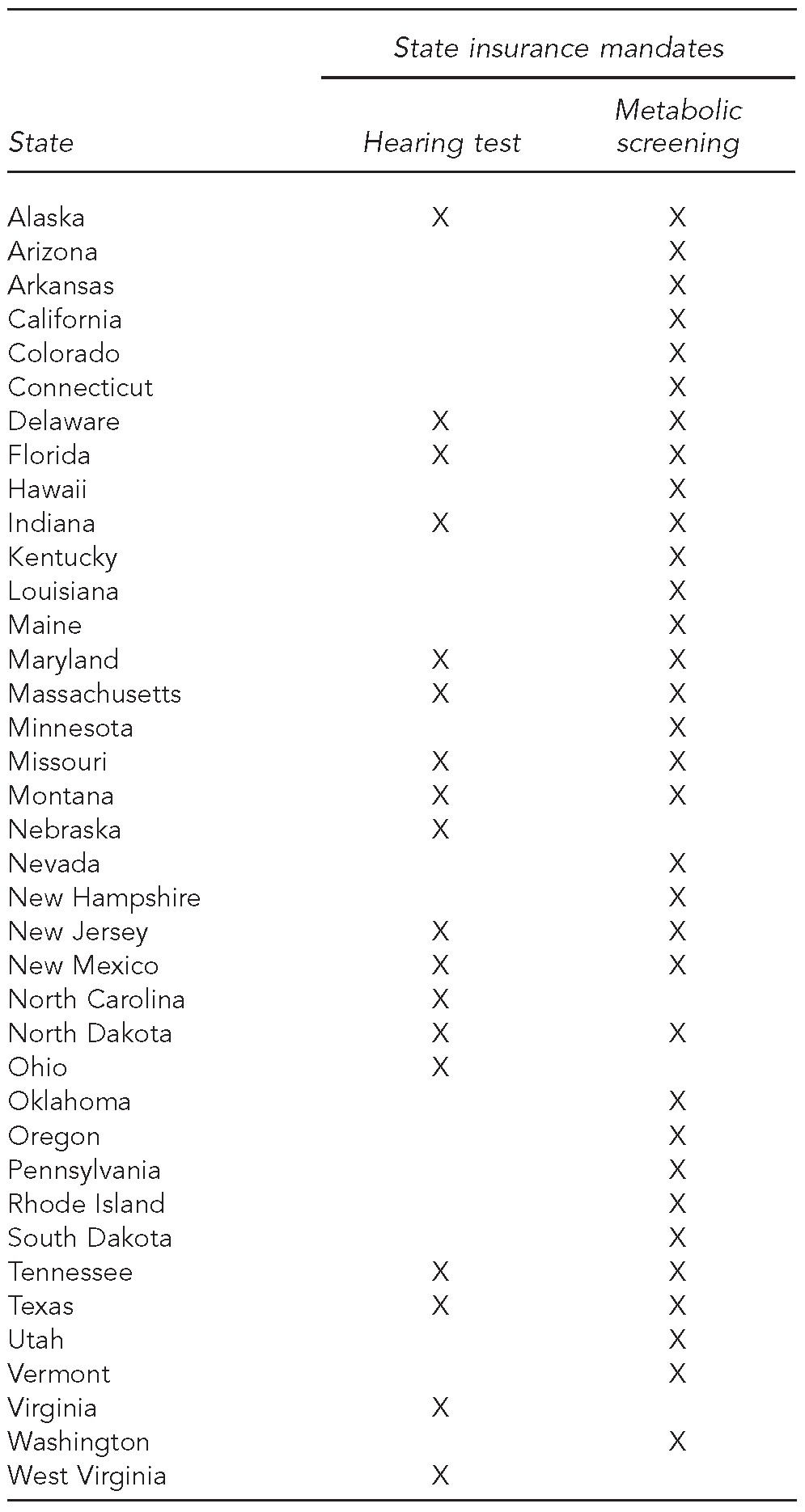
Alabama, Georgia, Idaho, Illinois, Iowa, Kansas, Michigan, Mississippi, New York, South Carolina, Washington, DC, Wisconsin, and Wyoming did not have state insurance mandates for hearing and metabolic screening as of 2010. Source: Bunce VC, Wieske JP. Health insurance mandates in the states, 2010. Alexandria (VA): Council for Affordable Health Insurance; 2010.
Billing and collection models
We found four basic models for states' billing and collection of NBS fees. In the three models in which a screening charge applies, the birthing hospital normally includes the charge in its bundled or diagnosis-related group charge for newborn care. While most states use their state public health laboratory or the laboratory of a larger neighboring state, some important variations exist, such as the use of commercial laboratories and those based at academic health centers. The CCHD and hearing screenings may be billed separately, particularly when an outside vendor provides the service. The count of states was an estimate based on program status at the time of this analysis, but is subject to change over time.
Model 1. The state agency bills the birthing facility for NBS test kits (17 states).
This process shifts the risk of loss for non-reimbursed charges from the state agency to the birthing facility. The facility then includes the charge, along with a handling fee (and sometimes an additional fee for hearing screening), in the diagnosis-related group or bundled charge for newborn care. Bundled fees are renegotiated at contractually determined intervals, but none of our key informants suggested a problem with reimbursement for the NBS fees as part of the bundled payment.
Model 2. The state agency bills the birthing facility when it submits the completed test kits for analysis (21 states).
Subsequent steps follow the pattern described in Model 1. There does not appear to be a problem with the hospitals paying as billed, so this model also shifts the risk of loss from nonpayment to the birthing facility. However, additional claims may be filed for point-of-service tests (e.g., CCHD and newborn hearing testing).
Model 3. The state agency bills either the birthing facility or third-party payers depending on the newborn's third-party coverage (nine states).
In some states, the state agency bills Medicaid for its covered newborns and bills the hospital for newborns without Medicaid numbers, which can take months to identify. State agencies that lack the technical capacity to bill a range of commercial carriers directly may contract with a vendor for this service. It is very unusual for a state agency to bill the parent of an uninsured child for NBS; states give parents options for state coverage in such cases. Uninsured newborns should be extremely rare anyway, regardless of their parents' immigration status, because of the newborns' coverage options as birthright citizens.
Model 4. States do not charge a fee and the state agency does not bill at all (three states and Washington, D.C.).
Kansas, New York, and Pennsylvania (with regard to six core conditions), as well as Washington, D.C., follow this model. Kansas funds NBS with an assessment on state-licensed managed care organizations, but hearing screening is billed separately. New York and Pennsylvania use state appropriations and Title V funding; the latter also supports the Washington, D.C., program.
The effect of the first two models is that NBS coverage is well established in about two-thirds of states. Most birthing facilities perform hearing and CCHD screening, and pay for metabolic screenings either in advance or at the time when dried blood spot sample cards are submitted. Services performed or paid by facilities are included in the global newborn care payment negotiated with commercial and government payers; thus, the parents would never see an NBS-specific bill.
In a recent development, some NBS, most commonly hearing tests, have been performed by nonhospital staff and billed separately to the payer of record. For example, the pediatric services vendor Pediatrix (a division of the publicly traded corporation MEDNAX) states on its website that it provides about 700,000 hearing screens annually to newborns in more than 350 hospitals.34 The Web page captioned “Pay Your Bill” includes the following statement: “Depending on your insurance coverage, you may have some financial responsibility for the cost of the hearing screen.” Families with coverage under the Affordable Care Act mandate should not have a cost-sharing requirement, but they may not be aware of their coverage. The issue has been the subject of formal and informal investigations in some states;35 however, interviewees were not aware of specific payment issues and arrangements.
A second cost-sharing issue arises when the clinical laboratory bills a family's insurer rather than the birthing facility for metabolic screening tests. This practice appears to be standard in Florida and Pennsylvania for tests other than the six tests covered without charge. Again, depending on the billing laboratory's participation status and insurance coverage, the risk of noncompliance with the Affordable Care Act coverage mandate exists. The total charge, while relatively low compared with other hospital fees, could be unaffordable for low-income parents.
State program directors reported that clinical laboratories other than the state NBS laboratories charged up to $20.00 for each of eight Current Procedural Terminology (CPT) codes for metabolic screening alone. The Medicare average (a common benchmark for reimbursement) for the metabolic screening panel is $138.34, according to the Centers for Medicare & Medicaid Services website.36
Related issues
For point-of-care tests, birthing facility performance of NBS may exceed statutory mandates. Data from the Maternal and Child Health Bureau's Title V Information System show a very high rate of newborn hearing screening in the 13 states where it is not universally required, only half a percentage point lower than in states with mandatory testing (98.4% vs. 99.0%).37 One possible explanation for this finding is that facilities may find it more efficient to test all children who have no contraindications to testing, even when the test is not mandated.
DISCUSSION
Once the Affordable Care Act coverage mandate takes full effect (i.e., when no grandfathered plans remain exempt from the ACA mandates), the proportion of screenings that default to state funding should be negligible. However, screening coverage alone will not relieve states of their need for NBS program funding. A number of additional factors are involved, including the service mix funded by the screening fee, the use of alternative funding sources, and state coverage mandates. The Affordable Care Act coverage mandate does not address costs of counseling, follow-up testing, medical food and formula, and future health care. Insurance carriers could decline to cover these services if their cost is included in the NBS fee, but the issue does not appear to have arisen, perhaps because NBS charges are typically included in negotiated newborn care reimbursements. If CPT code-based fees were billed separately, the total would exceed the highest current state fee. Therefore, carriers may prefer established practices. However, as NBS expands to include conditions with later onset, the burden of ongoing monitoring is likely to increase, raising the possibility of new problems with third-party coverage.
Some states' fees are not adequate to support program costs, particularly for tests beyond the scope of tandem mass spectrometry. In addition, several key informants questioned the ability of state laboratories to capture the fees owed. Where state fees or collections are inadequate, screening programs must compete with other worthy initiatives for federal or state funding.
Limitations
This study was subject to several limitations in the validity and generalizability of our findings. Timely and accurate cost information was lacking for most aspects of NBS programs and services, so cost data were only estimates based on available evidence. The full array of NBS billing and collections practices, as with those for other U.S. health services, were subject to extensive variation and could only be characterized with regard to available documentation. Similarly, the structural fragmentation of U.S. health care and of many state NBS programs may have given rise to oversights and omissions. A further limitation arose because of the dynamic nature of NBS programs, which can be characterized, at best, in a limited temporal context.
CONCLUSION
As NBS support shifts to third-party payers, state and federal officials should monitor the status of resources for follow-up care, counseling, and other services needed by newborns with positive diagnoses. If reimbursement rates are set to reflect screening costs alone, their contribution to comprehensive program support will cease, and new revenue sources will need to be identified. In some cases, essential benefits in commercial coverage or Medicaid would fill these gaps, but commercially insured families who remain in grandfathered plans may not have access to needed coverage. The persistent issue of coverage for medical formula and food—expensive items that fall outside the normal range of health benefits—would also need to be addressed. Only 20 states include medical formula and food in their benchmark plans' essential health benefits.38
About half of U.S. births are covered by Medicaid.39 If NBS reimbursement is raised to cover program costs, an increase in total reimbursement for newborn care would be necessary to avoid shortchanging the birthing hospital on other costs. The relationship between this increased cost and decreases in Title V support would need careful evaluation because of states' responsibility for partial support of their Medicaid programs.
The well-documented differences in states' approaches to NBS funding arise from politics and advocacy as well as clinical assessments. Actions that are likely to increase state Medicaid expenditures or commercial insurance premiums are subjects of heated debate. Thus, even if a shift to third-party billing for all screening costs makes sense for states, implementation of such a change could encounter serious resistance and jeopardize funding for other NBS program elements. If NBS programs are to succeed in reducing the burden of targeted conditions, they must have adequate funding not only for screenings, but also for the full range of services required to meet the long-term needs of affected children and their families. An updated assessment of both costs and benefits would provide critically needed evidence for effective policy at the state and federal levels.
Footnotes
This study was approved by the University of Kentucky Institutional Review Board.
REFERENCES
- 1. Pub. L. No. 111-148, 42 U.S.C. §300GG (2010)
- 2.Newborn screening: toward a uniform screening panel and system. Genet Med. 2006;8(Suppl 1):1S–252S. doi: 10.1097/01.gim.0000223891.82390.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services (US), Office of the Assistant Secretary for Planning and Evaluation. Increased coverage of preventive services with zero cost sharing under the Affordable Care Act [cited 2015 Jan 28] Available from: http://aspe.hhs.gov/health/reports/2014/preventiveservices/ib_preventiveservices.pdf.
- 4.Henry J. Kaiser Family Foundation. 2014 employer health benefits survey: section 13: grandfathered health plans [cited 2015 Jan 28] Available from: http://kff.org/report-section/ehbs-2014-section-thirteen-grandfathered-health-plans.
- 5.Brosco JP, Paul DB. The political history of PKU: reflections on 50 years of newborn screening. Pediatrics. 2013;132:987–9. doi: 10.1542/peds.2013-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grob R. New Brunswick (NJ): Rutgers University Press; 2011. Testing baby: the transformation of newborn screening, parenting, and policymaking. [Google Scholar]
- 7.Association of Public Health Laboratories. NewSTEPs state profiles [cited 2015 Sep 10] Available from: https://data.newsteps.org/newsteps-web/stateprofile/input.action.
- 8.National Newborn Screening and Global Resource Center. Newborn screening [cited 2015 Sep 10] Available from: http://genes-r-us.uthscsa.edu/resources/consumer/statemap.htm.
- 9.Kuehn BM. After 50 years, newborn screening continues to yield public health gains. JAMA. 2013;309:1215–7. doi: 10.1001/jama.2013.2087. [DOI] [PubMed] [Google Scholar]
- 10.Martin GR, Beekman RH, 3rd, Mikula EB, Fasules J, Garg LF, Kemper AR, et al. Implementing recommended screening for critical congenital heart disease. Pediatrics. 2013;132:e185–92. doi: 10.1542/peds.2012-3926. [DOI] [PubMed] [Google Scholar]
- 11.Utah State University, National Center for Hearing Assessment and Management. Enacted universal newborn hearing screening legislation [cited 2015 Jan 28] Available from: http://www.infanthearing.org/legislative/mandates.html.
- 12.Peterson C, Grosse SD, Glidewell J, Garg LF, Van Naarden Braun K, Knapp MM, et al. A public health economic assessment of hospitals' cost to screen newborns for critical congenital heart disease. Public Health Rep. 2014;129:86–93. doi: 10.1177/003335491412900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ky. Rev. Stat. Ann. §214.155 (2013)
- 14. Code of Delaware Regulations Title 16, §4103 (2014)
- 15. 410 Ind. Admin. Code 3-3-2.5 (2014)
- 16. Oregon Administrative Rules Ch. 333, Div. 24, as amended.
- 17.Wilcken B. Newborn screening: gaps in the evidence. Science. 2013;342:197–8. doi: 10.1126/science.1243944. [DOI] [PubMed] [Google Scholar]
- 18.Government Accountability Office (US) Newborn screening: characteristics of state programs. GAO 03-449. 2003 [cited 2015 Jan 28] Available from: http://www.gao.gov/assets/240/237601.pdf.
- 19.PricewaterhouseCoopers. White Plains (NY): March of Dimes Foundation; 2002. Newborn screening programs: an overview of costs and financing. [Google Scholar]
- 20.Baily MA, Murray TH. Ethics, evidence, and cost in newborn screening. Hastings Cent Rep. 2008;38:23–31. doi: 10.1353/hcr.0.0009. [DOI] [PubMed] [Google Scholar]
- 21.Langer A, Holle R, John J. Specific guidelines for assessing and improving the methodological quality of economic evaluations of newborn screening. BMC Health Serv Res. 2012;12:300–11. doi: 10.1186/1472-6963-12-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colgan S, Gold L, Wirth K, Ching T, Poulakis Z, Rickards F, et al. The cost-effectiveness of universal newborn screening for bilateral permanent congenital hearing impairment: systematic review. Acta Pediatr. 2012;12:171–80. doi: 10.1016/j.acap.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics. 2006;117(5 Pt 2):S287–95. doi: 10.1542/peds.2005-2633H. [DOI] [PubMed] [Google Scholar]
- 24.Therrell BL., Jr US newborn screening policy dilemmas for the twenty-first century. Mol Genet Metab. 2001;74:64–74. doi: 10.1006/mgme.2001.3238. [DOI] [PubMed] [Google Scholar]
- 25. 677 Wisconsin Admin. Code Ch. DHS 115 (2009)
- 26. Code of Vermont Rules 13-140-057 (2014)
- 27.Johnson K, Lloyd-Puryear MA, Mann MY, Ramos LR, Therrell BL. Financing state newborn screening programs: sources and uses of funds. Pediatrics. 2006;117(5 Pt 2):S270–9. doi: 10.1542/peds.2005-2633F. [DOI] [PubMed] [Google Scholar]
- 28.Therrell BL, Williams D, Johnson K, Lloyd-Puryear MA, Mann MY, Ramos LR. Financing newborn screening: sources, issues, and future considerations. J Public Health Manag Pract. 2007;13:207–13. doi: 10.1097/00124784-200703000-00020. [DOI] [PubMed] [Google Scholar]
- 29. Ohio Rev. Code Ann. 3701.501 (2014)
- 30. 12 Virginia Admin. Code 5-71-100 (2014)
- 31. Kansas Stat. Ann. §65-180 (2013)
- 32. S.C. Code Ann. § 44-37-40(H) (2013)
- 33.Hawaii Department of Health. Thalassemias and other hemoglobinopathies protocol: Hawaii [cited 2015 Jan 28] Available from: http://health.hawaii.gov/genetics/files/2013/04/thalprotocol.pdf.
- 34.Pediatrix Medical Group. Newborn hearing screen program [cited 2015 Jan 28] Available from: http://www.pediatrix.com/body_hs.cfm?id=2967&oTopID=50.
- 35.MEDNAX, Inc. About us [cited 2015 Jan 28] Available from: http://www.mednax.com/about-us.
- 36.Centers for Medicare & Medicaid Services (US) Clinical laboratory fee schedule [cited 2015 Sep 10] Available from: https://www.cms.gov/medicare/medicare-fee-for-service-payment/clinicallabfeesched/clinlab.html.
- 37.Department of Health and Human Services (US), Health Resources and Services Administration. Title V information system [cited 2015 Jan 28] Available from: https://mchdata.hrsa.gov/TVISreports.
- 38.Grace AM, Noonan KG, Cheng TL, Miller D, Verga B, Rubin D, et al. The ACA's pediatric essential health benefit has resulted in a state-by-state patchwork of coverage with exclusions. Health Aff (Millwood) 2014;33:2136–43. doi: 10.1377/hlthaff.2014.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markus AR, Andres D, West KD, Garro N, Pellegrini C. Medicaid covered births, 2008 through 2010, in the context of implementation of health reform [published erratum appears in Womens Health Issues 2013;23:e411] Womens Health Issues. 2013;23:e273–80. doi: 10.1016/j.whi.2013.06.006. [DOI] [PubMed] [Google Scholar]


