Abstract
Dispersal plays a crucial role in many aspects of species' life histories, yet is often difficult to measure directly. This is particularly true for many insects, especially nocturnal species (e.g. moths) that cannot be easily observed under natural field conditions. Consequently, over the past five decades, laboratory tethered flight techniques have been developed as a means of measuring insect flight duration and speed. However, these previous designs have tended to focus on single species (typically migrant pests), and here we describe an improved apparatus that allows the study of flight ability in a wide range of insect body sizes and types. Obtaining dispersal information from a range of species is crucial for understanding insect population dynamics and range shifts. Our new laboratory tethered flight apparatus automatically records flight duration, speed, and distance of individual insects. The rotational tethered flight mill has very low friction and the arm to which flying insects are attached is extremely lightweight while remaining rigid and strong, permitting both small and large insects to be studied. The apparatus is compact and thus allows many individuals to be studied simultaneously under controlled laboratory conditions. We demonstrate the performance of the apparatus by using the mills to assess the flight capability of 24 species of British noctuid moths, ranging in size from 12–27 mm forewing length (~40–660 mg body mass). We validate the new technique by comparing our tethered flight data with existing information on dispersal ability of noctuids from the published literature and expert opinion. Values for tethered flight variables were in agreement with existing knowledge of dispersal ability in these species, supporting the use of this method to quantify dispersal in insects. Importantly, this new technology opens up the potential to investigate genetic and environmental factors affecting insect dispersal among a wide range of species.
Keywords: Flight behavior, flight capability, flight mill, Lepidoptera, migration, Noctuidae
Introduction
Dispersal is a key facet of species' ecology and evolution, and it has profound effects on population dynamics, gene flow, and range size (Clobert et al. 2001; Bowler and Benton 2005; Lester et al. 2007). Increasing our understanding of dispersal is of particular importance in an environment of accelerating climate change and habitat fragmentation (Hughes et al. 2007; Gibbs et al. 2009) because dispersal is important for range shifting (Pearson and Dawson 2003) and meta‐population dynamics (Hanski et al. 2000). However, obtaining direct measures of dispersal ability can be challenging, especially in insects, making it important to develop new tools for measuring species' flight capability.
Over the past 50 years, a variety of laboratory techniques has been developed to measure flight ability of insects under controlled and experimental conditions, including methods for measuring free‐flying insects (Kennedy and Booth 1963) as well as tethered individuals (Dingle 1965). Insects can be tethered in ways that allow them to change their orientation (in so‐called “flight simulators”, allowing identification of consistent seasonal migration directions (Mouritsen and Frost 2002; Nesbit et al. 2009). Other tethered‐flight techniques enable insects to repeatedly take‐off and land and thus allow assessment of flight propensity (Gatehouse and Hackett 1980), as well as assessment of migratory tendency through presence or absence of prolonged flight (Attisano et al. 2013). Insects can also be tethered on a flight mill that allows them to fly round in a circle to assess maximum flight duration and distance within a set period, e.g. over the course of a night for nocturnal migrants (Chambers et al. 1976; Beerwinkle et al. 1995; Zhang et al. 2009). Here, we extend these previous methods, and we describe and test a new tethered flight apparatus for quantifying flight ability in moths. This technique involves a roundabout‐style apparatus allowing flight distance, duration and speed to be quantified on the same individual insect. The key attributes of the apparatus are; compact multiple units allowing many individuals to be recorded simultaneously; very low friction bearings and magnetic suspension system to minimize the degree of friction associated with turning the arm during flight; and a lightweight but rigid tethered flight arm, allowing a wide range of species to be flown (from 10 mm to 40 mm forewing length, ~10–1000 mg mass). The system for attachment of the insect to the flight mill by a rigid wire handle attached to the top of the thorax allows for ease of handling, facilitating weighing and feeding and minimizing stress to the insect during preparation for flight. This system records flight distance to the nearest 10 cm every 5 sec, providing the most fine‐scale flight data currently available. We have produced bespoke software for downloading and summarizing flight data.
Here, we describe the apparatus and illustrate its capabilities by using it to examine differences in flight ability of 24 species of British noctuid moths. We have chosen this family to illustrate the potential of the apparatus because the family includes species with a wide range of different dispersal abilities, body sizes, and life histories. We describe the improved tethered flight mill system and the measures of flight that are recorded. We demonstrate that differences in flight mill performance reflect differences in dispersal abilities under natural conditions, and discuss how the apparatus could be used to better understand dispersal ability in insects in the future.
Methods
Tethered flight mills and their operation
An illustration of a flight mill is shown in Figure 1 (Patent: Lim et al. 2013). Each mill consists of a lightweight arm suspended between two magnets. This magnet suspension provides an axis with very little resistance, so even relatively weak fliers can turn the mill successfully. The novel aspect of our design that permits interspecific comparison of flight is the mill arm, which is very lightweight but suitably rigid due to a unique construction method (Patent: Lim et al. 2013). The insect is attached to one end of the arm as shown in Figure 1B and flies in a circular trajectory with a circumference of 50 cm. A disk with a banded pattern is attached to the axis so that it turns with the arm, and a light detector detects the movement of the bands to record the distance flown and the flight speed. The tethered flight mill system currently has 16 channels (arms) allowing 16 individual insects to be flown simultaneously (but can be extended to include more channels). Flight data are automatically downloaded to a computer. The embedded microcontroller board records the distance flown by the insect to the nearest 10 cm and updates the computer with the distance travelled in five second intervals. An example of the data generated by the flight mill can be found in Appendix S1.
Figure 1.
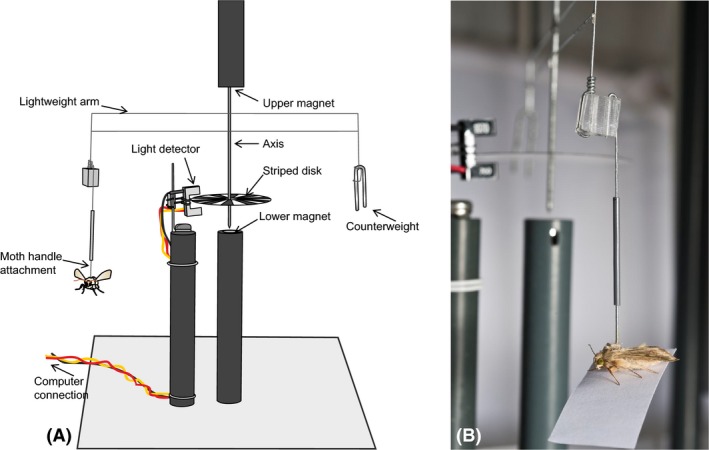
Tethered flight mill. (A) Labeled diagram of an individual flight mill. (B) Close up of the method of attaching the moth to the flight mill. Flight mills are patented (PCT/GB2014/052466). Moth shown is Helicoverpa armigera (species mean weight 0.200 g, wing length 15–20 mm).
To prevent damage to moths when preparing them for the flight mills, individuals were kept inactive in a domestic fridge and then restrained under netting to fix the attachment (Fig. 2). Scales were removed from the upper surface of the thorax using sticky tape, and then “handles” attached with contact adhesive. This system of having a short handle attached to the moth facilitates weighing and feeding prior to the insect being attached to the flight mills. Data recorded by the flight mills are measures of distance flown (m), time spent flying (s), and flight speed (m/s) (Table 1). These data can be used to analyse measures of distance, duration and speed of specific flights (e.g. the first flight of the night, or the longest flight), and derive additional variables. Flight data for each individual moth are processed using a script written in Matlab (The MathWorks Inc. 2012) to extract the beginning and end time of each individual flight and calculate each flight's duration, distance, and average speed. Because flight duration is always rounded up to the nearest 5 sec by the recording equipment, any small movement by an insect on the mill is recorded as a flight of 5 sec, therefore in our validation of the apparatus, we only analysed data for flights of 10 sec or longer. The maximum speed (calculated from the greatest distance travelled in any 5 sec interval) is also extracted. These flight data are processed in R (R Core Team 2013) to extract a total of 16 tethered flight variables (listed in Table 1).
Figure 2.
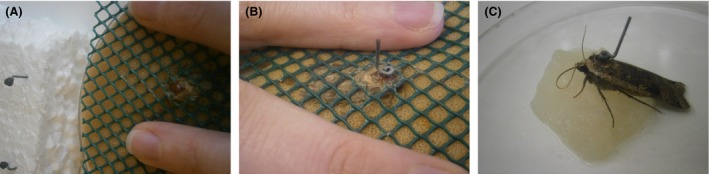
Preparing moths for tethered flight. (A) Removal of scales from thorax. (B) Attachment of flight handle with contact adhesive. (C) Feeding with honey solution.
Table 1.
Measured and derived tethered flight performance variables extracted from flight mill data. Raw data are distance, duration, average speed, and maximum speed of individual flights ≥10 sec
| Tethered flight variable | Definition | Units | PCA label |
|---|---|---|---|
| Total distance | Sum of distance covered by all flights | Metres | Distance 1 |
| Total duration | Sum of duration of all flights | Seconds | Duration 1 |
| Number of flights | Count of flights | Numeric | NumFlights |
| Average flight distance | Mean of distances of flights | Metres | Distance 2 |
| Average flight duration | Mean of duration of flights | Seconds | Duration 2 |
| Average flight speed | Mean of the speeds of individual flights (calculated as distance/duration) | Metres/sec | Speed 1 |
| Maximum speed attained | Greatest distance attained in any 5 sec interval/5 – of the whole night | Metres/sec | Speed 2 |
| First flight distance | Distance of first flight of the night | Metres | Distance 3 |
| First flight duration | Duration of first flight of the night | Seconds | Duration 3 |
| First flight average speed | Speed of first flight of the night (calculated as distance/duration) | Metres/sec | Speed 3 |
| First flight max speed | Greatest speed attained in any 5 sec interval of the first valid flight | Metres/sec | Speed 4 |
| Furthest flight distance | Distance travelled in the flight of greatest distance of the whole night | Metres | Distance 4 |
| Longest flight distance | Distance travelled in the flight of greatest duration of the whole night | Metres | Distance 5 |
| Longest flight duration | Duration of the flight with greatest duration | Seconds | Duration 4 |
| Longest flight average speed | Speed of the flight with greatest duration (calculated as distance/duration) | Metres/sec | Speed 5 |
| Longest flight max speed | Greatest speed attained in any 5 sec interval of the flight of greatest duration | Metres/sec | Speed 6 |
Validating flight mill data
Noctuid moths captured in light‐traps on site at Rothamsted Research, Harpenden, UK (51.809°N, −0.356°W) during summer 2013 were used in flight mill trials. Visual inspection of wing wear was used to ensure only recently emerged adults were flown, to constrain any variation in flight according to adult age. Following Thomas (1983), wing wear was assessed on a four point scale; fresh (4), good (3), poor (2), and worn (1), and only category 3 and 4 insects were used (Fig. 3A and B).
Figure 3.

Wing wear categories as per Thomas (1983) demonstrated in Apamea monoglypha. (A) Fresh (4). (B) Good (3). (C) Poor (2). (D) Worn (1).
Insects were kept in a domestic fridge and flown the following night after being caught. About two hours prior to flight, moths were removed from the fridge, weighed, and then given 20% honey solution ad libitum. They were then reweighed to verify feeding and attached to the flight mill with a piece of paper to hold on to and left until the lights were switched off at 21:00 BST. Each moth was flown on only one night. The flight mills were housed in a controlled environment insectary room at 18°C and 18L: 6D, which is equivalent to midsummer in the UK. Lights gradually changed during the hour before and after the night‐time dark period to simulate dawn and dusk. Multivariate analyses were carried out to examine which of the 16 tethered flight variables recorded by the apparatus (Table 1) were the most biologically informative.
In order to test the assumption that tethered flight performance reflects natural dispersal behavior in the wild, all study species were assigned to a mobility category based on two sources of information. First, we examined Rothamsted Insect Survey suction trap (Macaulay et al. 1988) data on the occurrence of moths in traps 12.2 m above the ground over the period 2000–2009 (Wood et al. 2009). We used the presence of a study species in the top 25% of all species caught 12.2 m above the ground to infer a strong likelihood of the study species to engage in long distance dispersal (Wood et al. 2009).
Secondly, we carried out a survey asking experts to classify the study species according to whether species were relatively sedentary, mobile or very mobile. The experience and opinion of lepidopterists has been shown to be a valid tool in quantifying dispersal ability (Stevens et al. 2010; Burke et al. 2011). Five experts designated each of the 24 study species into one of three dispersal categories, based on the experts' opinion and knowledge of the species' relative dispersal ability. The categories were sedentary (0), mobile (1), and very mobile (2; Table 2).
Table 2.
Responses to expert survey on noctuid moth mobility. Five experts categorized species as relatively sedentary, mobile, or very mobile which corresponds to 0, 1 or 2 mobility points in the table below
| Species | Expert 1 | Expert 2 | Expert 3 | Expert 4 | Expert 5 | Mean points |
|---|---|---|---|---|---|---|
| Agrotis exclamationis | 2 | 1 | 1 | 1 | 0 | 1 |
| Agrotis puta | 2 | 1 | 1 | 1 | 1 | 1.2 |
| Amphipoea oculea | 1 | 1 | 0 | 1 | 1 | 0.8 |
| Amphipyra pyramidea | 1 | 1 | 0 | 1 | 1 | 0.8 |
| Apamea monoglypha | 2 | 2 | 1 | 1 | 1 | 1.4 |
| Autographa gamma | 2 | 2 | 2 | 2 | 2 | 2 |
| Axylia putris | 1 | 1 | 0 | 1 | 0 | 0.6 |
| Hoplodrina alsines | 2 | 1 | 0 | 1 | 0 | 0.8 |
| Hoplodrina ambigua | 2 | 1 | 1 | 1 | 2 | 1.4 |
| Hydraecia micacea | 1 | 1 | 0 | 1 | 0 | 0.6 |
| Lacanobia oleracea | 1 | 1 | 0 | 1 | 0 | 0.6 |
| Mesapamea secalis | 2 | 1 | 0 | 1 | 0 | 0.8 |
| Mesapamea didyma | 2 | 1 | 0 | 1 | 0 | 0.8 |
| Mythimna impura | 2 | 1 | 0 | 1 | 0 | 0.8 |
| Mythimna pallens | 2 | 1 | 0 | 1 | 0 | 0.8 |
| Noctua comes | 2 | 2 | 1 | 1 | 0 | 1.2 |
| Noctua janthe | 2 | 2 | 1 | 1 | 1 | 1.4 |
| Noctua pronuba | 2 | 2 | 2 | 2 | 2 | 2 |
| Ochropleura plecta | 1 | 1 | 1 | 1 | 1 | 1 |
| Omphaloscelis lunosa | 2 | 1 | 0 | 1 | 1 | 1 |
| Phlogophora meticulosa | 2 | 2 | 0 | 2 | 2 | 1.6 |
| Xestia c‐nigrum | 2 | 1 | 1 | 1 | 2 | 1.4 |
| Xestia triangulum | 1 | 1 | 1 | 1 | 0 | 0.8 |
| Xestia xanthographa | 2 | 1 | 0 | 1 | 0 | 0.8 |
We combined the two sources of information on dispersal ability to place the 24 study species into three categories: “low”, “medium”, and “high” mobility (Table 3). An ANOVA was used to compare tethered flight variables among moth species assigned to these three mobility categories.
Table 3.
Summary table of individual moth species flown on tethered flight mills. All individuals were males
| Species | N flown | Suction trap score | Expert opinion | Score | Mobility category | Total distance (m) | Maximum speed (m/sec) |
|---|---|---|---|---|---|---|---|
| Agrotis exclamationis | 18 | 1 | 1 | 2.0 | Medium | 6935 | 1.458 |
| Agrotis puta | 8 | 1 | 1.2 | 2.2 | Medium | 597 | 0.743 |
| Amphipoea oculea | 11 | 0.8 | 0.8 | Low | 1580 | 0.962 | |
| Amphipyra pyramidea | 14 | 0.8 | 0.8 | Low | 12352 | 1.799 | |
| Apamea monoglypha | 39 | 1 | 1.4 | 2.4 | High | 9036 | 2.059 |
| Autographa gamma | 13 | 1 | 2 | 3.0 | High | 5168 | 1.535 |
| Axylia putris | 14 | 0.6 | 0.6 | Low | 2474 | 0.979 | |
| Hoplodrina alsines | 13 | 0.8 | 0.8 | Low | 2647 | 1.152 | |
| Hoplodrina ambigua | 13 | 1.4 | 1.4 | Medium | 1166 | 0.974 | |
| Hydraecia micacea | 23 | 0.6 | 0.6 | Low | 2647 | 1.163 | |
| Lacanobia oleracea | 16 | 0.6 | 0.6 | Low | 3756 | 1.352 | |
| Mesapamea didyma | 10 | 1 | 0.8 | 1.8 | Medium | 3598 | 1.112 |
| Mesapamea secalis | 16 | 1 | 0.8 | 1.8 | Medium | 3574 | 1.046 |
| Mythimna impura | 11 | 0.8 | 0.8 | Low | 1581 | 0.807 | |
| Mythimna pallens | 19 | 0.8 | 0.8 | Low | 2675 | 0.882 | |
| Noctua comes | 26 | 1.2 | 1.2 | Medium | 6548 | 1.474 | |
| Noctua janthe | 13 | 1.4 | 1.4 | Medium | 4489 | 1.215 | |
| Noctua pronuba | 37 | 1 | 2 | 3.0 | High | 11596 | 1.623 |
| Ochropleura plecta | 20 | 1 | 1.0 | Low | 626 | 0.697 | |
| Omphaloscelis lunosa | 16 | 1 | 1.0 | Low | 1693 | 1.286 | |
| Phlogophora meticulosa | 10 | 1 | 1.6 | 2.6 | High | 9501 | 1.877 |
| Xestia c‐nigrum | 59 | 1 | 1.4 | 2.4 | High | 5903 | 1.17 |
| Xestia triangulum | 12 | 0.8 | 0.8 | Low | 5254 | 1.478 | |
| Xestia xanthographa | 25 | 1 | 0.8 | 1.8 | Medium | 4193 | 0.936 |
Mobility category was assigned by summing scores from suction trap data and expert survey. One point was assigned if species were in the top 25% of species caught in Rothamsted Insect Survey (RIS) suction traps (mean yearly catch over period 2000–2009). Expert opinion was the mean value of responses where five experts were asked to assign species to categories of low (0), medium (1), and high (2) mobility (see Table 2). “Score” sums these two methods of classification and mobility category was assigned according to thresholds: ≤1 = Low, >1 to ≤2 = Medium and >2 = High. Species mean values for the tethered flight variables “Total distance flown overnight” and “maximum speed” are also shown.
Results
Characterizing dispersal ability with tethered flight
Significantly more males were caught than females in light traps, and so our sample sizes for flight mill validation were higher for males (495 individuals) than females (122 individuals). Given that there is likely to be intraspecific variation in flight between males and females (Berwaerts et al. 2006), and in order to maximize the number of species we studied, all flight trials were based only on males. In order to obtain robust measures for species, and to account for intra‐specific variation in flight, we only included species with ≥8 individuals flown (hence we measured 456 individuals in total, median = 15 individuals per species, from 24 species; Table 3). Many of the 16 tethered flight variables were highly correlated (Fig. 4) and a Principal Components Analysis confirmed redundancy in measures (Fig. 5), but that measurement of flight distance/duration and flight speed characterized different aspects of dispersal. A Canonical Variates Analysis (Table 4) indicated that measures of flight speed best distinguished among moth study species. Thus we concluded that that “total distance flown overnight” and “maximum speed” were the best tethered flight variables to analyse that captured most of the variation in flight in our study species.
Figure 4.
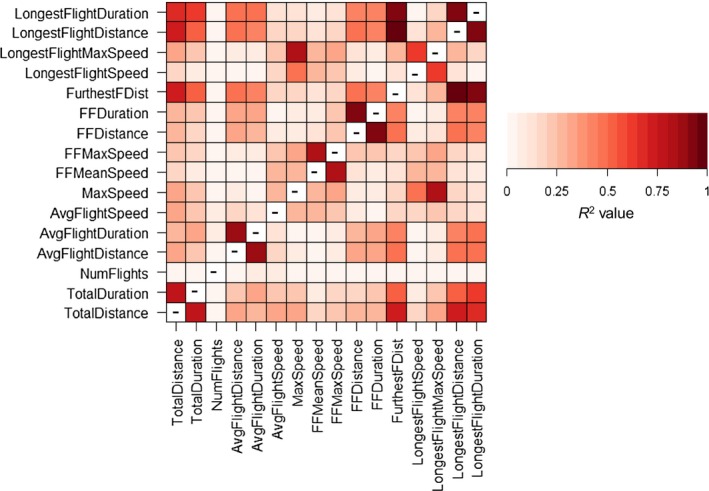
Matrix of pair‐wise correlations of the sixteen tethered flight variables outlined in Table 1. A dash indicates a cell where a correlation value has not been computed.
Figure 5.
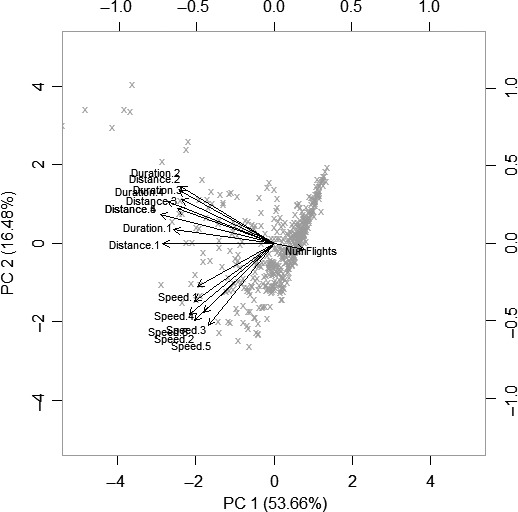
Principal components analysis biplot of the 16 tethered flight mill variables listed in Table 1. The two first principal components are plotted with the proportion of variance explained by each component printed next to the axes label which together explain >70% of variation in the data. Crosses indicate the 456 male individuals in the data set; the top and right axes show principal component scores of the individuals. The arrows indicate the principal component loadings of the different tethered flight variables.
Table 4.
Canonical Variates Analysis was performed on the 16 tethered flight variables (outlined in Table 1)
| Tethered flight measurement | CV1 (45.46) | CV2 (14.75) | CV3 (10.57) | CV4 (7.26) | CV5 (7.1) |
|---|---|---|---|---|---|
| AvgFlightDistance | −0.0002 | 0.0001 | 0.0004 | 0 | 0.0001 |
| AvgFlightDuration | 0.0001 | −0.0002 | −0.0004 | 0 | −0.0002 |
| AvgFlightSpeed | 0.8207 | −3.5807 | −0.5541 | −2.5477 | 2.1785 |
| FFDistance | 0.0001 | 0.0003 | −0.0002 | −0.0004 | 0.0002 |
| FFDuration | −0.0001 | −0.0002 | 0.0002 | 0.0002 | −0.0001 |
| FFMaxSpeed | 0.3871 | 1.3461 | −1.1428 | 0.7091 | −0.8464 |
| FFMeanSpeed | −1.0561 | −1.3326 | 0.902 | 0.8578 | 3.5797 |
| FurthestFDist | −0.0001 | −0.0001 | −0.0001 | 0.0005 | 0 |
| LongestFlightDistance | −0.0001 | −0.0001 | −0.0001 | 0.0005 | 0 |
| LongestFlightDuration | 0.0001 | 0.0002 | 0 | −0.0007 | 0.0001 |
| LongestFlightMaxSpeed | 1.1193 | 0.4216 | 1.392 | −1.12 | 1.3157 |
| LongestFlightSpeed | 0.0129 | 0.9717 | 0.4125 | −1.8829 | 0.8313 |
| MaxSpeed | 1.302 | 0.5732 | −1.5167 | 1.1944 | −1.9183 |
| NumFlights | 0.0095 | −0.0076 | 0.0325 | −0.0066 | 0.0087 |
| TotalDistance | 0.0001 | 0.0001 | 0.0001 | −0.0004 | −0.0003 |
| TotalDuration | 0 | −0.0002 | 0.0001 | 0.0003 | 0.0002 |
Loadings values of the variables in the first five canonical variates are shown. Values in brackets next to CV number are the percentage variance in the dataset accounted for by that canonical variate.
Validating flight mill data
Individuals from the 24 study species were assigned to mobility categories (low, medium or high) according to their species scores in Table 3. Mobility category had a significant effect on both flight distance and speed (total distance flown: F 2,21 = 8.69, P = 0.002; maximum speed: F 2,21 = 4.61, P = 0.022; Fig. 6). A Tukey post‐hoc test confirmed that the medium and low mobility groups had significantly shorter flight distances than the high group, and the low mobility group had slower flight speeds than the high group. Information on total distance and maximum speed of the study species are plotted as boxplots (Fig. 6).
Figure 6.
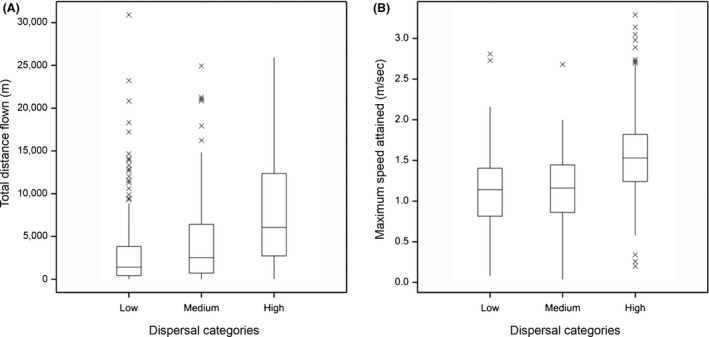
Boxplots showing (A) total distance flown and (B) maximum speed attained on tethered flight mills of 456 individuals assigned to three dispersal categories according to their species (Table 3). Boxes span the interquartile range of values, with the line dissecting the box indicating the median. Whiskers extend to 1.5 times the interquartile range beyond the quartiles. Beyond this outliers are plotted as a cross.
Discussion
In this study, we describe and test a new tethered flight system that has enabled us to fly a wide variety of noctuid moth species in controlled laboratory conditions (Patent: Lim et al. 2013). While other studies have used tethered flight mills to examine intra‐specific variation in flight performance, e.g. in relation to sex, population, age and levels of sexual maturity within species (Mcanelly 1986; Schumacher et al. 1997; Berwaerts et al. 2006; Taylor et al. 2010), this is the first study that has compared flight performances across a number of species spanning a range of sizes. Our novel flight mill design with magnetic suspension of the axis and a unique lightweight but rigid arm is sufficiently lightweight and low friction for the smallest species but strong enough for the largest species, and so facilitates the study of a wide range of species. Our apparatus currently allows the testing of 16 individuals at the same time, and the software extracts 16 flight variables for subsequent analysis. This enables the user to look at many aspects of the speed, distance and duration of flights and pattern of flight performance overnight. For our study species, measures of “total distance flown overnight” and “maximum speed” were the most informative for distinguishing among the 24 study species, but the range of flight variables recorded provides substantial flexibility in the types of experimental studies that could be carried out.
Flight mill validation
We showed that the tethered flight data obtained on the apparatus are representative of natural flight ability of species, supporting the usefulness of the apparatus in investigations of insect dispersal. Species placed in the high mobility category (such as Noctua pronuba and Autographa gamma; group mean flight distance = 8178 m) had mean flight distances 2.5 times that of species in the low mobility category (such as Axylia putris and Hydraecia micaea; group mean = 3263 m). Four of the five species in the “high” mobility group (A. gamma, N. pronuba, Phlogophora meticulosa and Xestia c‐nigrum) are migrants (Waring et al. 2009; Chapman et al. 2010), whereas there is very little published information on dispersal ability in other species. This lack of dispersal information was reflected in the expert survey information (Table 2) where there was some lack of consensus on which moths belonged in the “low” and “medium” categories. This lack of consensus may explain why our analyses were generally less capable of distinguishing between the low and medium groups of species, compared with the high group. All the study species are noctuids and are relatively mobile compared with some other macro‐moth families (e.g. Geometridae), but nonetheless there is variation in dispersal ability among these species which was evident in flight mill data. We therefore conclude that the tethered flight mills are an important new tool to elucidate dispersal ability in a wider range of species than has been possible previously.
Limitations of the flight mill system
Our tethered flight mill system has some limitations as a tool to assess dispersal, which are common to most tethered flight techniques. The tether restricts natural flight somewhat as it may obstruct wing‐flapping, especially in species which employ a “clap‐and‐fling” style of flight, e.g. butterflies (Srygley and Thomas 2002). Our preliminary flight observations concluded that geometrid moths' wing‐flapping was obstructed by the tether, but noctuid moths did not appear to be hindered. Flying on a tether also means that the insects do not have to produce sufficient lift to overcome their body weight and thus are not expending as much energy as free flying insects (Riley et al. 1997).
It is more complex to interpret how distances flown on the flight mill might relate to dispersal distances in the wild. It is difficult to simulate all the cues that an insect may require to fly, which is especially important if flight propensity is of interest (Colvin and Gatehouse 1993), and so insects may not behave naturally when tethered. For example, moths may not receive appropriate cues to take off, or once in flight, the absence of appropriate cues may prolong the insect's flight and delay landing. In addition, the lack of tarsal contact with the ground and the inability to land will likely encourage insects to fly for greatly extended periods compared to natural flight (Gatehouse and Hackett 1980), and thus the flight mill measures are more likely to be representative of upper flight limits than normal flight activity. Conversely, the added physical effort of pushing the flight mill while flying may cause the insect to tire and cease flight more quickly than in the wild; however, this may be countered by the lower energy expenditure resulting from the lift provided by the tether.
Despite these criticisms, tethered flight mills are an invaluable tool in studying the flight performance of nocturnal and/or high flying insects for which no observation of natural flight duration and movement pathways may be possible. Tethered flight mills are valuable tools to demonstrate differences in dispersal ability among different groups, as evidenced by this study and others (Blackmer et al. 2004; Taylor et al. 2010).
Potential for using flight mill system in new investigations
The tethered flight mills provide a platform to explore the relationship between measures of dispersal ability (such as flight speed and duration), and physiological, genetic and environmental factors that promote or inhibit flight. Insects can be flown after being caught from the wild, enabling assessment of the amount of variation in dispersal ability present in wild populations. Insects can also be flown having been reared under controlled conditions, which enables the effects of food availability, climate and disease levels during development on dispersal propensity to be assessed. The “handle” by which the moths are attached to the mill is small and light compared to many other set‐ups, enabling moths to be flown on sequential nights, and therefore age‐related changes in flight behavior can be quantified. Genetic and epigenetic factors affecting dispersal ability can also be assessed and compared across species.
In addition to the flight mill apparatus outlined in this paper, we also have flight mills with longer arm lengths that we have used to fly large, powerfully flying species such as the European hornet (Vespa crabro), hawk moths (Sphingidae), bumblebees (Bombus terrestris), and honeybees (Apis mellifera); and flight mills with extremely small and lightweight arms that have been used to quantify the flight ability of small, weak‐flying insects including brown planthoppers (Nilaparvata lugens) and mosquitoes (Aedes aegypti), weighing <1 mg. We are currently developing calibration methods that will enable the comparison of distances flown on different arm types, thereby opening up new possibilities to compare a much wider range of taxa. We conclude that our new tethered flight apparatus provides a robust technique to assess the flight ability of insects. This new technique opens up the potential to quantify the dispersal abilities of a much wider range of species for which current knowledge of dispersal is lacking, and to address a plethora of scientific questions about factors affecting insect dispersal.
Data Accessibility
Data will be uploaded to http://datadryad.org/.
The flight mills are subject to a patent (Patent number: PCT/GB2014/052466), but collaboration is welcomed or we recommend you contact the Knowledge Exchange and Commercialization team at Rothamsted (andrew.spencer@rothamsted.ac.uk).
Conflict of Interest
None declared.
Supporting information
Appendix S1. Example of raw data generated by the tethered flight mill for 8 moths in one night.
Acknowledgments
We are grateful to S. Clark for statistical advice and J. Helps for assistance with R. Rothamsted Research receives grant aided support from the Biotechnology and Biological Sciences Research Council. H.B.C.J. was funded by a BBSRC Quota studentship awarded to J.W.C. and J.K.H.
References
- Attisano, A. , Tregenza T., Moore A. J., and Moore P. J.. 2013. Oosorption and migratory strategy of the milkweed bug, Oncopeltus fasciatus . Anim. Behav. 86:651–657. [Google Scholar]
- Beerwinkle, K. R. , Lopez J. D., Cheng D., Lingren P. D., and Meola R. W.. 1995. Flight potential of feral Helicoverpa‐zea (Lepidoptera, noctuidae) males measured with a 32‐channel, computer monitored, flight‐mill system. Environ. Entomol. 24:1122–1130. [Google Scholar]
- Berwaerts, K. , Aerts P., and Van Dyck H.. 2006. On the sex‐specific mechanisms of butterfly flight: flight performance relative to flight morphology, wing kinematics, and sex in Pararge aegeria. Biol. J. Linn. Soc. 89:675–687. [Google Scholar]
- Blackmer, J. L. , Naranjo S. E., and Williams L. H.. 2004. Tethered and untethered flight by Lygus hesperus and Lygus lineolaris (Heteroptera: Miridae). Environ. Entomol. 33:1389–1400. [Google Scholar]
- Bowler, D. E. , and Benton T. G.. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80:205–225. [DOI] [PubMed] [Google Scholar]
- Burke, R. , Fitzsimmons J., and Kerr J.. 2011. A mobility index for Canadian butterfly species based on naturalists' knowledge. Biodivers. Conserv. 20:2273–2295. [Google Scholar]
- Chambers, D. L. , Sharp J. L., and Ashley T. R.. 1976. Tethered insect flight: a system for automated data processing of behavioral events. Behav. Res. Meth. Instrument 8:352–356. [Google Scholar]
- Chapman, J. W. , Nesbit R. L., Burgin L. E., Reynolds D. R., Smith A. D., Middleton D. R., et al. 2010. Flight orientation behaviors promote optimal migration trajectories in high‐flying insects. Science 327:682–685. [DOI] [PubMed] [Google Scholar]
- Clobert, J. , Danchin E., Dhondt A. A., and Nichols J. D. 2001. Dispersal. Pp. 452 Oxford University Press, Oxford. [Google Scholar]
- Colvin, J. , and Gatehouse A. G.. 1993. The reproduction‐flight syndrome and the inheritence of tethered‐flight activity in the cotton‐bollworm moth, Heliothis armigera . Physiol. Entomol. 18:16–22. [Google Scholar]
- Dingle, H. 1965. The relation between age and flight activity in the milkweed bug, Oncopeltus. J. Exp. Biol. 42:269–283. [Google Scholar]
- Gatehouse, A. G. , and Hackett D. S.. 1980. A technique for studying flight behaviour of tethered spodoptera‐exempta moths. Physiol. Entomol. 5:215–222. [Google Scholar]
- Gibbs, M. , Saastamoinen M., Coulon A., and Stevens V. M.. 2009. Organisms on the move: ecology and evolution of dispersal. Biol. Lett. 6:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski, I. , Alho J., and Moilanen A.. 2000. Estimating the parameters of survival and migration of individuals in metapopulations. Ecology 81:239–251. [Google Scholar]
- Hughes, C. L. , Dytham C., and Hill J. K.. 2007. Modelling and analysing evolution of dispersal in populations at expanding range boundaries. Ecol. Entomol. 32:437–445. [Google Scholar]
- Kennedy, J. S. , and Booth C. O.. 1963. Free flights of aphids in laboratory. J. Exp. Biol. 40:67–000. [Google Scholar]
- Lester, S. E. , Ruttenberg B. I., Gaines S. D., and Kinlan B. P.. 2007. The relationship between dispersal ability and geographic range size. Ecol. Lett. 10:745–758. [DOI] [PubMed] [Google Scholar]
- Lim, K. S. , Wolf M., Jones H., and Black I. 2013. Flight mill. Patent number: PCT/GB2014/052466.
- Macaulay, E. D. M. , Tatchell G. M., and Taylor L. R.. 1988. The Rothamsted Insect Survey 12‐metre suction trap. Bull. Entomol. Res. 78:121–129. [Google Scholar]
- McAnelly, M. L. , and Rankin M. A.. 1986. Migration in the grasshopper melanoplus sanguinipes (Fab.) II. Interactions between flight and reproduction. Biol. Bull., 170:378–392. [Google Scholar]
- Mouritsen, H. , and Frost B. J.. 2002. Virtual migration in tethered flying monarch butterflies reveals their orientation mechanisms. Proc. Natl Acad. Sci. USA 99:10162–10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbit, R. L. , Hill J. K., Woiwod I. P., Sivell D., Bensusan K. J., and Chapman J. W.. 2009. Seasonally adaptive migratory headings mediated by a sun compass in the painted lady butterfly, Vanessa cardui. Anim. Behav. 78:1119–1125. [Google Scholar]
- Pearson, R. G. , and Dawson T. P.. 2003. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 12:361–371. [Google Scholar]
- R Core Team . 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Riley, J. R. , Downham M. C. A., and Cooter R. J.. 1997. Comparison of the performance of Cicadulina leafhoppers on flight mills with that to be expected in free flight. Entomol. Exp. Appl. 83:317–322. [Google Scholar]
- Schumacher, P. , Weyeneth A., Weber D. C., and Dorn S.. 1997. Long flights in Cydia pomonella L (Lepidoptera: Tortricidae) measured by a flight mill: Influence of sex, mated status and age. Physiol. Entomol. 22:149–160. [Google Scholar]
- Srygley, R. B. , and Thomas A. L. R.. 2002. Unconventional lift‐generating mechanisms in free‐flying butterflies. Nature 420:487–489. [DOI] [PubMed] [Google Scholar]
- Stevens, V. M. , Turlure C., and Baguette M.. 2010. A meta‐analysis of dispersal in butterflies. Biol. Rev. 85:625–642. [DOI] [PubMed] [Google Scholar]
- Taylor, R. A. J. , Bauer L. S., Poland T. M., and Windell K. N.. 2010. Flight performance of Agrilus planipennis (Coleoptera: Buprestidae) on a flight mill and in free flight. J. Insect Behav. 23:128–148. [Google Scholar]
- The MathWorks Inc. (2012) MATLAB 8.0 and Statistics Toolbox 8.1. The MathWorks Inc., Natick, MA, USA. [Google Scholar]
- Thomas, J. A. 1983. A quick method for estimating butterfly numbers during surveys. Biol. Conserv. 27:195–211. [Google Scholar]
- Waring, P. , Townsend M., and Lewington R.. 2009. Field guide to the moths of Great Britain and Ireland, 2nd edn British Wildlife Publishing, Oxford. [Google Scholar]
- Wood, C. R. , Reynolds D. R., Wells P. M., Barlow J. F., Woiwod I. P., and Chapman J. W.. 2009. Flight periodicity and the vertical distribution of high‐altitude moth migration over southern Britain. Bull. Entomol. Res. 99:525–535. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Wu K., Wyckhuys K. A. G., and Heimpel G. E.. 2009. Trade‐offs between flight and fecundity in the soybean aphid (Hemiptera : Aphididae). J. Econ. Entomol. 102:133–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Example of raw data generated by the tethered flight mill for 8 moths in one night.
Data Availability Statement
Data will be uploaded to http://datadryad.org/.
The flight mills are subject to a patent (Patent number: PCT/GB2014/052466), but collaboration is welcomed or we recommend you contact the Knowledge Exchange and Commercialization team at Rothamsted (andrew.spencer@rothamsted.ac.uk).


