Abstract
Hepatitis C virus (HCV) is a global health problem affecting a large fraction of the world’s population: This virus is able to determine both hepatic and extrahepatic diseases. Mixed cryoglobulinemia, a B-cell “benign” lymphoproliferative disorders, represents the most closely related as well as the most investigated HCV-related extrahepatic disorder. Since this virus is able to determine extrahepatic [non-Hodgkin’s lymphoma (NHL)] as well as hepatic malignancies (hepatocellular carcinoma), HCV has been included among human cancer viruses. The most common histological types of HCV-associated NHL are the marginal zone, the lymphoplasmacytic and diffuse large cell lymphomas. The role of the HCV in the pathogenesis of the B-cell lymphoproliferative disorders is confirmed also by the responsiveness of the NHL to antiviral therapy. The purpose of this review is to provide an overview of the recent literature and a meta analysis of the epidemiology data, to explain the role of HCV in the development of NHL’s lymphoma. Furthermore, the possibility to treat these HCV-related NHL with the antiviral therapy or with other therapeutic options, like chemotherapy, is also discussed.
Keywords: Hepatitis C virus, Non-Hodgkin’s lymphoma, Hepatitis C virus genotypes, Alpha-interferon
Core tip: The goal of this article is to review the epidemiological data from different countries to perform an up-to-date meta-analysis of the risk to developing non-Hodgkin’s lymphomas in hepatitis C virus (HCV)-infected patients. Finally, we highlighted the clinical and the biological data necessary to optimize the cure of the patients affected by HCV-positive non-Hodgkin’s lymphomas.
INTRODUCTION
Non-Hodgkin’s lymphomas (NHL) are neoplastic diseases of the lymphoid tissue. Given the high heterogeneity in terms of histological and clinical characteristics, anatomical location, and putative aetiologies, several causative factors have been reported including inherited or acquired immunodeficiency, exposure to some toxic substances (pesticides) or radiation, smoking habits, and, in the last few years, infectious factors. In fact, the Epstein-Barr virus (EBV) has been shown to be involved in the development of the Burkitt’s lymphoma[1-3] and of other haematological malignancies (immunoblastic lymphoma, Hodgkin’s disease, nasopharyngeal carcinoma), the human retrovirus HTLV-I in the T-cell leukemia-lymphoma[4,5], and the double stranded DNA human herpes virus 8 in the Kaposi sarcoma[6,7], primary effusion lymphoma and multicentric Castleman disease[8]. But not only viruses are involved in pathogenesis of NHL, even the Gram-negative microaerophilic bacterium Helicobacter Pylori is thought to be the cause of gastric mucosa associated lymphoid (MALT) lymphoma[9,10]. However, although these infectious agents are widespread (EBV infects near 100% of all populations and remains in B-cell throughout the life span), only a very small fraction of virus-carriers develops lymphomas. This indicates the key role of some, not yet understood, host factors: Maybe genetic factors like HLA antigens or cytokine signalling pathways or acquired factors like exposure to toxic substances (ethanol, drugs, etc.) or immunosuppression secondary to therapy for rheumatological disorders or to chemotherapy for malignancies.
Hepatitis C virus (HCV) is a RNA virus belonging to the flaviviruses discovered in 1990 and involved in acute and chronic liver disease. The genome consists of a single-positive-stranded RNA molecule enveloped by a lipid bilayer within which two different glycoproteins are anchored[11]. The viral genome contains three distinct regions[12]: (1) a short 5′ non-coding region with two domains: A stem-loop structure involved in HCV replication and the internal ribosome entry site the structure responsible for attachment of the ribosome and polyprotein translation[13]; (2) A large, unique open reading frame of more than 9000 nucleotides, which encodes a single polyprotein precursor, that is cleaved co- and post-translationally to give the structural and non-structural viral proteins; and (3) The 3’ non-translated region endowed with high variability in the length and structure. The HCV shows a high genetic diversity since, similarly to all RNA positive-strand viruses, the RNA-dependent RNA polymerase lacks a 3’-5’ exonuclease proofreading activity for removal of the misincorporated bases. Therefore, the viral replication is error-prone, and this determines a large number of variants (quasispecies virus population)[14]. The frequency of the nucleotide mutations ranges from 1.4 × 103 to 1.9 × 103 substitutions per nucleotide per year. The HCV is classified into six genotypes with a different distribution by geographical region and between patient groups; each genotype contains a variable number of genetically distinct “subtypes”. At the nucleotide level, the genotypes differ from each other by 31% to 33%, while subtypes from 20% to 25%[15]. The peculiar characteristic of HCV is the ability to infect not only the liver cells but also the lymphocytes[16] and, likely, other cells and tissues[17,18]. This is due on the fact that liver cells and lymphocytes share the same HCV receptor, i.e., the CD81. The lymphotropism might explain the several extra hepatic manifestations of the chronic HCV infection[19-26], among which mixed cryoglobulinemia (MC) is the most common[27-32]. MC is a disease characterised by the presence in the serum of immuno-complexes able to precipitate with cold temperature and to re-dissolve with rewarming[33]. The main clinical manifestations of this disease are the skin lesions (purpura) secondary to vasculitis, which is caused by the deposition of the cryoglobulins in the small and medium sized blood vessels[34]. In addition to skin lesions, MC may involve several organs and tissues, determining peripheral neuropathy and/or glomerulonephritis. Since cryoglobulins are the production of monoclonal B-cells and lymphoid infiltrates are present frequently in the bone marrow[35] of these patients, MC should be considered as a smouldering lymphoma. Accordingly, even the first cases of MC described by Melzer, later, by other researchers[36-38] developed lymphomas months or years after the onset of the symptoms of MC[39]. These reports suggest that chronic HCV infection induces clonal B-cell proliferation, which can evolve from a “benign” lymphoproliferative disorder to an overt malignant lymphoma[40]. Since, according to some estimates, near 170 million of people are carriers of the virus[41], the clinical impact of the extrahepatic disorders, leading to neoplastic diseases of the hemopoietic system in addition to the liver diseases, makes HCV a major public health problem.
THE EPIDEMIOLOGY OF HCV-POSITIVE NHL: META-ANALYSIS UP-DATING
The first studies, which described the association of HCV and lymphoproliferative disorders, were performed recording the prevalence of anti-HCV antibodies in small-unselected groups of patients affected by lymphomas[42-46]. These preliminary reports excluded the association between HCV infection and Hodgkin’s disease, while showed a strong association between NHL and HCV, especially in low-grade lymphomas. However, this association was found mainly in Italy and other researchers from the North of Europe did not confirm these findings. Therefore, some authors considered this relationship as due to the high prevalence of HCV in the Italian general population. In the following years, several studies addressed the possible association between HCV and NHL[47] and many papers have been published from different areas of the world. At present, more than 10000 cases of NHL have been screened for the presence of HCV infection and several meta-analyses on the relationship between HCV and lymphoma have been published[48,49].
In this review, the most recent meta-analysis have been updated to include only the studies (until the end of 2011) with a control groups. Unfortunately, these control groups were heterogeneous, in fact, some papers included patients with hematological diseases other than NHL, other studies included cases with solid cancers, or cases undergoing an invasive procedure (like surgery or endoscopy) or population-based samples, other studies enrolled volunteer blood donors. Only recently, some authors designed these studies as case-control or as cohort studies with well-defined inclusion criteria. Therefore, these authors are able to estimate the odds ratios or the relative risks (RRs) adjusted for age, sex, and other confounding factors. In the present review, we discarded the studies including the patients with other lymphoproliferative diseases as control group since also these diseases might be correlated with HCV. In addition, we considered eligible for meta-analysis only the studies with at least one of the following requirements: (1) Sex- and age-adjusted RRs; (2) Cases and controls matched by age and sex; and (3) A measure of age and of the male/female ratio in both cases and controls.
If the authors did not provide RRs, we calculated the crude RRs (with 95%CIs) according to the Wald method, assuming the items 2 and 3 were available. In the analysis on NHL and HCV infection, we discarded the papers including less than 100 cases of NHL, while we included all the prospective studies (case-control or cohort studies) regardless of the number of NHL enrolled. Several problems of comparability were found in the retrieved studies since not all authors shared the definition of lymphoma. For instance, some authors included chronic lymphocytic leukemia (CLL) among NHL cases, whereas others did not. Since CLL patients show a prevalence of HCV infection lower than that observed in the general population, the inclusion or the exclusion of this very common lymphoproliferative disorder has a great impact on the epidemiological studies. In addition, the CLL cells and the small lymphocytic lymphoma (SLL) have the same immuno-phenotype[50,51], but SLL was included among NHL by all authors[51]. Most authors excluded the cases with human immunodeficiency virus (HIV) infection; therefore, we did not review the studies including HIV patients. To avoid bias, we discarded also the studies including only selected populations, non-representative of general population[52,53]. Another problem was the method of checking and confirming the HCV infection: In the first papers, most authors used only the enzyme-linked immunosorbent assay (ELISA) whereas, more recently, most authors used recombinant immunoblot assay (RIBA). To increase the complexity of the analysis, some authors enrolled only the patients with active HCV replication, i.e., with detectable levels of serum HCV-RNA. Since the first generation ELISAs showed low sensitivity and specificity, in this review we included only the studies employing second or third generation ELISAs. However, we did not consider the detection of HCV-RNA as a requirement for including a study.
Statistical methods: We calculated the summary RR and corresponding 95%CI with the models of DerSimonian and Laird, which incorporate both within and between-study variability, as a weighted average of the estimated RRs, by giving each study a weight proportional to its precision. The heterogeneity among studies was evaluated using the Q statistics. The Begg’s and Egger’s asymmetry tests were used to assess the publication bias.
Figure 1 indicates the results of studies on HCV and NHL. The highest prevalence of HCV infection in the general population (over 20%) was found in Egypt. A rather high prevalence (5%-10%) was found in Italy and in Japan, while most countries (South Korea, Northern Europe, United States, Australia, and Canada) showed a prevalence below 5%. The 19 case-controls studies included in this review enrolled altogether 9038 cases and 12224 controls. The pooled RR from this large group was 2.4 (95%CI: 2.0-3.0), and most of them (11/19) showed a RRs significantly elevated (Figure 1). The RRs of the cohort studies was 2.0 (95%CI: 1.4-3.0). The overall RR estimation was 2.3 (95%CI: 1.8-2.9) with no significant heterogeneity between study designs. The different prevalence of the HCV infection in the control groups determined the great heterogeneity in the results. In fact, the studies performed in areas with a high HCV prevalence (above 5%) showed a more elevated RR (> 3) than those performed in areas with a low HCV prevalence (RR < 2). A significant heterogeneity emerged also for the publication period: In fact, the studies published up to 2003 indicated higher RRs when compared with the studies carried out thereafter. In addition, there are regional variations: people infected by HCV from Japan and from the Mediterranean basin show a relative risk of NHL from 2 to 4 times higher than people of Northern Europe[54].
Figure 1.
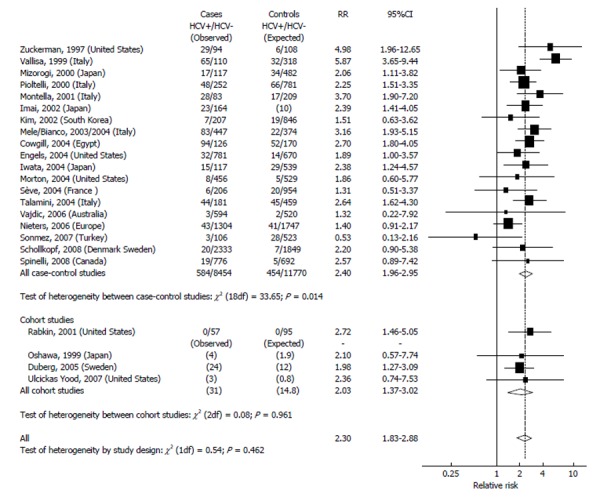
Relative risk estimates and corresponding 95%CI of non-Hodgkin’s lymphoma by hepatitis C virus seropositivity in case-control and cohort studies.
The mechanisms by which lymphoma is induced by HCV are still limited. The HCV-induced transformation process of B-cell may occur in three ways: (1) Chronic stimulation of B-Cell Receptor or other receptors placed on the surface of B-cells by the viral antigens (in absence of cell infection) with secondary proliferation; (2) Infection and persistent replication of HCV inside B-cells with oncogenic effects by some viral proteins; and (3) Temporary intracellular virus replication with damage of B-cells[55]. However, since an active replication of HCV in human B or T lymphocytes in vivo (with evidence of the HCV-RNA negative strands) has never been demonstrated, a direct oncogenic effect by HCV inside B cells is unlikely. In addition, viral proteins, indicative of active replication, could never be demonstrated in the neoplastic lymphoid tissue of the HCV-NHL. Based on these considerations, it is likely that the neoplastic transformation is determined by the chronic antigen stimulation of B cells by viral surface proteins[56]. There are several experimental data supporting this theory: (1) the B lymphocytes from HCV patients show a higher level of activation markers[56] than normal lymphocyte; and (2) the long-term exposure to the epitopes of HCV lead to selection and expansion of a oligoclonal B-cells, which evolve in clonal B-cells and finally in an overt HCV-NHL.
In conclusions, HCV infection seems to be associated with a 2.5-fold increase in the risk of developing NHL. The fraction of the NHL secondary to HCV infection may be 10%-15% in areas where HCV prevalence is high, but it is smaller in the countries of low prevalence. Based on epidemiological and experimental evidence, IARC recently concluded that there was sufficient evidence in humans to indicate the HCV infection as a cause of non-Hodgkin lymphomas, in addition to the previously recognized causal association with hepatocellular carcinoma[57].
THE THERAPY OF HCV-POSITIVE NHL
As previously indicated, the HCV-positive NHL are heterogeneous in terms of histological features and clinical aspects. The most common HCV-related NHL are indolent lymphomas (marginal-zone), but several aggressive and, rarely, very aggressive NHL are reported. Since the relationship between viral replication and monoclonal lympho-proliferation is by now consolidated, the antiviral therapy could appear to be an attractive therapeutic option, in analogy to the antibiotic therapy employed to treat MALT lymphoma associated with Helicobacter Pylori infection[7]. However, before starting antiviral treatment of “bona fide” HCV-related NHL, several points should be taken in consideration, including: (1) Is the NHL really related with HCV infection? How the haematologist can be sure that a given NHL is HCV-associated? (2) Which is the best therapeutic approach? (3) Is the chemotherapy safe? In the case of the need to plan a chemotherapy, are the HCV-positive NHL exposed to higher risks than HCV-negative cases? and (4) The outcome of the HCV + NHL is the same as compared with the HCV-NHL with the same histotype?
The HCV-related NHL show some typical, histological, clinical, laboratory and molecular characteristics. The most common histological types of HCV+NHL are lymphoplasmacytic, primary nodal marginal zone, splenic marginal zone, MALT marginal zone, while other histotypes are less closely associated with HCV[58-61]. The clinical course of the disease is generally indolent. The most common feature of true HCV + NHL is the longstanding presence of MC[62], and the late appearance of overt NHL, often after years from the onset of the clinical symptoms of MC. From a biological point of view, the HCV-related NHL often show a monoclonal IgMk component and the presence of several auto-antibodies (mainly anti-thyroid). From a molecular point of view, these patients use a restricted IgHV gene repertoire[63], with a strong bias for the IGHV1-69 and V3-A27[64,65]. In addition, the same set of V region genes, VH1-69 and Vk3 -A27 encode for the monoclonal IgMk component (if present). Finally the bcl-2/IgH translocation has been described in some studies, although not confirmed by others, present in HCV + NHL, at least of the lymphoplasmacytic subtype[66-68].
To choose the best therapeutic strategy several factors should be taken in consideration. Firstly the tumour burden: If there are large or huge nodal or extra nodal masses, chemotherapy becomes the first choice; on the contrary, if the tumour burden is low (confined to enlarged spleen and mild lymphoid infiltration of bone marrow) antiviral therapy is more indicated. A second factor to be considered is the course of the disease: If the course is indolent and lymphoma discovered occasionally during the follow-up of the patient, the antiviral therapy is more attractive, while if the patient show progressive and rapid node or spleen enlargement, chemotherapy is again the best choice. A third factor should be always taken in consideration, i.e., the presence or the absence of a chronic liver disease (CLD), and, if present, the severity of such a CLD. This means that the patient should undergo a complete hepatological evaluation including ultrasonography and, if indicated, endoscopy and liver biopsy. If the patient is affected by chronic C hepatitis without evidence of cirrhosis, the antiviral therapy should be indicated, while an advanced chronic liver disease with severe portal hypertension could be a contra-indication for antiviral and chemotherapy as well. A fourth factor to be considered is the presence and the quality of clinical symptoms. In fact the symptoms could be tumour-related (fever, weight loss, asthenia, etc.) or MC-related (vasculitis, neuropathy, arthralgias, etc.), in the former case chemotherapy is indicated while in the latter antiviral treatment could be the right choice. Finally, some specific contra-indications to antiviral therapy should be considered, often not familiar to haematologists[69], like deep depression[70,71] or immunological disorders[72].
The presence of HCV replication, i.e., detectable levels of HCV-RNA, without liver disease, cannot be considered a contra-indication for chemotherapy. In fact, the experience in the treatment of HCV + cryoglobulinemia[73] shows that when these patients undergo either anti-CD20 therapy, or other intensive immunosuppressive treatment, tough a mild elevation of HCV-RNA levels has been noticed, the liver function never worsens. On the contrary, some author reported a mild improvement in some cases. Despite few papers focused on this topic, the literature data confirm this point of view: Faggioli et al[74], in a small series of cases, did not detect any acute hepatitis due to the reactivation of HCV replication. These data were confirmed by other authors in larger cohorts of patients: Takai et al[75] found that, after chemotherapy, the fraction of NHL patients who developed liver function test alterations was higher in non-hepatitis virus carriers (12%) than in HCV-bearing patients (10%), while a significant proportion of HBsAg carriers (36%) showed post-chemotherapy liver injuries. To further confirm these data, Visco et al[76], during the follow-up of 136 HCV-positive diffuse large cell lymphomas, found that only 5 cases (4%) discontinued the chemotherapy due to severe liver function impairment. It is noteworthy that 9 cases (7%) of the series had liver cirrhosis, and 26 cases had chronic hepatitis (19%). Altogether, this means that even in presence of HCV-related chronic liver disease, chemotherapy is feasible with a reasonable margin of safety.
Contradictory data on the outcome of HCV-positive NHL are present is the literature. A first Japanese paper of Tomita et al[77] showed that the cases affected by HCV-positive aggressive NHL have the same prognosis as HCV-negative aggressive NHL, at least in the subjects without an advanced chronic liver disease. On the contrary, Besson et al[78], grouping together two large GELA studies (NHL93 and NHL98), found that the proportion of patients with high and high-intermediate IPI was higher among HCV-positive patients, and that, at 2 years, the OS and PFS of HCV-positive cases was 56% vs 80% and 53% vs 75%,respectively. These surprising results could be explained, at least in part, by taking into account a possible selection bias of cases. In fact, the prevalence of HCV in these two cohorts of cases affected by NHL is largely lower (0.46%) of that found in the general population of France, where the prevalence is 2.8%. Since, as previously indicated, the prevalence of HCV-infection is always higher in NHL than in the general population[79], the very low number of HCV-positivity in the two groups of patients indicates the possibility of a selective enrolment in the trial of high-risk HCV-positive cases only, while standard- or low-risk cases were discarded. Nearly at the same time, Visco et al[76] following his large cohort of HCV-positive NHL showed that the OS and PFS of HCV-positive cases were similar to HCV-negatives. The question is still open and further controlled clinical trials should be needed to have definitive answers.
As shown in Table 1, antiviral therapy of HCV-NHL yielded different outcomes, according to the various authors. Since the number of cases is usually rather limited, several histotypes were enrolled with obvious different response rates, which makes published data are often contradictory. Moreover, several authors included cases with liver disease while others excluded these cases, and, finally, the presence of cryoglobulinemia is scattered among the cases and not recorded by all the authors. From 1996 to 2011, only 112 cases of HCV-positive NHL underwent antiviral therapy, the first three groups have been treated with interferon alone, thereafter with interferon and ribavirin and the last three groups with PEG-interferon (PEG-IFN) and ribavirin. The different antiviral power of these three regimens increases the difficult to interpret the results. In the first studies, the complete remission of the lymphomas was obtained in large fractions of patients (75% range: 64% to 84%), but most cases relapsed within few months. Much better results were achieved in the patients affected by splenic lymphoma with villous lymphocytes[80], in fact all HCV-positive cases entered complete remission upon treatment with interferon alone or with interferon and ribavirin, while HCV-negative lymphomas with villous lymphocytes controls did not benefit from antiviral therapy. The results obtained by Hermine et al[80] were confirmed subsequently by other studies[81-83]. These results suggest to perform a systematic screening for HCV in the patients affected by the marginal-zone lymphomas, since in the HCV-RNA positive cases, the antiviral therapy should be considered the treatment of choice. Several studies have documented the regression of different histotypes of NHL after antiviral treatment, such as lymphoplasmacytic lymphoma[84-86], mantle-cell lymphoma[87], nodal marginal zone lymphomas[88] or extranodal marginal zone lymphoma of MALT tissue (MALT lymphomas)[89]. In the last three published studies all the patients were treated with the same antiviral regimen (PEG-IFN plus ribavirin), allowing better interpretation of the homogeneous results. In all three papers the haematological response significantly (P < 0.005) correlates to the disappearance of HCV-RNA, and the sustained virological response was more frequently obtained in patients with genotype 2 or 3 more than genotype 1 or 4, which are usually found in HCV-chronic hepatitis without NHL. Given the high antiviral power of the treatment, the relapse rate is lower in these three studies (30%) than that previously recorded. At present, no data are available on the triple therapy in HCV-NHL.
Table 1.
Main studies of antiviral therapy in patients with hepatitis C virus infection and non-Hodgkin’s lymphoma (reports with single cases were discarded)
| Ref. | n | Lymphoma histology (n) | Disease sites BM-S-LN-PB | MC type II (n) | Antiviral therapy (n) | SVR (n) | NHL response (n) | Response duration (mo) |
| Mazzaro et al[94] | 6 | LPL (6) | 6-0-2-0 | 4 | IFN (6) | 4 | CR (3) PR (1) | 12 (8-18) |
| Moccia et al[95] | 3 | SMZL (3) | 1-3-0-0 | NR | IFN (3) | 2 | CR (2) NR (1) | 24 (5-40) |
| Hermine et al[80] | 9 | SLVL (9) | 6-9-5-9 | 6 | IFN (7) IFN-RBV (2) | 7 | CR (7) PR (1) | 27 (15-40) |
| NR (1) | ||||||||
| Arcaini et al[96] | 4 | SMZL (4) | 4-4-1-2 | NR | IFN + RBV (4) | 3 | CR (2) PR (1) | 36 (1-16) |
| Kelaidi et al[82] | 8 | SMZL (4) MZL/MALT (4) | 7-6-2-6 | 8 | IFN (2) IFN + RBV (6) | 5 | CR (5) PR (1) | 6 |
| Pitini et al[97] | 2 | SMZL (2) | 2-2-1-2 | NR | IFN (2) | 2 | CR (2) | 9 |
| Saadoun et al[83] | 18 | SLVL (18) | 10-18-8-10 | 18 | IFN (8) IFN + RBV (10) | 14 | CR (14) PR (4) | 62 |
| Tursi et al[89] | 16 | MZL/MALT (16) | NR | NR | IFN + RBV (16) | 11 | CR (11) | NotR |
| Vallisa et al[86] | 13 | SMZL (4) MALT (4) FL (1) LPL (4) | 5-4-0-6 | 5 | PEG-IFN + RBV (13) | 7 | CR (7) PR (2) | 14 (2-24) |
| Mazzaro et al[85] | 18 | SLVL (1), FL (1), LPL (16) | 16-2-2-16 | 13 | IFN + RBV (8) | 3 | CR (3) PR (2) | 18 (8-32) |
| PEG-IFN + RBV (10) | 6 | CR (6) PR (2) | ||||||
| Paulli et al[98] | 2 | MZL/MALT (2) | Cutaneous | 2 | PEG-IFN + RBV | 2 | CR (1) PR (1) | NotR |
| Pellicelli et al[88] | 9 | SMZL (3) MZL (4) FL (2) | NR | 4 | PEG-IFN + RBV (9) | 7 | CR (5) PR (2) | 12 |
MZL: Marginal zone lymphoma; SMZL: Splenic marginal zone lymphoma; SLVL: Splenic lymphoma with villous lymphocytes; FL: Follicular lymphoma; LPL: Lymphoplasmacytic lymphoma; BM: Bone marrow; S: Spleen; LN: Lymph nodes; PB: Peripheral blood; IFN: Alfa2a/Alfa2b interferon 3 times a week; RBV: Ribavirin; PEG-IFN: Pegylated alfa2a/alfa2b interferon; CR: Complete remission; PR: Partial remission; NR: No response; SVR: Sustained virological response; NotR: Not reported.
CONCLUSION
In addiction of acute and chronic liver diseases, the HCV infection determines many extra hepatic manifestations. Among them, the ability of the virus to interact with B cells leads to antigen-driven B-cell transformation, which ultimately may determine MC and finally a frank NHL. Based on clinical and biological considerations, the antiviral therapy should be considered as the treatment of choice in HCV-associated lymphomas, especially in the presence of MC. However, the traditional antiviral therapy, based on PEG-IFN plus RIBA, is fading given the low efficacy and the numerous and severe side effects. At present, a new era is born for the management of HCV infection: The new strong direct antiviral agents[90-93] opened the gate for a complete eradication of viral infection. These new drugs, described as lacking in side effects, can be used even in heavily pretreated patients or in cases with advanced liver disease with high possibility of success. It is likely that these new treatment options will be able to reduce drastically the number of the chronic carriers of HCV, as consequence, the number of HCV-related NHL.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 22, 2015
First decision: July 10, 2015
Article in press: December 8, 2015
P- Reviewer: Kim SJ, Yamakawa M S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
References
- 1.Vereide D, Sugden B. Proof for EBV’s sustaining role in Burkitt’s lymphomas. Semin Cancer Biol. 2009;19:389–393. doi: 10.1016/j.semcancer.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hecht JL, Aster JC. Molecular biology of Burkitt’s lymphoma. J Clin Oncol. 2000;18:3707–3721. doi: 10.1200/JCO.2000.18.21.3707. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy G, Komano J, Sugden B. Epstein-Barr virus provides a survival factor to Burkitt’s lymphomas. Proc Natl Acad Sci USA. 2003;100:14269–14274. doi: 10.1073/pnas.2336099100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallo RC. Research and discovery of the first human cancer virus, HTLV-1. Best Pract Res Clin Haematol. 2011;24:559–565. doi: 10.1016/j.beha.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Kalyanaraman VS, Sarngadharan MG, Robert-Guroff M, Miyoshi I, Golde D, Gallo RC. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982;218:571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- 6.Cai Q, Verma SC, Lu J, Robertson ES. Molecular biology of Kaposi‘s sarcoma-associated herpesvirus and related oncogenesis. Adv Virus Res. 2010;78:87–142. doi: 10.1016/B978-0-12-385032-4.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen KW, Damania B. Kaposi sarcoma-associated herpesvirus (KSHV): molecular biology and oncogenesis. Cancer Lett. 2010;289:140–150. doi: 10.1016/j.canlet.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leroy S, Moshous D, Cassar O, Reguerre Y, Byun M, Pedergnana V, Canioni D, Gessain A, Oksenhendler E, Fieschi C, et al. Multicentric Castleman disease in an HHV8-infected child born to consanguineous parents with systematic review. Pediatrics. 2012;129:e199–e203. doi: 10.1542/peds.2010-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology. 1992;20:29–34. doi: 10.1111/j.1365-2559.1992.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 10.Roggero E, Zucca E, Pinotti G, Pascarella A, Capella C, Savio A, Pedrinis E, Paterlini A, Venco A, Cavalli F. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med. 1995;122:767–769. doi: 10.7326/0003-4819-122-10-199505150-00006. [DOI] [PubMed] [Google Scholar]
- 11.Brass V, Moradpour D, Blum HE. Molecular virology of hepatitis C virus (HCV): 2006 update. Int J Med Sci. 2006;3:29–34. doi: 10.7150/ijms.3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollinger FB. NANBH viruses. In: Hollinger FB, Robinson WS, Purcell RH, Gerin JL, Ticehurst J, et al., editors. Viral hepatitis, biological and clinical features, specific diagnosis and prophylaxis. New York: Raven Press; 1991. pp. 139–173. [Google Scholar]
- 13.Reed KE, Rice CM. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Curr Top Microbiol Immunol. 2000;242:55–84. doi: 10.1007/978-3-642-59605-6_4. [DOI] [PubMed] [Google Scholar]
- 14.Martell M, Esteban JI, Quer J, Vargas V, Esteban R, Guardia J, Gómez J. Dynamic behavior of hepatitis C virus quasispecies in patients undergoing orthotopic liver transplantation. J Virol. 1994;68:3425–3436. doi: 10.1128/jvi.68.5.3425-3436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmonds P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, Halfon P, Inchauspé G, Kuiken C, Maertens G, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–973. doi: 10.1002/hep.20819. [DOI] [PubMed] [Google Scholar]
- 16.Zignego AL, Macchia D, Monti M, Thiers V, Mazzetti M, Foschi M, Maggi E, Romagnani S, Gentilini P, Bréchot C. Infection of peripheral mononuclear blood cells by hepatitis C virus. J Hepatol. 1992;15:382–386. doi: 10.1016/0168-8278(92)90073-x. [DOI] [PubMed] [Google Scholar]
- 17.Lerat H, Berby F, Trabaud MA, Vidalin O, Major M, Trépo C, Inchauspé G. Specific detection of hepatitis C virus minus strand RNA in hematopoietic cells. J Clin Invest. 1996;97:845–851. doi: 10.1172/JCI118485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crovatto M, Pozzato G, Zorat F, Pussini E, Nascimben F, Baracetti S, Grando MG, Mazzaro C, Reitano M, Modolo ML, et al. Peripheral blood neutrophils from hepatitis C virus-infected patients are replication sites of the virus. Haematologica. 2000;85:356–361. [PubMed] [Google Scholar]
- 19.Cosserat J, Cacoub P, Blétry O. Immunological disorders in C virus chronic hepatitis. Nephrol Dial Transplant. 1996;11 Suppl 4:31–35. doi: 10.1093/ndt/11.supp4.31. [DOI] [PubMed] [Google Scholar]
- 20.Andreone P, Gramenzi A, Cursaro C, Bernardi M, Zignego AL. Monoclonal gammopathy in patients with chronic hepatitis C virus infection. Blood. 1996;88:1122. [PubMed] [Google Scholar]
- 21.Ganne-Carrie N, Medini A, Coderc E, Seror O, Christidis C, Grimbert S, Trinchet JC, Beaugrand M. Latent autoimmune thyroiditis in untreated patients with HCV chronic hepatitis: a case-control study. J Autoimmun. 2000;14:189–193. doi: 10.1006/jaut.1999.0360. [DOI] [PubMed] [Google Scholar]
- 22.Ramos-Casals M, García-Carrasco M, Cervera R, Rosas J, Trejo O, de la Red G, Sánchez-Tapias JM, Font J, Ingelmo M. Hepatitis C virus infection mimicking primary Sjögren syndrome. A clinical and immunologic description of 35 cases. Medicine (Baltimore) 2001;80:1–8. doi: 10.1097/00005792-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Koike K, Moriya K, Ishibashi K, Yotsuyanagi H, Shintani Y, Fujie H, Kurokawa K, Matsuura Y, Miyamura T. Sialadenitis histologically resembling Sjogren syndrome in mice transgenic for hepatitis C virus envelope genes. Proc Natl Acad Sci USA. 1997;94:233–236. doi: 10.1073/pnas.94.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilli M, Penna A, Zerbini A, Vescovi P, Manfredi M, Negro F, Carrozzo M, Mori C, Giuberti T, Ferrari C, et al. Oral lichen planus pathogenesis: A role for the HCV-specific cellular immune response. Hepatology. 2002;36:1446–1452. doi: 10.1053/jhep.2002.37199. [DOI] [PubMed] [Google Scholar]
- 25.Silvestri F, Barillari G, Fanin R, Zaja F, Infanti L, Patriarca F, Baccarani M, Pipan C, Falasca E, Botta GA. Risk of hepatitis C virus infection, Waldenström’s macroglobulinemia, and monoclonal gammopathies. Blood. 1996;88:1125–1126. [PubMed] [Google Scholar]
- 26.Santini GF, Crovatto M, Modolo ML, Martelli P, Silvia C, Mazzi G, Franzin F, Moretti M, Tulissi P, Pozzato G. Waldenström macroglobulinemia: a role of HCV infection? Blood. 1993;82:2932. [PubMed] [Google Scholar]
- 27.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 28.Ferri C, Greco F, Longombardo G, Palla P, Moretti A, Marzo E, Mazzoni A, Pasero G, Bombardieri S, Highfield P. Association between hepatitis C virus and mixed cryoglobulinemia [see comment] Clin Exp Rheumatol. 1991;9:621–624. [PubMed] [Google Scholar]
- 29.Misiani R, Bellavita P, Fenili D, Borelli G, Marchesi D, Massazza M, Vendramin G, Comotti B, Tanzi E, Scudeller G. Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med. 1992;117:573–577. doi: 10.7326/0003-4819-117-7-573. [DOI] [PubMed] [Google Scholar]
- 30.Ferri C, La Civita L, Longombardo G, Zignego AL. Hepatitis C virus and mixed cryoglobulinaemia. Br J Rheumatol. 1994;33:301. doi: 10.1093/rheumatology/33.3.301. [DOI] [PubMed] [Google Scholar]
- 31.Mazzaro C, Tulissi P, Moretti M, Mazzoran L, Pussini E, Crovatto M, Santini GF, Pozzato G. Clinical and virological findings in mixed cryoglobulinaemia. J Intern Med. 1995;238:153–160. doi: 10.1111/j.1365-2796.1995.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 32.Adinolfi LE, Utili R, Attanasio V, Zampino R, Ragone E, Tripodi MF, Ruggiero G. Epidemiology, clinical spectrum and prognostic value of mixed cryoglobulinaemia in hepatitis C virus patients: a prospective study. Ital J Gastroenterol. 1996;28:1–9. [PubMed] [Google Scholar]
- 33.Meltzer M, Franklin EC, Elias K, McCluskey RT, Cooper N. Cryoglobulinemia--a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med. 1966;40:837–856. doi: 10.1016/0002-9343(66)90200-2. [DOI] [PubMed] [Google Scholar]
- 34.Grey HM, Kohler PF. Cryoimmunoglobulins. Semin Hematol. 1973;10:87–112. [PubMed] [Google Scholar]
- 35.Perl A, Gorevic PD, Ryan DH, Condemi JJ, Ruszkowski RJ, Abraham GN. Clonal B cell expansions in patients with essential mixed cryoglobulinaemia. Clin Exp Immunol. 1989;76:54–60. [PMC free article] [PubMed] [Google Scholar]
- 36.Invernizzi F, Galli M, Serino G, Monti G, Meroni PL, Granatieri C, Zanussi C. Secondary and essential cryoglobulinemias. Frequency, nosological classification, and long-term follow-up. Acta Haematol. 1983;70:73–82. doi: 10.1159/000206699. [DOI] [PubMed] [Google Scholar]
- 37.Gorevic PD, Kassab HJ, Levo Y, Kohn R, Meltzer M, Prose P, Franklin EC. Mixed cryoglobulinemia: clinical aspects and long-term follow-up of 40 patients. Am J Med. 1980;69:287–308. doi: 10.1016/0002-9343(80)90390-3. [DOI] [PubMed] [Google Scholar]
- 38.Monteverde A, Rivano MT, Allegra GC, Monteverde AI, Zigrossi P, Baglioni P, Gobbi M, Falini B, Bordin G, Pileri S. Essential mixed cryoglobulinemia, type II: a manifestation of a low-grade malignant lymphoma? Clinical-morphological study of 12 cases with special reference to immunohistochemical findings in liver frozen sections. Acta Haematol. 1988;79:20–25. doi: 10.1159/000205684. [DOI] [PubMed] [Google Scholar]
- 39.Silvestri F, Pipan C, Barillari G, Zaja F, Fanin R, Infanti L, Russo D, Falasca E, Botta GA, Baccarani M. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87:4296–4301. [PubMed] [Google Scholar]
- 40.Mazzaro C, Zagonel V, Monfardini S, Tulissi P, Pussini E, Fanni M, Sorio R, Bortolus R, Crovatto M, Santini G, et al. Hepatitis C virus and non-Hodgkin’s lymphomas. Br J Haematol. 1996;94:544–550. doi: 10.1046/j.1365-2141.1996.6912313.x. [DOI] [PubMed] [Google Scholar]
- 41.Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55 Suppl 1:S10–S15. doi: 10.1093/cid/cis361. [DOI] [PubMed] [Google Scholar]
- 42.Ferri C, Caracciolo F, La Civita L, Monti M, Longombardo G, Greco F, Zignego AL. Hepatitis C virus infection and B-cell lymphomas. Eur J Cancer. 1994;30A:1591–1592. doi: 10.1016/0959-8049(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 43.Ferri C, La Civita L, Caracciolo F, Zignego AL. Non-Hodgkin’s lymphoma: possible role of hepatitis C virus. JAMA. 1994;272:355–356. doi: 10.1001/jama.1994.03520050033023. [DOI] [PubMed] [Google Scholar]
- 44.Pozzato G, Mazzaro C, Crovatto M, Modolo ML, Ceselli S, Mazzi G, Sulfaro S, Franzin F, Tulissi P, Moretti M. Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood. 1994;84:3047–3053. [PubMed] [Google Scholar]
- 45.Ferri C, Caracciolo F, Zignego AL, La Civita L, Monti M, Longombardo G, Lombardini F, Greco F, Capochiani E, Mazzoni A. Hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Br J Haematol. 1994;88:392–394. doi: 10.1111/j.1365-2141.1994.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 46.Negri E, Little D, Boiocchi M, La Vecchia C, Franceschi S. B-cell non-Hodgkin’s lymphoma and hepatitis C virus infection: a systematic review. Int J Cancer. 2004;111:1–8. doi: 10.1002/ijc.20205. [DOI] [PubMed] [Google Scholar]
- 47.Gisbert JP, García-Buey L, Arranz R, Blas C, Pinilla I, Khorrami S, Acevedo A, Borque MJ, Pajares JM, Fernández-Rañada JM, et al. The prevalence of hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Eur J Gastroenterol Hepatol. 2004;16:135–138. doi: 10.1097/00042737-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of epidemiological studies. Cancer Sci. 2004;95:745–752. doi: 10.1111/j.1349-7006.2004.tb03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15:2078–2085. doi: 10.1158/1055-9965.EPI-06-0308. [DOI] [PubMed] [Google Scholar]
- 50.Flanagan MB, Sathanoori M, Surti U, Soma L, Swerdlow SH. Cytogenetic abnormalities detected by fluorescence in situ hybridization on paraffin-embedded chronic lymphocytic leukemia/small lymphocytic lymphoma lymphoid tissue biopsy specimens. Am J Clin Pathol. 2008;130:620–627. doi: 10.1309/H9AREV6E2JTMEC6J. [DOI] [PubMed] [Google Scholar]
- 51.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amin J, Dore GJ, O’Connell DL, Bartlett M, Tracey E, Kaldor JM, Law MG. Cancer incidence in people with hepatitis B or C infection: a large community-based linkage study. J Hepatol. 2006;45:197–203. doi: 10.1016/j.jhep.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, Engels EA. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 54.Giordano TP IARC. Monographs on the Evaluation of carcinogenic risks to Humans Volume 100 Part B: A review of human carcinogens: Biological agents. IARC Press: Lyon; 2012. Available from: http://monographs.iarc.fr/ENG/Monographs/vol100B/mono100B.pdf. [Google Scholar]
- 55.Sung VM, Shimodaira S, Doughty AL, Picchio GR, Can H, Yen TS, Lindsay KL, Levine AM, Lai MM. Establishment of B-cell lymphoma cell lines persistently infected with hepatitis C virus in vivo and in vitro: the apoptotic effects of virus infection. J Virol. 2003;77:2134–2146. doi: 10.1128/JVI.77.3.2134-2146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosa D, Saletti G, De Gregorio E, Zorat F, Comar C, D’Oro U, Nuti S, Houghton M, Barnaba V, Pozzato G, et al. Activation of naïve B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci USA. 2005;102:18544–18549. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franceschi S, Lise M, Trépo C, Berthillon P, Chuang SC, Nieters A, Travis RC, Vermeulen R, Overvad K, Tjønneland A, et al. Infection with hepatitis B and C viruses and risk of lymphoid malignancies in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Epidemiol Biomarkers Prev. 2011;20:208–214. doi: 10.1158/1055-9965.EPI-10-0889. [DOI] [PubMed] [Google Scholar]
- 58.Gasparotto D, De Re V, Boiocchi M. Hepatitis C virus, B-cell proliferation and lymphomas. Leuk Lymphoma. 2002;43:747–751. doi: 10.1080/10428190290016845. [DOI] [PubMed] [Google Scholar]
- 59.Zuckerman E, Zuckerman T, Levine AM, Douer D, Gutekunst K, Mizokami M, Qian DG, Velankar M, Nathwani BN, Fong TL. Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann Intern Med. 1997;127:423–428. doi: 10.7326/0003-4819-127-6-199709150-00002. [DOI] [PubMed] [Google Scholar]
- 60.Khoury T, Chen S, Adar T, Jacob EO, Mizrahi M. Hepatitis C infection and lymphoproliferative disease: accidental comorbidities? World J Gastroenterol. 2014;20:16197–16202. doi: 10.3748/wjg.v20.i43.16197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rasul I, Shepherd FA, Kamel-Reid S, Krajden M, Pantalony D, Heathcote EJ. Detection of occult low-grade b-cell non-Hodgkin’s lymphoma in patients with chronic hepatitis C infection and mixed cryoglobulinemia. Hepatology. 1999;29:543–547. doi: 10.1002/hep.510290224. [DOI] [PubMed] [Google Scholar]
- 62.Newkirk MM, Mageed RA, Jefferis R, Chen PP, Capra JD. Complete amino acid sequences of variable regions of two human IgM rheumatoid factors, BOR and KAS of the Wa idiotypic family, reveal restricted use of heavy and light chain variable and joining region gene segments. J Exp Med. 1987;166:550–564. doi: 10.1084/jem.166.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivanovski M, Silvestri F, Pozzato G, Anand S, Mazzaro C, Burrone OR, Efremov DG. Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood. 1998;91:2433–2442. [PubMed] [Google Scholar]
- 64.Marasca R, Vaccari P, Luppi M, Zucchini P, Castelli I, Barozzi P, Cuoghi A, Torelli G. Immunoglobulin gene mutations and frequent use of VH1-69 and VH4-34 segments in hepatitis C virus-positive and hepatitis C virus-negative nodal marginal zone B-cell lymphoma. Am J Pathol. 2001;159:253–261. doi: 10.1016/S0002-9440(10)61691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perotti M, Ghidoli N, Altara R, Diotti RA, Clementi N, De Marco D, Sassi M, Clementi M, Burioni R, Mancini N. Hepatitis C virus (HCV)-driven stimulation of subfamily-restricted natural IgM antibodies in mixed cryoglobulinemia. Autoimmun Rev. 2008;7:468–472. doi: 10.1016/j.autrev.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Zignego AL, Giannelli F, Marrocchi ME, Mazzocca A, Ferri C, Giannini C, Monti M, Caini P, Villa GL, Laffi G, et al. T(14; 18) translocation in chronic hepatitis C virus infection. Hepatology. 2000;31:474–479. doi: 10.1002/hep.510310230. [DOI] [PubMed] [Google Scholar]
- 67.Zignego AL, Ferri C, Giannelli F, Giannini C, Caini P, Monti M, Marrocchi ME, Di Pietro E, La Villa G, Laffi G, et al. Prevalence of bcl-2 rearrangement in patients with hepatitis C virus-related mixed cryoglobulinemia with or without B-cell lymphomas. Ann Intern Med. 2002;137:571–580. doi: 10.7326/0003-4819-137-7-200210010-00008. [DOI] [PubMed] [Google Scholar]
- 68.Zuckerman E, Zuckerman T, Sahar D, Streichman S, Attias D, Sabo E, Yeshurun D, Rowe J. bcl-2 and immunoglobulin gene rearrangement in patients with hepatitis C virus infection. Br J Haematol. 2001;112:364–369. doi: 10.1046/j.1365-2141.2001.02573.x. [DOI] [PubMed] [Google Scholar]
- 69.Cooper C, Lester R, Thorlund K, Druyts E, El Khoury AC, Yaya S, Mills EJ. Direct-acting antiviral therapies for hepatitis C genotype 1 infection: a multiple treatment comparison meta-analysis. QJM. 2013;106:153–163. doi: 10.1093/qjmed/hcs214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loftis JM, Patterson AL, Wilhelm CJ, McNett H, Morasco BJ, Huckans M, Morgan T, Saperstein S, Asghar A, Hauser P. Vulnerability to somatic symptoms of depression during interferon-alpha therapy for hepatitis C: a 16-week prospective study. J Psychosom Res. 2013;74:57–63. doi: 10.1016/j.jpsychores.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schäfer A, Scheurlen M, Kraus MR. [Managing psychiatric side effects of antiviral therapy in chronic hepatitis C] Z Gastroenterol. 2012;50:1108–1113. doi: 10.1055/s-0031-1281682. [DOI] [PubMed] [Google Scholar]
- 72.Tran HA, Malcolm Reeves GE, Gibson R, Attia JR. Development of thyroid diseases in the treatment of chronic hepatitis C with alpha-interferon may be a good prognosticator in achieving a sustained virological response: a meta-analysis. J Gastroenterol Hepatol. 2009;24:1163–1168. doi: 10.1111/j.1440-1746.2009.05874.x. [DOI] [PubMed] [Google Scholar]
- 73.Ferri C, Cacoub P, Mazzaro C, Roccatello D, Scaini P, Sebastiani M, Tavoni A, Zignego AL, De Vita S. Treatment with rituximab in patients with mixed cryoglobulinemia syndrome: results of multicenter cohort study and review of the literature. Autoimmun Rev. 2011;11:48–55. doi: 10.1016/j.autrev.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Faggioli P, De Paschale M, Tocci A, Luoni M, Fava S, De Paoli A, Tosi A, Cassi E. Acute hepatic toxicity during cyclic chemotherapy in non Hodgkin’s lymphoma. Haematologica. 1997;82:38–42. [PubMed] [Google Scholar]
- 75.Takai S, Tsurumi H, Ando K, Kasahara S, Sawada M, Yamada T, Hara T, Fukuno K, Takahashi T, Oyama M, et al. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol. 2005;74:158–165. doi: 10.1111/j.1600-0609.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 76.Visco C, Arcaini L, Brusamolino E, Burcheri S, Ambrosetti A, Merli M, Bonoldi E, Chilosi M, Viglio A, Lazzarino M, et al. Distinctive natural history in hepatitis C virus positive diffuse large B-cell lymphoma: analysis of 156 patients from northern Italy. Ann Oncol. 2006;17:1434–1440. doi: 10.1093/annonc/mdl131. [DOI] [PubMed] [Google Scholar]
- 77.Tomita N, Kodama F, Takabayashi M, Kawano T, Yamaji S, Fujimaki K, Fujisawa S, Kanamori H, Motomura S, Ishigatsubo Y. Clinical features and outcome in HCV-positive aggressive non-Hodgkin’s lymphoma. Leuk Lymphoma. 2003;44:1159–1164. doi: 10.1080/1042819031000083055. [DOI] [PubMed] [Google Scholar]
- 78.Besson C, Canioni D, Lepage E, Pol S, Morel P, Lederlin P, Van Hoof A, Tilly H, Gaulard P, Coiffier B, et al. Characteristics and outcome of diffuse large B-cell lymphoma in hepatitis C virus-positive patients in LNH 93 and LNH 98 Groupe d’Etude des Lymphomes de l’Adulte programs. J Clin Oncol. 2006;24:953–960. doi: 10.1200/JCO.2005.01.5016. [DOI] [PubMed] [Google Scholar]
- 79.Sève P, Renaudier P, Sasco AJ, Dumontet C, Salles G, Coiffier B, Zoulim F, Broussolle C, Trépo C. Hepatitis C virus infection and B-cell non-Hodgkin’s lymphoma: a cross-sectional study in Lyon, France. Eur J Gastroenterol Hepatol. 2004;16:1361–1365. doi: 10.1097/00042737-200412000-00021. [DOI] [PubMed] [Google Scholar]
- 80.Hermine O, Lefrère F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, Delmas B, Valensi F, Cacoub P, Brechot C, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 81.Vallisa D, Bertè R, Rocca A, Civardi G, Giangregorio F, Ferrari B, Sbolli G, Cavanna L. Association between hepatitis C virus and non-Hodgkin’s lymphoma, and effects of viral infection on histologic subtype and clinical course. Am J Med. 1999;106:556–560. doi: 10.1016/s0002-9343(99)00069-8. [DOI] [PubMed] [Google Scholar]
- 82.Kelaidi C, Rollot F, Park S, Tulliez M, Christoforov B, Calmus Y, Podevin P, Bouscary D, Sogni P, Blanche P, et al. Response to antiviral treatment in hepatitis C virus-associated marginal zone lymphomas. Leukemia. 2004;18:1711–1716. doi: 10.1038/sj.leu.2403443. [DOI] [PubMed] [Google Scholar]
- 83.Saadoun D, Suarez F, Lefrere F, Valensi F, Mariette X, Aouba A, Besson C, Varet B, Troussard X, Cacoub P, et al. Splenic lymphoma with villous lymphocytes, associated with type II cryoglobulinemia and HCV infection: a new entity? Blood. 2005;105:74–76. doi: 10.1182/blood-2004-05-1711. [DOI] [PubMed] [Google Scholar]
- 84.Patriarca F, Silvestri F, Fanin R, Zaja F, Sperotto A, Baccarani M. Long-lasting complete remission of hepatitis C virus (HCV) infection and HCV-associated immunocytoma with alpha-interferon treatment. Br J Haematol. 2001;112:370–372. doi: 10.1046/j.1365-2141.2001.02571.x. [DOI] [PubMed] [Google Scholar]
- 85.Mazzaro C, De Re V, Spina M, Dal Maso L, Festini G, Comar C, Tirelli U, Pozzato G. Pegylated-interferon plus ribavirin for HCV-positive indolent non-Hodgkin lymphomas. Br J Haematol. 2009;145:255–257. doi: 10.1111/j.1365-2141.2008.07565.x. [DOI] [PubMed] [Google Scholar]
- 86.Vallisa D, Bernuzzi P, Arcaini L, Sacchi S, Callea V, Marasca R, Lazzaro A, Trabacchi E, Anselmi E, Arcari AL, et al. Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non-Hodgkin’s lymphoma: a multicenter Italian experience. J Clin Oncol. 2005;23:468–473. doi: 10.1200/JCO.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Levine AM, Shimodaira S, Lai MM. Treatment of HCV-related mantle-cell lymphoma with ribavirin and pegylated interferon Alfa. N Engl J Med. 2003;349:2078–2079. doi: 10.1056/NEJM200311203492121. [DOI] [PubMed] [Google Scholar]
- 88.Pellicelli AM, Marignani M, Zoli V, Romano M, Morrone A, Nosotti L, Barbaro G, Picardi A, Gentilucci UV, Remotti D, et al. Hepatitis C virus-related B cell subtypes in non Hodgkin’s lymphoma. World J Hepatol. 2011;3:278–284. doi: 10.4254/wjh.v3.i11.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tursi A, Brandimarte G, Torello M. Disappearance of gastric mucosa-associated lymphoid tissue in hepatitis C virus-positive patients after anti-hepatitis C virus therapy. J Clin Gastroenterol. 2004;38:360–363. doi: 10.1097/00004836-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 90.Rodriguez-Torres M, Lawitz E, Kowdley KV, Nelson DR, Dejesus E, McHutchison JG, Cornpropst MT, Mader M, Albanis E, Jiang D, et al. Sofosbuvir (GS-7977) plus peginterferon/ribavirin in treatment-naïve patients with HCV genotype 1: a randomized, 28-day, dose-ranging trial. J Hepatol. 2013;58:663–668. doi: 10.1016/j.jhep.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 91.Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–413. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 92.Suzuki F, Toyota J, Ikeda K, Chayama K, Mochida S, Hayashi N, Ishikawa H, Miyagoshi H, Hu W, McPhee F, et al. A randomized trial of daclatasvir with peginterferon alfa-2b and ribavirin for HCV genotype 1 infection. Antivir Ther. 2014;19:491–499. doi: 10.3851/IMP2730. [DOI] [PubMed] [Google Scholar]
- 93.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 94.Mazzaro C, Franzin F, Tulissi P, Pussini E, Crovatto M, Carniello GS, Efremov DG, Burrone O, Santini G, Pozzato G. Regression of monoclonal B-cell expansion in patients affected by mixed cryoglobulinemia responsive to alpha-interferon therapy. Cancer. 1996;77:2604–2613. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2604::AID-CNCR26>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 95.Moccia F, Tognoni E, Boccaccio P. The relationship between splenic marginal zone B-cell lymphoma and chronic liver disease associated with hepatitis C virus infection. Ann Ital Med Int. 1999;14:288–293. [PubMed] [Google Scholar]
- 96.Arcaini L, Paulli M, Boveri E, Vallisa D, Bernuzzi P, Orlandi E, Incardona P, Brusamolino E, Passamonti F, Burcheri S, et al. Splenic and nodal marginal zone lymphomas are indolent disorders at high hepatitis C virus seroprevalence with distinct presenting features but similar morphologic and phenotypic profiles. Cancer. 2004;100:107–115. doi: 10.1002/cncr.11893. [DOI] [PubMed] [Google Scholar]
- 97.Pitini V, Arrigo C, Righi M, Scaffidi M, Sturniolo G. Systematic screening for HCV infection should be performed in patients with splenic marginal zone lymphoma. Br J Haematol. 2004;124:252–253. doi: 10.1046/j.1365-2141.2003.04751.x. [DOI] [PubMed] [Google Scholar]
- 98.Paulli M, Arcaini L, Lucioni M, Boveri E, Capello D, Passamonti F, Merli M, Rattotti S, Rossi D, Riboni R, et al. Subcutaneous ‘lipoma-like’ B-cell lymphoma associated with HCV infection: a new presentation of primary extranodal marginal zone B-cell lymphoma of MALT. Ann Oncol. 2010;21:1189–1195. doi: 10.1093/annonc/mdp454. [DOI] [PubMed] [Google Scholar]


