Abstract
The hepatitis C virus (HCV) infection is an important public health problem and it is associated with hepatic and extrahepatic manifestations. Autoimmune thyroid diseases are common in HCV infected patients and the standard interferon-based treatment is associated with an increase of the immune-mediated thyroid damage. Recent evidence in the literature analyzed critical points of the mechanisms of thyroid damage, focusing on the balance between the two sides of the interaction: The environment (virus infection with potential cross-reaction) and the host (susceptibility genes with consistent immune response). The spectrum of antiviral treatment for chronic HCV infection is rapidly expanding for the development of dual o triple therapy. The availability of interferon-free combined treatment with direct antiviral agents for HCV is very promising, in order to ameliorate the patient compliance and to reduce the development of thyroid autoimmunity.
Keywords: Hepatitis C virus, Thyroid autoimmunity, Interferon, Antiviral agents, Self-tolerance
Core tip: This review examines the relationship between the hepatitis C virus (HCV) infection and the thyroid autoimmunity, on the basis of recent evidence of the literature about the mechanisms of self tolerance and thyroid damage related to HCV. The advances in the HCV infection treatment have been discussed in the paper, with relevant clinical results.
INTRODUCTION
Hepatitis C virus (HCV) infection is a liver disease that may be associated with extra hepatic manifestations (EHM) (autoimmune disorders or malignant tumors), defining the HCV syndrome as result of multifactorial process with significant genetic predisposition and/or environmental triggering cofactors[1].
More than 50% of HCV-positive patients have symptoms of at least one EHM during the course of the disease that can be the first and only clinical signs of a chronic hepatitis C[2].
The loss of tolerance is the main mechanism that promotes autoimmune diseases and, particularly, autoimmune thyroid disorders (AITD)[3,4], with autoantibodies (Abs) or T lymphocytes (humoral or cellular response) reacting with self-antigens (Ags) (Figure 1).
Figure 1.
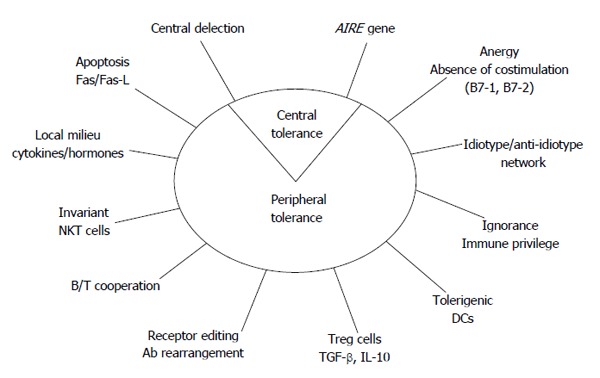
Potential mechanisms for self-tolerance control. AIRE: Autoimmune regulator gene; DCs: Dendritic cells; TGF: Transforming growth factor; IL: Interleukin; Ab: Antibody; NKT cells: Natural killer T cells.
The clinical spectrum of AITD includes hyper- [Graves’ disease (GD)] or hypo-function [Hashimoto’s thyroiditis (HT)] of the gland. The Abs against the thyroglobulin (Tg) and the thyrotropin-stimulating hormone (TSH)-receptor (TSH-r) in patients with GD were firstly identified 50 years ago[5,6]. The Abs bind and activate the TSH receptor in GD, whereas antibody-dependent cellular cytotoxicity to thyroglobulin and thyroid peroxidase (TPO) and T cells mediated injury in HT. An immune-mediated mechanism is present in painful subacute thyroiditis (without significant anti-thyroid autoantibodies) and in drug-induced thyroiditis (interferons).
T cells CD4+ are divided into regulatory T (Treg) cells and conventional T helper (Th) cells (with Th1 and Th2 lineages controlling cell-mediated and humoral immunity, respectively)[7-11]. In the central event of the immune response, the antigen-presenting cell (APC) presents the Ag bound to the human leukocyte antigen (HLA) class II to the CD4+ T cell, through the T cell receptor and additional costimulations (engagement of B7 with CD28 and CD40 with CD40 ligand). The Ag recognition for CD8+ T cells requires linear peptides that are processed and bound to HLA class I. The CD4+/CD8+ ratio, the HLA system and the costimulation have been involved in initiation, progression, and maintenance of AITD[12]. Since activated T cells stimulate B cells to proliferate and secrete antibodies (IgG), B cell tolerance mechanisms are considered as a secondary mechanism[13]. Tregs suppress immune responses against self or non-self Ags, producing immunosuppressive cytokines [interleukin-10 (IL-10), and transforming growth factor β (TGF-β)] and Tregs are dysfunctional in AITD patients[14,15]. Programmed death-1 negative co-stimulatory pathway mediate Treg activity, that is characterized by the expression of forkhead box protein 3 (FoxP3) and cytotoxic T-lymphocyte antigen 4 (CTLA-4).
At the peripheral site of chronic inflammation, the Th17 cells produce proinflammatory cytokines (IL-17, IL-21 and IL-22), as it has been demonstrated in AITD[16,17]. Local immunosuppressive regulatory cytokines (TGF-β and IL-10) may be involved in the maintenance of tolerance and prevention of AITD[18,19]. A decreased apoptosis of activated T cells, like in defects of interaction of Fas (CD95) and Fas ligand (Fas-L), has been studied in AITD[20]. The proportion of intrathyroidal natural killer T cell subset has been found lower in GD than in the peripheral blood of the same patients and of controls, contributing to the incomplete regulation of autoreactive T cells[13].
HOST-DEPENDENT FACTORS IN THYROID AUTOIMMUNITY
The aetiology of the AITD is unknown, but endogenous agents may predispose to the development to autoimmunity.
A genetic influence (the susceptibility genes) has been reported in the development of autoimmunity[21,22]. As matter of fact, the association with HLA class II molecules, the concordance studies in twins, the association with CTLA-4 and protein tyrosine phosphatase nonreceptor-type 22 and CD40 polymorphism (A/G49 and 1858C/T and CC genotype, respectively), the association of a microsatellite inside the FoxP3 gene, the linkage with chromosomal locations (14q31, 18q21, 20q11, Xp11, Xq21, 6p, 13q32 and 12q22) and the presence of anti-thyroid Abs in siblings of probands with AITD have been observed[23-32]. Moreover, the HLA class II (DRB1*0301) is also associated with chronic HCV infection[33]. Genome-wide association studies of autoimmune disease recently revealed multiple associations with the major immune cell subsets and uncovered insights into the control for regulatory Tregs[34].
AITD clearly increases with age, resulting from changes in immune regulation (endogenous factor). A sexual dimorphism in AITD has been described[3], with the highest ratio in females with HT (F:M = 4-10:1), suggesting an immunomodulatory role of sex steroids (respectively for androgens, estrogens and progesterone), mediated by specific receptor[35]. Males have an increased risk of advanced liver disease (cirrhosis and hepatocellular carcinoma) during HCV infection, in association with polymorphisms in sex steroid hormone synthesis and signaling[36,37].
A blunted hypothalamic-pituitary-adrenal axis may be associated to susceptibility to autoimmune/inflammatory disease[38], but no evidence of pituitary or adrenal involvement was present in a recent histopathologic study in HCV patients with thyroid disorders[39].
The main targets of the immune response in AITD are the Tg (two 330-kDa monomers, with the highest “immunogenicity score”), the TSH-r (60 kDa for the A subunit) and the TPO (homodimer of two 107-kDa subunits); no supporting data, at the moment, for the sodium/iodide symporter (NIS) and the pendrin[9]. Specific Tg peptides (representing major T-cell epitopes that can bind to the HLA-DRB-Arg74 pockets) and intron 1 polymorphism in the TSH-r gene (altering its splicing) has been associated with GD[40,41]. Cytotoxic CD8+ T cells recognized Tg or TPO peptide epitopes associated to HLA-A2 molecules in patients with HT[11].
Epigenetic modifications (including DNA methylation, histone modifications, and RNA interference by microRNA) can amplify a risk conferred by an inherited polymorphism resulting in a combined high risk for disease[42].
ENVIRONMENT AND VIRUS-DEPENDENT FACTORS IN THYROID AUTOIMMUNITY
Environmental risk factors include pollution, iodine intake (as in the cases of Jod-Basedow and Wolff-Chaikoff effect) and smoking. Stressful situations are well known inducers of AITD and, in particular, of hyperthyroidism[43]. Allostatic load during stress conditions is a well-known environmental factor favouring the development of AITD. A high number of drugs (lithium, amiodarone, interferons, anti-CD52 monoclonal antibody Campath-1H) may induce AITD[44-47]. In the past years, leukocyte-derived interferon (IFN) contaminated with γ-IFN demonstrated “in vivo” potent inducing properties of AITD in humans[48].
The HCV is one of the most important viruses associated with autoimmune diseases (both chronic liver inflammation and EHM). HCV may interferes with the functions and mechanisms of self-recognition both on the immune system and thyroid cells[49,50], where HCV may directly destroy thyroid tissue or mimic the structure of some components of thyroid gland, starting the autoimmune disease (Figure 2).
Figure 2.
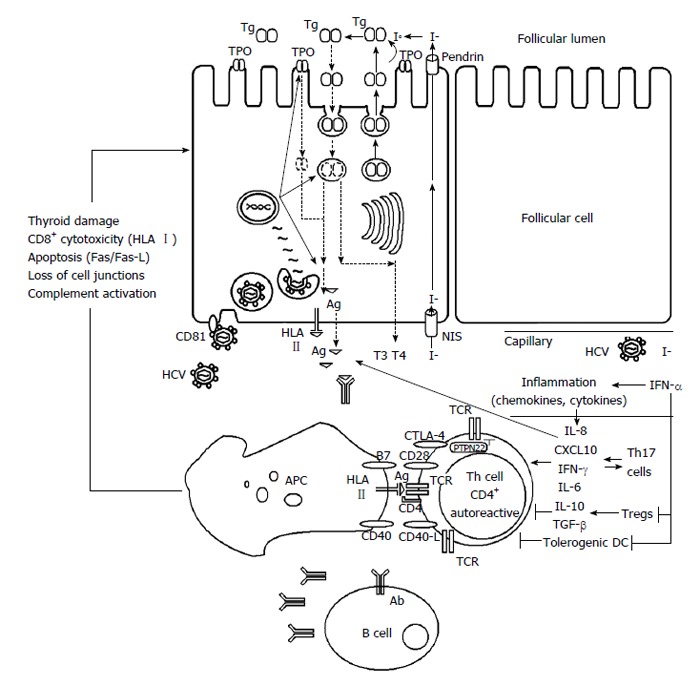
Development of thyroid autoimmunity in patients with chronic hepatitis C virus infection during interferon-α treatment. Ab: Antibody; Ag: Antigen; APC: Antigen presenting cell; CD: Cluster of differentiation; CTLA-4: Cytotoxic T-lymphocyte antigen 4; CXCL10: C-X-C motif chemokine; DC: Dendritic cell; HCV: Hepatitis C virus; HLA: Human leukocyte antigen; I-: Iodide; IFN: Interferon; IL: Interleukin; NIS: Sodium/iodide symporter; PTPN22: Protein tyrosine phosphatase nonreceptor-type 22; T3 and T4: Thyroid hormones; TCR: T cell receptor; Tg: Thyroglobulin; TGF: Transforming growth factor; Th: T helper; TPO: Thyroid peroxidase; Tregs: T regulatory cells.
The HCV prevalence is about 5%, strongly associated with health inequity[51,52]. HCV structure consists of three structural (core, E1 and E2) and seven non-structural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B) and six main HCV-RNA genotypes[53]. HCV has a significant lymphotropism: In fact, the lymphoid tissue is a site for the persistence of the infection and chronic immune stimulus[54,55]. The chronic stimulation results in: AutoAbs production (clonal B lymphocyte expansion and Th2 response), anti-apoptotic effects (translocation with Bcl-2 activation and prolonged survival of lymphocytes), drive for autoimmunity (binding of protein E2 to CD81, that mediate attachment on hepatocytes), increased cytokine and chemokine secretion (IFN-γ and Th1 response with IFN-γ inducible chemokines such as C-X-C motif chemokine 10 or CXCL10, in order to stop viral spread; IL-8) and upregulation of CXCL10 by NS5a[56-59]. However, no association has been found between chronic hepatitis C with increased CXCL10 and AITD[60].
DEVELOPMENT OF AITD DURING THE α-IFN TREATMENT FOR HCV CHRONIC HEPATITIS
The AITD during the α-IFN treatment for viral chronic hepatitis is an interesting clinical model for autoimmunity, since it includes both environmental and endogenous factors, together interacting. The mechanisms responsible for AITD in HCV patients have not been elucidated.
In the autoimmune model, initiating (susceptibility genes/environmental stimuli) and modulating factors (sex hormones/neuroendocrine influences) are involved in the whole complex of the autoimmune processes.
Age, female gender and pre-existing positive Abs are well-known risk factors for the development of AITD in the IFN-treated HCV patients[61-64].
HCV is associated with AITD (10%) and thyroid dysfunction (3%, with a hypothyroidism/hyperthyroidism ratio of about 2:1)[49,61-63,65-70]. AITD in patients with HCV are more frequent than in viral hepatitis B (5%) and in controls (2%-4%)[11,66].
The standard antiviral therapy with α-IFN for HCV-related chronic hepatitis may exacerbate or induce underlying latent thyroid disorders, increasing the incidence of AITD and dysfunction to 20%-40% and 11%-15%, respectively[49,61-63,65,67,68,70-72]. The “de novo” appearance of anti-thyroid Abs and overt dysfunctions in euthyroid subjects have been demonstrated after the α-IFN therapy, suggesting that this cytokine is a direct inducer of AITD[49,61,62,67,68,70,71].
Recombinant α-IFN administration induces an increase of endogenous γ-IFN and IL-6, supporting a sequence in the cytokine cascade that modulate the immune system and the neuroendocrine axis secretion[73]. At the thyroid level, IFNs (α, β and γ) are inhibitors of iodide uptake and hormone release on thyrocytes[74]. At the pituitary level, γ-IFN and IL-6 do not change TSH release[75], whereas at the hypothalamic level, γ-IFN stimulates somatostatin release[76] that suppresses TSH secretion. We examined the effect of α-IFN (3 million IU i.m. 3 times a week) on hypothalamic-pituitary-thyroid (HPT) axis in patients with viral chronic hepatitis and negative anti-thyroid Abs from a neuroendocrine point of view and we did not find a statistically relevant modification of thyroid hormones and TSH levels[77].
A case of De Quervain’s thyroiditis during α-IFN therapy for HCV-related chronic hepatitis, with persisting negative anti-thyroid Abs after α-IFN therapy, has been reported[78]. The common viral infections (Coxsackie virus, mumps, Epstein-Barr virus, adenovirus, cytomegalovirus) were negative, but we found an association with HLA-Bw35[79,80]. The patient presented the HCV, the typical HLA class I predisposition for the thyroid disease and an exogenous accelerating factor (α-IFN therapy). During viral infections, APCs present antigens to Th cells, in the presence of cytokines (i.e., α-IFN, IL-12), inducing them to differentiate towards the Th1 phenotype that causes cell damage[81].
In a second case report, a patient with HCV infection and negative anti-thyroid Abs before treatment but with the typical association for HT (HLA-DR5 antigen or HLA-DRB1.11/HLA-DRB1.12 alleles) in Caucasian developed HT during α-IFN treatment[82].
In a preliminary longitudinal (range 12-54 mo) study in patients with chronic hepatitis C and absence of thyroid disorders at the baseline (n = 15), the relationship between the HLA antigen susceptibility and the thyroid disorders during the α-IFN treatment was evaluated, with respect to control subjects (n = 107)[83]. The HCV genotype was 1b (20%), 2a (60%) and 3a (20%), with the distribution (1b:2a:3a) of 1:3:1 and absence of mixed genotype. It is well known that the HLA-B35, -DR3 (DRB1.03 allele) and -DR5 (DRB1.11/HLA-DRB1.12 alleles) are commonly associated with De Quervain’s thyroiditis, thyrotoxicosis/hyperthyroidism and hypothyroidism, respectively[80,84-92]. Arginine at position 74 of HLA-DRB1 chain (DRB-Arg74) may permit autoAg peptides to fit into the binding pocket, to be presented more efficiently to T cells[93]. On the other side, the HLA-A2 has been aspecifically associated with thyroid disorders (either hyper- or hypothyroidism) in patients with chronic hepatitis C during α-IFN therapy[30]. The HLA-A2 antigen (class I molecule) is involved in the restricted presentation of HCV peptides by the APC to the CTL (response strongly increased by α-IFN, with final outcome of target cell disruption both at the liver and thyroid gland level)[94-97].
Forty percent of HCV patients presented a double positive HLA result (HLA-A2/B-35, HLA-A2/DRB1.03, HLA-A2/DRB1.11 or HLA-B35/DRB1.11) before the treatment and five patients with double positive HLA received the α-IFN therapy. Four double positive HLA treated females developed clinical thyroid disorders, with the HLA system specifically associated with the particular kind of the thyroid disorder (P < 0.05). The HLA-A2 was not specific for thyroid disorder, being present in hypothyroidism, in thyrotoxicosis as well as in thyroiditis. The relationship between the thyroid disorders and the HCV genotype did not reveal significant association. In our group with 40% double positive HLA pre-treatment, the overall development of thyroid disorders after α-IFN was 36% (33% in patients with pre-treatment negative anti-thyroid Abs).
Previous studies have showed the association of AITD with female gender, older age and pre-existing positive anti-thyroid Abs, in α-IFN treated patients with HCV-related chronic hepatitis[26,49,61,66,69,70]. Our results suggest that the HLA system is a strong susceptibility factor to the development of AITD, in particular, in the patients with two Ags together (the double association of HLA class I and/or II). Therefore, the examination of HLA (HLA-A2, -B35, -DRB1.03, -DRB1.11) in HCV patients before α-IFN treatment may be an useful predictive tool to detect the predisposition to develop the specific AITD.
HCV virion attachment and entry in thyrocytes are mediated by CD81 (host) and E2 (virus), activating the local inflammatory response (as well as it occurs for hepatocytes). Moreover, HCV also replicates within the infected human thyroid cells in vitro[98]. The HCV infection of thyroid cells can trigger the autoimmune thyroiditis by induction of changes in self Ag expression, exposing of cryptic epitopes or molecular mimicry and leading to production of the proinflammatory IL-8 (a contributor to bystander activation)[11].
Even if important host effector molecules (such as the interferon-induced transmembrane proteins IFITM family of proteins) may act against HCV in the liver, restricting infection by targeting the endocytosed virion for lysosomal degradation[99], at the moment, no data in the literature describe the role of IFITM in AITD.
The molecular mimicry is the mainly investigated mechanism of induction of autoimmunity and we analyzed the frequency of the sequence homology between the thyroid and the HCV. We found 62.5%-100% homology, when the conservative substitutions were included in the analysis (ten out of ten identical/conservative amino acids in the sequence), between the HCV polyprotein and five thyroid Ags (Tg, TPO, TSHr, NIS and pendrin). The homology was not restricted to a single HCV genotype, with the highest degree between the NIS and the HCV1a-NS4a protein. The Tg had the highest number of homologies with the different HCV genotypes. The length of ten amino acids is consistent with the presentation of the self/viral Ags with the HLA class I to CD8+ lymphocytes (the HLA class II usually bind longer peptides)[100].
The aberrant expression of HLA class II on thyroid cells (with costimulation) and the local inflammation (with cytokine release) result in activation of autoreactive T cells by bystander mechanisms. Systemic inflammation (cytokines and chemokines, like IL-8) plays an important role in the immunopathogenesis of thyroiditis and antagonize the antiviral effects of IFN, facilitating HCV persistence in thyrocytes. The absence of HCV clearance from thyrocytes perpetuates the chronic inflammation and autoimmunity. α-IFN triggers AITD through an epigenetic mechanism involving variant of Tg and TSHr gene promoter[101,102]. Moreover, α-IFN locally enhances the expression of TSH-r, Tg, TPO and HLA class I molecules on thyrocytes and the secretion of the potent proinflammatory IL-2 cytokine[11].
α-IFN treatment for HCV-related chronic hepatitis acts an enhancer of AITD in susceptible patients. The standard dual therapy with pegylated α-IFN (pegIFN)/ribavirin has been recently increased to a triple therapy, based on new direct-acting antiviral drugs [NS3/4A serine protease inhibitor (PI), such as telaprevir or boceprevir].
The monitoring of the patients during the treatment avoids the side effects (typically flu-like symptoms with pegIFN or anemia with ribavirin, or irritability, allergic reactions, severe fatigue, bacterial infections)[103,104]. Thyroid function tests should be examined every 3 mo during the α-IFN based treatment[105,106]. Recently, α-IFN-free combined treatment with direct antiviral agents for HCV has been developed with or without ribavirin, ameliorating the patient compliance and reducing the risk for thyroid autoimmunity development. These agents are second generation PI (simeprevir, grazoprevir), NS5A inhibitor (daclatasvir, ledipasvir, ombitasvir, elbasvir) and NS5B polymerase inhibitor (sofosbuvir, paritaprevir, dasabuvir, beclabuvir, asunaprevir) that are strongly efficacious to eradicate the HCV infection (undetectable HCV-RNA after 24 wk from the beginning of therapy)[53,107-109].
CONCLUSION
In conclusion, the development of AITD in patients with chronic HCV-infection is a complex model for autoimmunity in which every component (the host and the environment) has a significant role.
The new approach with α-IFN-free combined treatment for chronic HCV-infection with direct antiviral agents is very promising in order to ameliorate the patient compliance and to reduce the risk of development of AITD.
Footnotes
Conflict-of-interest statement: No financial conflicts of interest or other relationships are present in the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 6, 2015
First decision: October 14, 2015
Article in press: December 4, 2015
P- Reviewer: Sargsyants N S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
References
- 1.Zignego AL, Gragnani L, Piluso A, Sebastiani M, Giuggioli D, Fallahi P, Antonelli A, Ferri C. Virus-driven autoimmunity and lymphoproliferation: the example of HCV infection. Expert Rev Clin Immunol. 2015;11:15–31. doi: 10.1586/1744666X.2015.997214. [DOI] [PubMed] [Google Scholar]
- 2.Jadali Z, Alavian SM. Autoimmune diseases co-existing with hepatitis C virus infection. Iran J Allergy Asthma Immunol. 2010;9:191–206. [PubMed] [Google Scholar]
- 3.Martocchia A, Stefanelli M, Cola S, Falaschi P. Sex steroids in autoimmune diseases. Curr Top Med Chem. 2011;11:1668–1683. doi: 10.2174/156802611796117595. [DOI] [PubMed] [Google Scholar]
- 4.Poletaev AB, Stepanyuk VL, Gershwin ME. Integrating immunity: the immunculus and self-reactivity. J Autoimmun. 2008;30:68–73. doi: 10.1016/j.jaut.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Adams DD, Purves HD. Abnormal responses in the assay of thyrotrophin. Proc Univer Otago Med School. 1956;34:11–12. [Google Scholar]
- 6.Roitt IM, Doniach D, Campbell PN, Hudson RV. Auto-antibodies in Hashimoto’s disease (lymphadenoid goitre) Lancet. 1956;271:820–821. doi: 10.1016/s0140-6736(56)92249-8. [DOI] [PubMed] [Google Scholar]
- 7.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 8.Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe M, Nakamura Y, Matsuzuka F, Takamura Y, Miyauchi A, Iwatani Y. Decrease of intrathyroidal CD161+Valpha24+Vbeta11+ NKT cells in Graves’ disease. Endocr J. 2008;55:199–203. doi: 10.1507/endocrj.k07e-006. [DOI] [PubMed] [Google Scholar]
- 12.Nada AM, Hammouda M. Immunoregulatory T cells, LFA-3 and HLA-DR in autoimmune thyroid diseases. Indian J Endocrinol Metab. 2014;18:574–581. doi: 10.4103/2230-8210.137524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLachlan SM, Rapoport B. Breaking tolerance to thyroid antigens: changing concepts in thyroid autoimmunity. Endocr Rev. 2014;35:59–105. doi: 10.1210/er.2013-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glick AB, Wodzinski A, Fu P, Levine AD, Wald DN. Impairment of regulatory T-cell function in autoimmune thyroid disease. Thyroid. 2013;23:871–878. doi: 10.1089/thy.2012.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez-Muñoz A, Vitales-Noyola M, Ramos-Levi A, Serrano-Somavilla A, González-Amaro R, Marazuela M. Levels of regulatory T cells CD69(+)NKG2D (+)IL-10 (+) are increased in patients with autoimmune thyroid disorders. Endocrine. 2015:Epub ahead of print. doi: 10.1007/s12020-015-0662-2. [DOI] [PubMed] [Google Scholar]
- 16.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Cai W, Gu R, Zhang Y, Zhang H, Tang K, Xu P, Katirai F, Shi W, Wang L, et al. Th17 cell plays a role in the pathogenesis of Hashimoto’s thyroiditis in patients. Clin Immunol. 2013;149:411–420. doi: 10.1016/j.clim.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Vural P, Degirmencioglu S, Erden S, Gelincik A. The relationship between transforming growth factor-beta1, vascular endothelial growth factor, nitric oxide and Hashimoto’s thyroiditis. Int Immunopharmacol. 2009;9:212–215. doi: 10.1016/j.intimp.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 19.de la Vega JR, Vilaplana JC, Biro A, Hammond L, Bottazzo GF, Mirakian R. IL-10 expression in thyroid glands: protective or harmful role against thyroid autoimmunity? Clin Exp Immunol. 1998;113:126–135. doi: 10.1046/j.1365-2249.1998.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimaoka Y, Hidaka Y, Okumura M, Takeoka K, Tada H, Amino N. Serum concentration of soluble Fas in patients with autoimmune thyroid diseases. Thyroid. 1998;8:43–47. doi: 10.1089/thy.1998.8.43. [DOI] [PubMed] [Google Scholar]
- 21.Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: from gene mapping to gene function. Endocr Rev. 2003;24:694–717. doi: 10.1210/er.2002-0030. [DOI] [PubMed] [Google Scholar]
- 22.Weetman AP. Autoimmune thyroid disease: propagation and progression. Eur J Endocrinol. 2003;148:1–9. doi: 10.1530/eje.0.1480001. [DOI] [PubMed] [Google Scholar]
- 23.Ban Y, Tozaki T, Tobe T, Ban Y, Jacobson EM, Concepcion ES, Tomer Y. The regulatory T cell gene FOXP3 and genetic susceptibility to thyroid autoimmunity: an association analysis in Caucasian and Japanese cohorts. J Autoimmun. 2007;28:201–207. doi: 10.1016/j.jaut.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Bech K, Lumholtz B, Nerup J, Thomsen M, Platz P, Ryder LP, Svejgaard A, Siersbaek-Nielsen K, Hansen JM, Larsen JH. HLA antigens in Graves’ disease. Acta Endocrinol (Copenh) 1977;86:510–516. doi: 10.1530/acta.0.0860510. [DOI] [PubMed] [Google Scholar]
- 25.Brix TH, Kyvik KO, Hegedus L. What is evidence of genetic factor in the aetiology of Graves’ disease? A brief review. Thyroid. 1998;8:627–634. doi: 10.1089/thy.1998.8.627. [DOI] [PubMed] [Google Scholar]
- 26.Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Immunologic features and HLA associations in chronic viral hepatitis. Gastroenterology. 1995;108:157–164. doi: 10.1016/0016-5085(95)90020-9. [DOI] [PubMed] [Google Scholar]
- 27.Hall R, Owen SG, Smart GA. Evidence for genetic predisposition to formation of thyroid autoantibodies. Lancet. 1960;2:187–188. doi: 10.1016/s0140-6736(60)91330-1. [DOI] [PubMed] [Google Scholar]
- 28.Hunt PJ, Marshall SE, Weetman AP, Bunce M, Bell JI, Wass JA, Welsh KI. Histocompatibility leucocyte antigens and closely linked immunomodulatory genes in autoimmune thyroid disease. Clin Endocrinol (Oxf) 2001;55:491–499. doi: 10.1046/j.1365-2265.2001.01356.x. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson EM, Huber AK, Akeno N, Sivak M, Li CW, Concepcion E, Ho K, Tomer Y. A CD40 Kozak sequence polymorphism and susceptibility to antibody-mediated autoimmune conditions: the role of CD40 tissue-specific expression. Genes Immun. 2007;8:205–214. doi: 10.1038/sj.gene.6364375. [DOI] [PubMed] [Google Scholar]
- 30.Kakizaki S, Takagi H, Murakami M, Takayama H, Mori M. HLA antigens in patients with interferon-alpha-induced autoimmune thyroid disorders in chronic hepatitis C. J Hepatol. 1999;30:794–800. doi: 10.1016/s0168-8278(99)80131-7. [DOI] [PubMed] [Google Scholar]
- 31.Tomer Y, Barbesino G, Greenberg DA, Concepcion E, Davies TF. A new Graves disease-susceptibility locus maps to chromosome 20q11.2. International Consortium for the Genetics of Autoimmune Thyroid Disease. Am J Hum Genet. 1998;63:1749–1756. doi: 10.1086/302146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanagawa T, Hidaka Y, Guimaraes V, Soliman M, DeGroot LJ. CTLA-4 gene polymorphism associated with Graves’ disease in a Caucasian population. J Clin Endocrinol Metab. 1995;80:41–45. doi: 10.1210/jcem.80.1.7829637. [DOI] [PubMed] [Google Scholar]
- 33.Höhler T, Gerken G, Notghi A, Knolle P, Lubjuhn R, Taheri H, Schneider PM, Meyer zum Büschenfelde KH, Rittner C. MHC class II genes influence the susceptibility to chronic active hepatitis C. J Hepatol. 1997;27:259–264. doi: 10.1016/s0168-8278(97)80169-9. [DOI] [PubMed] [Google Scholar]
- 34.Roederer M, Quaye L, Mangino M, Beddall MH, Mahnke Y, Chattopadhyay P, Tosi I, Napolitano L, Terranova Barberio M, Menni C, et al. The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell. 2015;161:387–403. doi: 10.1016/j.cell.2015.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 36.White DL, Tavakoli-Tabasi S, Kuzniarek J, Pascua R, Ramsey DJ, El-Serag HB. Higher serum testosterone is associated with increased risk of advanced hepatitis C-related liver disease in males. Hepatology. 2012;55:759–768. doi: 10.1002/hep.24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White DL, Liu Y, Garcia J, El-Serag HB, Jiao L, Tsavachidis S, Franco LM, Lee JS, Tavakoli-Tabasi S, Moore D, et al. Sex hormone pathway gene polymorphisms are associated with risk of advanced hepatitis C-related liver disease in males. Int J Mol Epidemiol Genet. 2014;5:164–176. [PMC free article] [PubMed] [Google Scholar]
- 38.Sternberg EM. Neuroendocrine regulation of autoimmune/inflammatory disease. J Endocrinol. 2001;169:429–435. doi: 10.1677/joe.0.1690429. [DOI] [PubMed] [Google Scholar]
- 39.Tran HA, Reeves GE, Lyons TJ, Attia JR. Histopathologic findings of autoimmunity in thyroid, pituitary, and adrenal diseases in chronic hepatitis C postmortem cases. Endocr Pract. 2010;16:566–569. doi: 10.4158/EP09359.OR. [DOI] [PubMed] [Google Scholar]
- 40.Menconi F, Huber A, Osman R, Concepcion E, Jacobson EM, Stefan M, David CS, Tomer Y. Tg.2098 is a major human thyroglobulin T-cell epitope. J Autoimmun. 2010;35:45–51. doi: 10.1016/j.jaut.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin X, Latif R, Bahn R, Tomer Y, Davies TF. Influence of the TSH receptor gene on susceptibility to Graves’ disease and Graves’ ophthalmopathy. Thyroid. 2008;18:1201–1206. doi: 10.1089/thy.2008.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol. 2014;9:147–156. doi: 10.1146/annurev-pathol-012513-104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winsa B, Adami HO, Bergström R, Gamstedt A, Dahlberg PA, Adamson U, Jansson R, Karlsson A. Stressful life events and Graves’ disease. Lancet. 1991;338:1475–1479. doi: 10.1016/0140-6736(91)92298-g. [DOI] [PubMed] [Google Scholar]
- 44.Chiovato L, Pinchera A. Stressful life events and Graves’ disease. Eur J Endocrinol. 1996;134:680–682. doi: 10.1530/eje.0.1340680. [DOI] [PubMed] [Google Scholar]
- 45.Coles AJ, Wing M, Smith S, Coraddu F, Greer S, Taylor C, Weetman A, Hale G, Chatterjee VK, Waldmann H, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1999;354:1691–1695. doi: 10.1016/S0140-6736(99)02429-0. [DOI] [PubMed] [Google Scholar]
- 46.Ruwhof C, Drexhage HA. Iodine and thyroid autoimmune disease in animal models. Thyroid. 2001;11:427–436. doi: 10.1089/105072501300176381. [DOI] [PubMed] [Google Scholar]
- 47.Weetman AP, McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocr Rev. 1994;15:788–830. doi: 10.1210/edrv-15-6-788. [DOI] [PubMed] [Google Scholar]
- 48.Burman P, Tötterman TH, Oberg K, Karlsson FA. Thyroid autoimmunity in patients on long term therapy with leukocyte-derived interferon. J Clin Endocrinol Metab. 1986;63:1086–1090. doi: 10.1210/jcem-63-5-1086. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh MC, Yu ML, Chuang WL, Shin SJ, Dai CY, Chen SC, Lin ZY, Hsieh MY, Liu JF, Wang LY, et al. Virologic factors related to interferon-alpha-induced thyroid dysfunction in patients with chronic hepatitis C. Eur J Endocrinol. 2000;142:431–437. doi: 10.1530/eje.0.1420431. [DOI] [PubMed] [Google Scholar]
- 50.Lunel F. Hepatitis C virus and autoimmunity: fortuitous association or reality? Gastroenterology. 1994;107:1550–1555. doi: 10.1016/0016-5085(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 51.Shaheen MA, Idrees M. Evidence-based consensus on the diagnosis, prevention and management of hepatitis C virus disease. World J Hepatol. 2015;7:616–627. doi: 10.4254/wjh.v7.i3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith B, Falck-Ytter Y. Guidelines for the screening, care and treatment of persons with hepatitis C infection. WHO Library Cataloguing-in-Publication Data: 2014. [Google Scholar]
- 53.Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385:1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferri C, Antonelli A, Mascia MT, Sebastiani M, Fallahi P, Ferrari D, Pileri SA, Zignego AL. HCV-related autoimmune and neoplastic disorders: the HCV syndrome. Dig Liver Dis. 2007;39 Suppl 1:S13–S21. doi: 10.1016/s1590-8658(07)80005-3. [DOI] [PubMed] [Google Scholar]
- 55.Calvaruso V, Craxì A. Immunological alterations in hepatitis C virus infection. World J Gastroenterol. 2013;19:8916–8923. doi: 10.3748/wjg.v19.i47.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosa D, Saletti G, De Gregorio E, Zorat F, Comar C, D’Oro U, Nuti S, Houghton M, Barnaba V, Pozzato G, et al. Activation of naïve B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci USA. 2005;102:18544–18549. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petracca R, Falugi F, Galli G, Norais N, Rosa D, Campagnoli S, Burgio V, Di Stasio E, Giardina B, Houghton M, et al. Structure-function analysis of hepatitis C virus envelope-CD81 binding. J Virol. 2000;74:4824–4830. doi: 10.1128/jvi.74.10.4824-4830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Apolinario A, Majano PL, Lorente R, Núñez O, Clemente G, García-Monzón C. Gene expression profile of T-cell-specific chemokines in human hepatocyte-derived cells: evidence for a synergistic inducer effect of cytokines and hepatitis C virus proteins. J Viral Hepat. 2005;12:27–37. doi: 10.1111/j.1365-2893.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 59.Akeno N, Blackard JT, Tomer Y. HCV E2 protein binds directly to thyroid cells and induces IL-8 production: a new mechanism for HCV induced thyroid autoimmunity. J Autoimmun. 2008;31:339–344. doi: 10.1016/j.jaut.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danilovic DL, Mendes-Correa MC, Chammas MC, Zambrini H, Barros RK, Marui S. Thyroid disturbance related to chronic hepatitis C infection: role of CXCL10. Endocr J. 2013;60:583–590. doi: 10.1507/endocrj.ej12-0321. [DOI] [PubMed] [Google Scholar]
- 61.Broussolle C, Steineur MP, Bailly F, Zoulim F, Trépo C. [Hepatitis C virus infection and thyroid diseases] Rev Med Interne. 1999;20:766–773. doi: 10.1016/s0248-8663(00)88683-x. [DOI] [PubMed] [Google Scholar]
- 62.Carella C, Mazziotti G, Morisco F, Manganella G, Rotondi M, Tuccillo C, Sorvillo F, Caporaso N, Amato G. Long-term outcome of interferon-alpha-induced thyroid autoimmunity and prognostic influence of thyroid autoantibody pattern at the end of treatment. J Clin Endocrinol Metab. 2001;86:1925–1929. doi: 10.1210/jcem.86.5.7459. [DOI] [PubMed] [Google Scholar]
- 63.Fernandez-Soto L, Gonzalez A, Escobar-Jimenez F, Vazquez R, Ocete E, Olea N, Salmeron J. Increased risk of autoimmune thyroid disease in hepatitis C vs hepatitis B before, during, and after discontinuing interferon therapy. Arch Intern Med. 1998;158:1445–1448. doi: 10.1001/archinte.158.13.1445. [DOI] [PubMed] [Google Scholar]
- 64.Huang MJ, Tsai SL, Huang BY, Sheen IS, Yeh CT, Liaw YF. Prevalence and significance of thyroid autoantibodies in patients with chronic hepatitis C virus infection: a prospective controlled study. Clin Endocrinol (Oxf) 1999;50:503–509. doi: 10.1046/j.1365-2265.1999.00686.x. [DOI] [PubMed] [Google Scholar]
- 65.Deutsch M, Dourakis S, Manesis EK, Gioustozi A, Hess G, Horsch A, Hadziyannis S. Thyroid abnormalities in chronic viral hepatitis and their relationship to interferon alfa therapy. Hepatology. 1997;26:206–210. doi: 10.1002/hep.510260127. [DOI] [PubMed] [Google Scholar]
- 66.Ganne-Carrie N, Medini A, Coderc E, Seror O, Christidis C, Grimbert S, Trinchet JC, Beaugrand M. Latent autoimmune thyroiditis in untreated patients with HCV chronic hepatitis: a case-control study. J Autoimmun. 2000;14:189–193. doi: 10.1006/jaut.1999.0360. [DOI] [PubMed] [Google Scholar]
- 67.Marazuela M, García-Buey L, González-Fernández B, García-Monzón C, Arranz A, Borque MJ, Moreno-Otero R. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-alpha therapy. Clin Endocrinol (Oxf) 1996;44:635–642. doi: 10.1046/j.1365-2265.1996.751768.x. [DOI] [PubMed] [Google Scholar]
- 68.Marcellin P, Pouteau M, Benhamou JP. Hepatitis C virus infection, alpha interferon therapy and thyroid dysfunction. J Hepatol. 1995;22:364–369. doi: 10.1016/0168-8278(95)80291-6. [DOI] [PubMed] [Google Scholar]
- 69.Oppenheim Y, Ban Y, Tomer Y. Interferon induced Autoimmune Thyroid Disease (AITD): a model for human autoimmunity. Autoimmun Rev. 2004;3:388–393. doi: 10.1016/j.autrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 70.Prummel MF, Laurberg P. Interferon-alpha and autoimmune thyroid disease. Thyroid. 2003;13:547–551. doi: 10.1089/105072503322238809. [DOI] [PubMed] [Google Scholar]
- 71.Rocco A, Gargano S, Provenzano A, Nardone M, De Sanctis GM, Altavilla N, Chircu LV, Grimaldi F. Incidence of autoimmune thyroiditis in interferon-alpha treated and untreated patients with chronic hepatitis C virus infection. Neuro Endocrinol Lett. 2001;22:39–44. [PubMed] [Google Scholar]
- 72.Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am. 2007;36:1051–1066; x-xi. doi: 10.1016/j.ecl.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gisslinger H, Gilly B, Woloszczuk W, Mayr WR, Havelec L, Linkesch W, Weissel M. Thyroid autoimmunity and hypothyroidism during long-term treatment with recombinant interferon-alpha. Clin Exp Immunol. 1992;90:363–367. doi: 10.1111/j.1365-2249.1992.tb05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamazaki K, Kanaji Y, Shizume K, Yamakawa Y, Demura H, Kanaji Y, Obara T, Sato K. Reversible inhibition by interferons alpha and beta of 125I incorporation and thyroid hormone release by human thyroid follicles in vitro. J Clin Endocrinol Metab. 1993;77:1439–1441. doi: 10.1210/jcem.77.5.8077347. [DOI] [PubMed] [Google Scholar]
- 75.McCann SM, Lyson K, Karanth S, Gimeno M, Belova N, Kamat A, Rettori V. Mechanism of action of cytokines to induce the pattern of pituitary hormone secretion in infection. Ann N Y Acad Sci. 1995;771:386–395. doi: 10.1111/j.1749-6632.1995.tb44697.x. [DOI] [PubMed] [Google Scholar]
- 76.Ryu SY, Jeong KS, Yoon WK, Park SJ, Kang BN, Kim SH, Park BK, Cho SW. Somatostatin and substance P induced in vivo by lipopolysaccharide and in peritoneal macrophages stimulated with lipopolysaccharide or interferon-gamma have differential effects on murine cytokine production. Neuroimmunomodulation. 2000;8:25–30. doi: 10.1159/000026449. [DOI] [PubMed] [Google Scholar]
- 77.Falaschi P, D’Urso R, Proietti A, Martocchia A, Pastore R, Angelucci L. Effect of r-interferon alpha administration on hypothalamus-pituitary-thyroid axis in chronic hepatitis. Life Sci. 1997;60:43–50. doi: 10.1016/s0024-3205(96)00587-5. [DOI] [PubMed] [Google Scholar]
- 78.Falaschi P, Martocchia A, D’Urso R, Proietti A. Subacute thyroiditis during interferon-alpha therapy for chronic hepatitis C. J Endocrinol Invest. 1997;20:24–28. doi: 10.1007/BF03347968. [DOI] [PubMed] [Google Scholar]
- 79.Hall R. Subacute (De Quervain’s) thyroiditis. In: Hall R, Besser GM, editors. Fundamentals of clinical endocrinology. Churchill Livingstone: Edimburgh; 1989. p. 101. [Google Scholar]
- 80.Nyulassy S, Hnilica P, Buc M, Guman M, Hirschová V, Stefanovic J. Subacute (de Quervain’s) thyroiditis: association with HLA-Bw35 antigen and abnormalities of the complement system, immunoglobulins and other serum proteins. J Clin Endocrinol Metab. 1977;45:270–274. doi: 10.1210/jcem-45-2-270. [DOI] [PubMed] [Google Scholar]
- 81.Romagnani S. Induction of TH1 and TH2 responses: a key role for the ‘natural’ immune response? Immunol Today. 1992;13:379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 82.Martocchia A, Labbadia G, Paoletti V, Gargano S, Grossi A, Trabace S, Musca A, Falaschi P. Hashimoto’s disease during interferon-alpha therapy in a patient with pre-treatment negative anti-thyroid autoantibodies and with the specific genetic susceptibility to the thyroid disease. Neuro Endocrinol Lett. 2001;22:49–52. [PubMed] [Google Scholar]
- 83.Grimaldi F, Martocchia A, Lulli P, Frugoni P, Fiore RF, Rossi C, Ferrari F, Labbadia G, Falaschi P. Frequenza degli alleli HLA nei pazienti affetti da epatite cronica HCV-correlata e predisposizione alla comparsa della patologia tiroidea immuno-mediata dopo trattamento antivirale. Int Emerg Med. 2009;4:S84. [Google Scholar]
- 84.Ohsako N, Tamai H, Sudo T, Mukuta T, Tanaka H, Kuma K, Kimura A, Sasazuki T. Clinical characteristics of subacute thyroiditis classified according to human leukocyte antigen typing. J Clin Endocrinol Metab. 1995;80:3653–3656. doi: 10.1210/jcem.80.12.8530615. [DOI] [PubMed] [Google Scholar]
- 85.Chen QY, Huang W, She JX, Baxter F, Volpe R, Maclaren NK. HLA-DRB1*08, DRB1*03/DRB3*0101, and DRB3*0202 are susceptibility genes for Graves’ disease in North American Caucasians, whereas DRB1*07 is protective. J Clin Endocrinol Metab. 1999;84:3182–3186. doi: 10.1210/jcem.84.9.5991. [DOI] [PubMed] [Google Scholar]
- 86.Dalton TA, Bennett JC. Autoimmune disease and the major histocompatibility complex: therapeutic implications. Am J Med. 1992;92:183–188. doi: 10.1016/0002-9343(92)90110-w. [DOI] [PubMed] [Google Scholar]
- 87.Heward JM, Allahabadia A, Daykin J, Carr-Smith J, Daly A, Armitage M, Dodson PM, Sheppard MC, Barnett AH, Franklyn JA, et al. Linkage disequilibrium between the human leukocyte antigen class II region of the major histocompatibility complex and Graves’ disease: replication using a population case control and family-based study. J Clin Endocrinol Metab. 1998;83:3394–3397. doi: 10.1210/jcem.83.10.5137. [DOI] [PubMed] [Google Scholar]
- 88.Kinney JS, Hurwitz ES, Fishbein DB, Woolf PD, Pinsky PF, Lawrence DN, Anderson LJ, Holmes GP, Wilson CK, Loschen DJ. Community outbreak of thyrotoxicosis: epidemiology, immunogenetic characteristics, and long-term outcome. Am J Med. 1988;84:10–18. doi: 10.1016/0002-9343(88)90002-2. [DOI] [PubMed] [Google Scholar]
- 89.Zamani M, Spaepen M, Bex M, Bouillon R, Cassiman JJ. Primary role of the HLA class II DRB1*0301 allele in Graves disease. Am J Med Genet. 2000;95:432–437. doi: 10.1002/1096-8628(20001218)95:5<432::aid-ajmg5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 90.Farid NR, Sampson L, Moens H, Barnard JM. The association of goitrous autoimmune thyroiditis with HLA-DR5. Tissue Antigens. 1981;17:265–268. doi: 10.1111/j.1399-0039.1981.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 91.Bogner U, Badenhoop K, Peters H, Schmieg D, Mayr WR, Usadel KH, Schleusener H. HLA-DR/DQ gene variation in nongoitrous autoimmune thyroiditis at the serological and molecular level. Autoimmunity. 1992;14:155–158. doi: 10.3109/08916939209083135. [DOI] [PubMed] [Google Scholar]
- 92.Pocecco M, Barbi E, De Campo C. [Autoimmune thyroid pathology. Study and follow-up of pediatric case reports] Pediatr Med Chir. 1986;8:691–694. [PubMed] [Google Scholar]
- 93.Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, Davies TF, Ban Y, Jacobson EM, Concepcion ES, Li CW, et al. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci USA. 2008;105:14034–14039. doi: 10.1073/pnas.0806584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sarobe P, Huarte E, Lasarte JJ, López-Díaz de Cerio A, García N, Borrás-Cuesta F, Prieto J. Characterization of an immunologically conserved epitope from hepatitis C virus E2 glycoprotein recognized by HLA-A2 restricted cytotoxic T lymphocytes. J Hepatol. 2001;34:321–329. doi: 10.1016/s0168-8278(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 95.Vertuani S, Bazzaro M, Gualandi G, Micheletti F, Marastoni M, Fortini C, Canella A, Marino M, Tomatis R, Traniello S, et al. Effect of interferon-alpha therapy on epitope-specific cytotoxic T lymphocyte responses in hepatitis C virus-infected individuals. Eur J Immunol. 2002;32:144–154. doi: 10.1002/1521-4141(200201)32:1<144::AID-IMMU144>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 96.Brazillet MP, Batteux F, Abehsira-Amar O, Nicoletti F, Charreire J. Induction of experimental autoimmune thyroiditis by heat-denatured porcine thyroglobulin: a Tc1-mediated disease. Eur J Immunol. 1999;29:1342–1352. doi: 10.1002/(SICI)1521-4141(199904)29:04<1342::AID-IMMU1342>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 97.Iwatani Y, Amino N, Hidaka Y, Kaneda T, Ichihara K, Tamaki H, Matsuzuka F, Fukata S, Kuma K, Miyai K. Decreases in alpha beta T cell receptor negative T cells and CD8 cells, and an increase in CD4+ CD8+ cells in active Hashimoto’s disease and subacute thyroiditis. Clin Exp Immunol. 1992;87:444–449. doi: 10.1111/j.1365-2249.1992.tb03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blackard JT, Kong L, Huber AK, Tomer Y. Hepatitis C virus infection of a thyroid cell line: implications for pathogenesis of hepatitis C virus and thyroiditis. Thyroid. 2013;7:863–870. doi: 10.1089/thy.2012.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Narayana SK, Helbig KJ, McCartney EM, Eyre NS, Bull RA, Eltahla A, Lloyd AR, Beard MR. The Interferon-induced Transmembrane Proteins, IFITM1, IFITM2, and IFITM3 Inhibit Hepatitis C Virus Entry. J Biol Chem. 2015;290:25946–25959. doi: 10.1074/jbc.M115.657346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martocchia A, Falaschi P. Amino acid sequence homologies between HCV polyprotein and thyroid antigens. Intern Emerg Med. 2007;2:65–67. doi: 10.1007/s11739-007-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stefan M, Jacobson EM, Huber AK, Greenberg DA, Li CW, Skrabanek L, Conception E, Fadlalla M, Ho K, Tomer Y. Novel variant of thyroglobulin promoter triggers thyroid autoimmunity through an epigenetic interferon alpha-modulated mechanism. J Biol Chem. 2011;286:31168–31179. doi: 10.1074/jbc.M111.247510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stefan M, Wei C, Lombardi A, Li CW, Concepcion ES, Inabnet WB, Owen R, Zhang W, Tomer Y. Genetic-epigenetic dysregulation of thymic TSH receptor gene expression triggers thyroid autoimmunity. Proc Natl Acad Sci USA. 2014;111:12562–12567. doi: 10.1073/pnas.1408821111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 104.Hunyady B, Kovács B, Battyáni Z. [Side-effects of pegylated interferon plus ribavirin therapy with or without protease inhibitor direct acting antiviral agents during treatment of chronic hepatitis C virus infection] Orv Hetil. 2011;152:1997–2009. doi: 10.1556/OH.2011.29266. [DOI] [PubMed] [Google Scholar]
- 105.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 106.Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003;38:639–644. doi: 10.1053/jhep.2003.50350. [DOI] [PubMed] [Google Scholar]
- 107.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 108.Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, Sulkowski MS, Wedemeyer H, Tam E, Desmond P, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604–1614. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 109.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]


