Abstract
Over the last years it has started a real revolution in the treatment of chronic hepatitis C. This occurred for the availability of direct-acting antiviral agents that allow to reach sustained virologic response in approximately 90% of cases. In the near future further progress will be achieved with the use of pan-genotypic drugs with high efficacy but without side effects.
Keywords: Direct-acting antiviral agents, Nucleoside inhibitors, Boceprevir, Sofosbuvir, Telaprevir, Hepatitis C, Simeprevir, Daclatasvir, Ledipasvir, Faldaprevir, Ritonavir, Ombitasvir, Dasabuvir
Core tip: This review analyzes the current therapies for chronic hepatitis C and the future challenges of the research. So it tries to give an update on the research of hepatitis C virus (HCV) infection, providing a critical view of the emerging therapies and their impact on the future management of HCV infection. Since novel treatments for HCV infection are highly efficacious but costly, priority should be given to patients with advanced hepatic fibrosis, which is a disease that cannot be deferred.
INTRODUCTION
The hepatitis C virus (HCV), identified in the 70s but cloned in 1989, is a single-stranded RNA virus belonging to the family Flaviviridae.
HCV is the main cause of progressive liver diseases and a public health problem worldwide. It is estimated that approximately 150-180 million people in the world are living with chronic hepatitis[1,2], 350 million of whom die each year from liver damage associated with the infection[3].
About 80% of people infected with HCV develop chronic hepatitis, of which 20%-40% will develop liver cirrhosis or hepatocellular carcinoma (HCC) 20-30 years after infection.
As a consequence, chronic HCV infection is the major reason of liver transplantation in developed countries[4-7].
According to the Global Burden Disease Study in Europe, the death rate for viral hepatitis is significantly higher than that for human immunodeficiency virus (HIV) and acquired immune deficiency syndrome; in particular in 2010, the number of deaths from viral hepatitis have been ten times bigger than that attributed to HIV. It is reasonable to think that this difference is due to the lack of effective therapies for HCV until a few years ago[8].
HCV is also one of the main causes of death[9]. The virus causes both liver damage and extra-hepatic manifestations, many of these syndromes are associated with the ability of HCV to replicate in peripheral blood mononuclear cells (PBMCs); an example is the mixed cryoglobulinemia, which is by far the most common extrahepatic disease closely connected with the infection.
Recently it was shown that antiviral treatment is associated with improved renal and cardiovascular outcomes in patients with cryoglobulinemia[4,6,10,11]. Newly approved oral anti-HCV drugs are very safe and effective, but unfortunately their cost will force to choose a priority of treatment. The intent should therefore be to identify and treat patients with a higher risk of morbidity and mortality due to HCV.
The availability of these new oral treatments can definitely heal patients and consequently it will cause a significant reduction in health care costs[2]. The aim of this review article is to give an update on the research of HCV infection, providing a critical view of the emerging therapies and their impact on the future management of HCV infection.
Natural history of chronic hepatitis C
The natural history of chronic hepatitis C is partly defined. The primary HCV infection is completely asymptomatic in 60%-70% of cases, but in 80% of patients the infection becomes chronic and is characterized by the persistence of the viral genome in the blood for at least 6 mo from the onset of acute infection. In a variable proportion of people carrying the virus, especially in the presence of strong necro-inflammation and/or co-factors of liver damage, the disease can evolve from the condition of chronic hepatitis to cirrhosis and HCC.
There are several factors that can change the course, severity and progression of the disease, including age at the time of infection, route of infection, viral load, co-infection with other hepatitis viruses or HIV, alterations of immune status, and the coexistence of other hepatolesive factors such as consumption of alcohol, iron overload, obesity, type 2 diabetes, resistance to insulin and genetic factors[12-14].
Chronic HCV infection in about 20% of cases progresses up to hepatic cirrhosis, end-stage liver disease and HCC, generally after 20-30 years from primary infection.
The progression of chronic disease leads, through a mechanism of chronic damage, to the loss of organ function, for progressive deposition of fibrotic tissue and disruption of the parenchymal structure, and results in liver fibrosis and cirrhosis.
Cirrhosis changes the normal liver architecture, and furthermore itself represents the most important risk factor for the development of HCC, in part by acting as a cofactor accelerating the process triggered by a primary carcinogen (HCV), and specially by increased hepatocyte regeneration. Once HCV infection progresses to cirrhosis, there is a 1%-5% annual risk of HCC[12].
The probability that a patient with compensated cirrhosis can evolve towards the decompensated form increases progressively over time.
Liver cirrhosis and its complications (portal hypertension and therefore esophageal varices, splenomegaly, ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome, hepato-pulmonary syndrome and HCC) are burdened with high morbidity and mortality.
It is also known that different variables influence the progression of the disease, for which the prognosis changes individually and is very hard to define[12-14].
Several studies have concluded that the eradication of HCV infection slows the progression of the disease, improves the survival, and reduces the incidence of liver failure and the risk of developing liver cancer[12-28]. The understanding of the natural history of chronic hepatitis C and its long-term consequences is essential to enable appropriate decisions on treatment, but unfortunately the natural history of HCV infection is still the subject of much controversy. In fact, according to some authors the disease is relentlessly progressive, with a high probability to evolve to cirrhosis and HCC, while according to others the course is variable, and most patients die as a consequence of co-morbidities, not the infection itself.
Because the infection has a significant role in causing chronic hepatitis, cirrhosis and HCC, the goal of treatment is to cure HCV infection, and consequently to prevent its complications.
Although the viral RNA genome does not integrate into the host genome, the infection becomes persistent in the majority of patients, and about 70%-90% of the infected people fail to clear the virus once acquired.
It is widely known that the antiviral treatment and the achievement of a sustained virological response (SVR - defined as an absence of detectable HCV-RNA 12 mo after therapy is complete) are associated with regression of fibrosis and clinical improvement. However, despite treatment, HCV may persist in liver tissue and extrahepatic locations like PBMCs, leading to late relapse, defined as reappearance of viremia after SVR has been achieved[29,30].
Treatment and SVR - what is the real purpose of antiviral therapy?
As mentioned above the primary goal of HCV therapy is the complete eradication of the virus, which is the SVR.
SVR was traditionally defined as HCV-RNA undetectable in serum for at least 24 wk after the end of treatment (SVR24); however, recent data suggest that the assessment at 12 wk after treatment (SVR12) is sufficient for defining this result.
Follow-up studies document that more than 99% of patients who achieve an SVR remain HCV-RNA negative 4-5 years after the end of treatment, and no signs of hepatitis have been documented.
SVR represents the main goal of antiviral therapy, indeed once achieved, the SVR is considered effective in the long term because late recurrences are rare; the SVR is associated with long-term health benefits, including improved quality of life.
SVR reduces risk for progression to cirrhosis, HCC, liver transplantation and liver-related mortality, and also decreases extra-hepatic manifestations of HCV infection (for example, cryoglobulinemic vasculitis).
Moreover it seems reasonable to assume that a lasting biochemical and virological response induced by treatment can also lead to improved liver fibrosis[31-39].
For decades the antiviral therapy of chronic HCV infection was based on the administration of interferon (IFN), initially as monotherapy and subsequently in combination with ribavirin (RBV). Dual therapy with “pegylated IFN (PEG-IFN) and RBV” achieves SVR rates of 40% to 50% in patients with genotype 1, and about 80% in those with genotypes 2, 3, 5 and 6; the results for genotype 4 are intermediates.
In 2011, the first direct-acting antivirals boceprevir and telaprevir have been approved in combination with PEG-IFN and RBV. These drugs are protease inhibitors (PIs) and increase SVR rates in both naive patients and in experienced patients, compared to dual therapy[40-46]; however, they were dropped due to their significant toxicity.
With the advent of new oral antiviral regimens, with better efficacy and tolerability, and a shorter treatment duration, the number of patients that can be treated is expected to increase significantly, and also the SVR rates will improve to approximately 95% or plus[47].
HCV and host: The HCV replication cycle and mechanisms of action of the new direct acting antiviral agents
HCV is classified within the Flaviviridae family, as the only member of a distinct genus called Hepacivirus[48].
The lack of detailed information on the viral replication cycle has significantly prevented the development of direct acting antivirals.
For decades the antiviral therapy of chronic HCV infection was based on the administration of IFN, initially alone and then in combination with RBV, but this regimen was effective in only 50% of patients with genotype 1, with significant side effects[49-54].
In the last decade the development of in vitro models of viral replication has thus represented a turning point for the understanding of the different stages of the replication cycle, and quickly has led to the design and introduction of direct acting antivirals (DAAs)[55].
However, because of huge variability of the virus, new drugs cannot be administered as monotherapy because it would quickly lead to the selection of drug-resistant viral variants.
HCV indeed is characterized by an extremely high degree of variability. The genetic heterogeneity of HCV gives an adaptive advantage as the simultaneous presence of multiple genomic variants allows rapid selection of mutants that better adapt to environmental changes (for example resistance to drugs or the immune response); this genetic heterogeneity is the basis of chronic infection, and is probably involved in the phenomena of evasion of the immune response and in the limited efficacy of treatment[56-59].
The HCV replication cycle occurs in the cytoplasm, and can be summarized as follows: (1) entry into the host cell and release of viral genomic RNA into the cytoplasm; (2) translation of RNA, processing of the viral polyprotein and formation of a replication complex associated with intracellular membrane; (3) using positive RNA for the synthesis of an intermediate negative RNA for the production of new positive RNA molecules with different destination; and (4) release of viral progeny into circulation from infected cells. The infectious viral structure is comprised of envelope glycoproteins in a lipid bilayer, that contain the viral core protein and RNA[60-63].
After cell entry, the viral RNA is translated through the host machinery into a polyprotein, which is cleaved during and after translation by both host and viral-encoded proteases into 10 mature viral proteins, including several non-structural (NS) proteins. One of the viral proteases involved in this post-translational processing is a heterodimeric complex of the NS3 and NS4A proteins (NS3/NS4A). NS3 has the proteolytic activity and NS4 is a membrane protein that acts as a cofactor. Synthesis of new viral RNA occurs in a highly structured replication complex that consists of NS3, NS4A, NS4B, NS5A, and NS5B. NS5B is an RNA-dependent RNA polymerase that is essential for viral replication. NS5A has a presumptive role in the organization of the replication complex and in regulating replication. It is also involved in assembly of the viral particle that is released from the host cell (Figure 1)[64-69].
Figure 1.
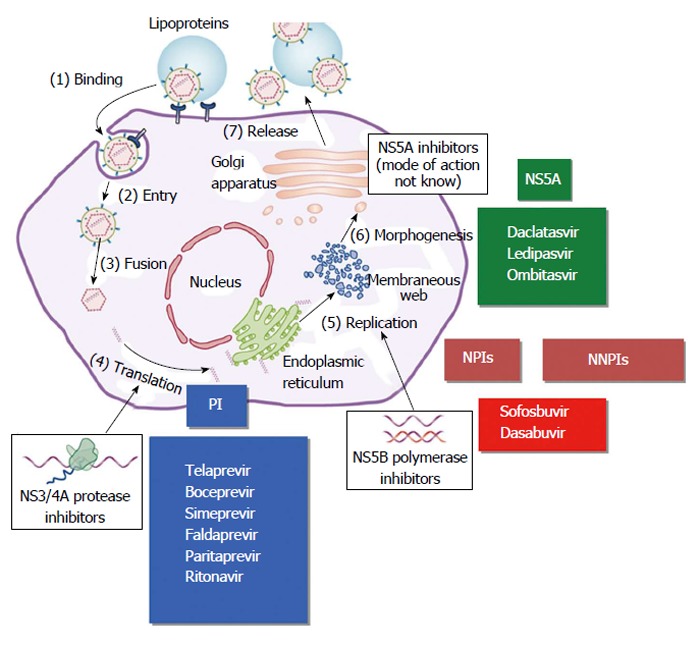
Hepatitis C virus replicative cycle and main targets for direct acting antiviral agents. Modified from Manns and Cornberg. Lancet Infectious Diseases 2013. PIs: Protease inhibitors; NPIs: Nucleoside polymerase inhibitors; NNPIs: Non-nucleoside polymerase inhibitors.
Therapies
Increased knowledge of the HCV replication cycle and genomic diversity has driven the development of antiviral agents specifically targeting well-conserved proteins required for efficient viral replication. Aside from PEG-IFN, HCV-specific therapeutic agents that have gained widespread use or reached late-stage clinical trials include NS3 PIs, nucleoside and nucleotide analogues, and other NS5B polymerase inhibitors.
DAAs
After year of IFN-based therapy, the introduction of DAAs has increased the number of patients who respond to treatment, and has changed radically the treatment of chronic HCV genotype-1 infection[43,70-72].
Thanks to the discovery of key viral replication targets such as the NS3/4A protease, NS5A, and the NS5B RNA polymerase, other potent antiviral inhibitors were licensed in 2014.
These new regimens include the addition of simeprevir (SMV) (a second-generation PI), daclatasvir (an NS5A inhibitor), and sofosbuvir (an uridine nucleotide prodrug NS5B polymerase inhibitor), in combination with PEG-IFN and RBV for 12-24 wk[73,74].
The main targets of the DAAs are the HCV-encoded proteins that are vital to the viral replication. The DAAs have a high barrier to resistance and ideally, they should also be active against all HCV genotypes. Furthermore, these drugs are well tolerated and have few drug interactions.
There are four classes of DAAs, which are defined by their mechanism of action and therapeutic target[75] (Figure 2 and Table 1): (1) NS3/4A PIs; (2) NS5B nucleoside polymerase inhibitors (NPIs); (3) NS5B non-NPIs (NNPIs); and (4) NS5A inhibitors.
Figure 2.
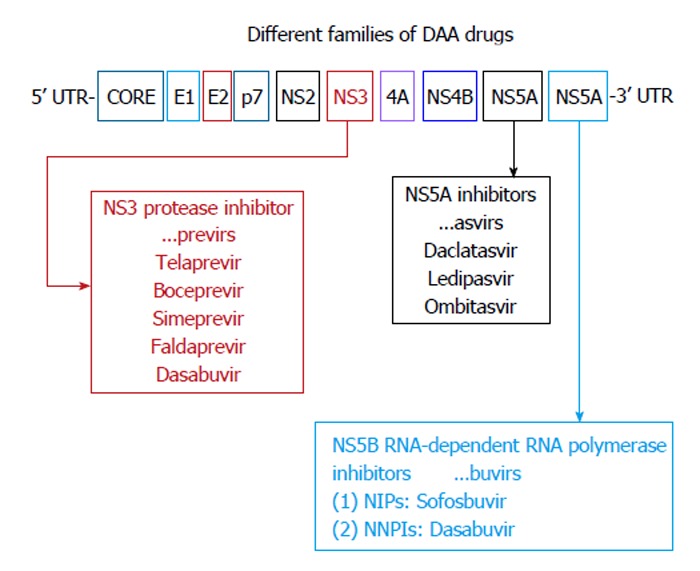
Direct acting antiviral agents. Modified from Alexopoulou et al[121]. Interferon-based combination treatment for chronic hepatitis C in the era of direct acting antivirals. Annals of Gastroenterology 2015; 28: 55-65. NPIs: Nucleoside polymerase inhibitors; NNPIs: Non-nucleoside polymerase inhibitors; DAA: Direct acting antiviral.
Table 1.
Classification of new antiviral drugs
| NS3/4A PIs | First-generation protease inhibitors |
| Telaprevir | |
| Boceprevir | |
| Second-generation protease inhibitors | |
| Simeprevir | |
| Faldaprevir | |
| Paritaprevir | |
| Ritonavir | |
| NS5B NPIs | Sofosbuvir |
| NS5B NNPIs | Dasabuvir |
| NS5A inhibitors | Daclatasvir |
| Ledipasvir | |
| Ombitasvir |
PIS: Protease inhibitors; NPIs: Nucleoside polymerase inhibitors; NNPIs: Non-nucleoside polymerase inhibitors.
NS3/4A PIS
NS3/4A PIs are drugs that inhibit the NS3/4A serine protease, an enzyme involved in post-translational processing and replication of HCV[76].
There are two generation of PIs.
First-generation PIs (telaprevir and boceprevir)
The first-generation PIs telaprevir and boceprevir were the first DAAs available for the treatment of CHC[77].
The addition of PIs to PEG-IFN and RBV has become the new standard of care for the treatment of genotype 1 infection, and so, in 2011, has increased the efficacy of PEG-IFN and RBV in patients with chronic HCV genotype 1 infection.
Telaprevir and boceprevir were approved for the treatment of chronic HCV genotype 1 infection by the Food and Drug Administration (FDA) and European Medicines Agency in combination with PEG-IFN-α and RBV in adults with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous IFN and RBV therapy[78].
Telaprevir and boceprevir are NS3/4A PIs, and they both have the same molecular target: The HCV NS3/4A serine protease.
They have an high antiviral potency only against genotypes 1 and 2, but a low barrier to resistance[79].
Monotherapy with these agents resulted in the selection of drug resistant variants, so they should always be used in triple combinations together with PEG-IFN and RBV in a triple therapy regimen to reduce the frequencies of resistant mutants and viral breakthrough, and they can improve the SVR rates by 15% to 20% compared with PEG-IFN-α and RBV[42,43,80].
Viral resistance may develop even in triple combinations with PEG-IFN and RBV, and due to this problem strict stopping rules are applied in triple therapy-based regimens.
Response to HCV therapy in genotype 1 can be predicted by identifying the single nucleotide polymorphisms located in the region of interleukin-28B (IL-28B) gene through genome-wide association studies.
High response rates have been reported in patients with CC genotype of IL-28B as compared to CT or TT IL-28B-genotype (70% vs 25%-30%). Testing for IL-28B genotype is thus a useful tool in the management of patients[81].
First-generation PIs increase the number of patients with genotype 1 infection who respond to treatment, however, the side effect profiles of these triple combination therapies are not favourable, because it can cause clinically significant adverse events.
The most common side effects of telaprevir are anaemia, pruritis, nausea, diarrhoea, and anorectal discomfort. Around 4% of patients develop severe dermatitis, necessitating cessation of treatment.
Drug reactions like eosinophilia and systemic symptoms or Stevens-Johnson syndrome are rare, but have been reported. Boceprevir causes dysguesia and anaemia[43].
Several drug-drug interactions can occur, so the use of first-generation PIs has been significantly restricted[82].
Second-generation PIs
Second-generation PIs offer several benefits, for example, few drug-drug interactions and less frequent and less severe side effects.
In addition, second-generation PIs also appear to have increased efficacy against genotype 1 HCV[83]; as treatment options have progressed and improved, HCV- 1, HCV-2 and HCV-4 are considered to be easy to treat[84] but HCV genotype 3 infection has become the most difficult to treat.
SMV: SMV was the first available second-generation PI with antiviral activity against genotypes 1, 2, 4, 5 and 6[85].
SMV is administered orally as a daily pill, and has limited drug-drug interactions.
No dose recommendation can be given for patients with Child-Pugh class B or C cirrhosis, because higher SMV exposure (particularly in Child-Pugh C patients) may be associated with increased frequency of adverse reactions. No dose adjustment is required in the setting of renal impairment, because SMV is eliminated by the liver[85]. SMV is well tolerated, and adverse reactions in patients receiving SMV in combination with PEG-IFN-α and RBV are rash, pruritus and nausea. Because SMV is an inhibitor of the transporters OATP1B1 and MRP2, mild, transient hyperbilirubinaemia not accompanied by changes in other liver parameters was observed in approximately 10% of cases. SMV is oxidatively metabolized by CYP3A subfamily, which consists mainly of hepatic and intestinal CYP3A4 metabolism[86]. Co-administration of SMV with inhibitors of cytochrome P450 3A (CYP3A) is not recommended.
In post-liver transplant patients with HCV infection, co-administration of SMV with cyclosporine resulted in significantly elevated SMV levels, so it is not recommended[87]. SMV can be safely administered with tacrolimus or sirolimus. SMV was approved by the FDA for genotype 1 treatment in November 2013 under the name of “OLYSIO”, in Japan it was licensed in September 2013, finally in Europe in May 2014 (European Medical Agency approval).
In phase II of COSMOS trial, sofosbuvir (SOF; 400 mg daily) was administered in combination with SMV (SMV 150 mg daily) with or without RBV for 12 wk or 24 wk in genotype 1 patients. SVR12 rates were not different between 12 or 24 wk of treatment, with or without RBV, and comparing naive patients to experienced (95% vs 91%)[87,88].
In this small study, the regimen SOF plus SMV with or without RBV was well tolerated; the most common side effects were headache, fatigue, and nausea, and only four (2%) patients discontinued treatment due to these events.
Although the results of this study are encouraging, due to the small number of patients and the future availability of other oral regimes with better antiviral efficacy and fewer side effects, this regimen should be considered as a second-line option.
Two phase III trials of SMV/SOF without RBV are ongoing (OPTIMIST-1 and -2)[89]. These studies provide us much bigger data about SOF/SIM regimen, and investigate the efficacy and safety of SMV 150 mg in combination with sofosbuvir 400 mg in HCV genotype 1 infected naïve or experienced patients, with and without cirrhosis.
SMV/SOF treatment led to high SVR12 rates in patients infected with HCV GT-1 subtype, regardless of treatment duration or the addition of RBV. SVR12 rates were high, regardless of baseline characteristics, including HCV GT-1 subtype, IL-28B allele, or Q80K polymorphism. On-treatment virologic response, including RVR, was not predictive of SVR. Two ongoing phase III trials are investigating SMV/SOF without RBV (OPTIMIST-1 and -2).
Baseline predictive factors significantly associated with virologic relapse were male sex, body weight ≥ 75 kg, IL-28B non-CC allele, cirrhosis, baseline HCV RNA ≥ 800000 IU/mL, and prior treatment failure. Current SOF regimens are highly efficacious, even in patients with multiple traditional negative predictors of diminished efficacy; SVR12 rates are comparatively lower in patients who have five or six negative predictors[90,91].
The approval of the treatment scheme “SMV plus PEG-IFN/RBV” is based on a clinical trial program comprising three phase III studies, with more than 1000 patients with genotype 1.
The studies, QUEST-1, QUEST-2 and PROMISE, have evaluated the use of SMV in combination with PEG-IFN/RBV in naive patients (Quest-1 and 2)[92,93] and relapsed patients (PROMISE[94]) after an IFN-based treatment. All three studies have shown that SMV, in combination with PEG-IFN/RBV, gets significant SVR rates when compared to PEG-IFN/RBV.
A triple therapy with SMV, PEG-IFN and RBV has been recommended for genotype 1 also after the data of other four phase III trials: CONCERTO-1, -2, -3 and -4[95-98].
Faldaprevir: Faldaprevir is one of the new-generation NS3/4A PIs in development. It is a pan-genotypic potent NS3/NS4 PI (antiviral activity against genotypes 1, 2, 4, 5 and 6 in vitro). It was used in genotype 1 infection in two combinations: (1) a triple regimen with faldaprevir, PEG-IFN and RBV for a total of 24 wk[98,99]; and (2) IFN-free regimens with faldaprevir and deleobuvir with or without RBV[100,101].
In both combinations faldaprevir provides high SVR rates, but IFN-containing regimens registered most cases of breakthrough and relapse, while with the IFN-free combination of faldaprevir and deleobuvir with RBV, very encouraging results were obtained[102].
Faldaprevir is administered orally, once a day. The most common adverse events are gastrointestinal dysfunction, rash and photosensitivity skin. Faldaprevir in combination with PEG-IFN and RBV appears to be associated with fewer adverse events than the first PIs telaprevir and boceprevir.
Paritaprevir and ritonavir: Paritaprevir is an HCV protease inhibitor that is given with low dose ritonavir for a pharmacologic boosting effect.
Ritonavir is a protease inhibitor that does not have anti-HCV activity but it is a pharmacoenhancer that is included to increase levels of paritaprevir through inhibition of CYP3A-mediated metabolism.
Paritaprevir and ritonavir are available as a fixed-dose combination with ombitasvir and given with the non-nucleoside NS5B inhibitor dasabuvir. This regimen is given with and without RBV for the treatment of HCV GT-1 subtype[103].
NS5A INHIBITORS
The NS5A is a multifunctional non-structural protein involved both in viral replication and in the assembly of HCV[104]. However, the precise molecular mechanisms of HCV NS5A inhibitors are unclear.
NS5A inhibitors have high antiviral activity against a lot of genotypes, but a low genetic barrier. They significantly reduce HCV-RNA levels and enhance SVR when given in conjunction with PEG-IFN and RBV. They also result in very high SVR rates among patients with genotype 1 infection when given in combination with other direct-acting antivirals with or without RBV[105].
Daclatasvir
Daclatasvir is a pangenotypic NS5A inhibitor that is available for use in Europe. According to EASL guidelines daclatasvir should be administered orally (60 mg once daily) with low potential for drug-drug interactions. It is well tolerated. Dose adjustments are not needed in patients with Child B or C disease. Side effects with daclatasvir are fatigue, headache, and nausea. Little information has been released on daclatasvir drug-drug interactions.
In previously untreated patients infected with genotype 2 or 3, SVR was reported in 94%-100% of patients treated with the combination of daclatasvir plus sofosbuvir. In the ALLY-3 study[106], 133 patients with genotype 3 infection were treated for 12 wk with 400 mg of sofosbuvir and 60 mg of daclatasvir for 12 wk. Ninety-one percent of previously untreated patients had an SVR compared with 86% of treatment-experienced patients.
In the COMMAND GT2/3 study, Dore et al[107] compared the efficacy and safety of daclatasvir plus PEG-IFN-α-2a/RBV administered for either 12 or 16 wk with a standard 24-wk course of PEG-IFN-α-2a/RBV in HCV GT-2 or GT-3 subtype. Daclatasvir has been given with PEG-IFN-α-2a/RBV for 12 or 16 wk to previously untreated patients with genotype 2 or 3 infection. Around 83% of patients infected with genotype 2 and 70% of patients with genotype 3 infection have been reported to achieve SVR[107]. In another open-label study, the drug’s effectiveness has been demonstrated[108].
Other NS5A inhibitors
Other NS5A inhibitors available in the United States are ledipasvir and ombitasvir, and they are each available in fixed-dose combinations with other direct-acting antivirals.
Ledipasvir: Ledipasvir is the first NS5A inhibitor available in the United States. Ledipasvir and sofosbuvir are co-formulated in a single tablet in a fixed-dose combination (90 mg ledipasvir/400 mg sofosbuvir) that is administered once daily with or without food. This combination is well tolerated, and ledipasvir has the same drug interactions as sofosbuvir. This regimen is administered with or without RBV, depending on the patient population, in genotype 1 infection.
Ombitasvir: Ombitasvir (also known as ABT-267) is available as a fixed-dose combination with the PIs paritaprevir and ritonavir (12.5 mg ombitasvir/75 mg paritaprevir/50 mg ritonavir). This single tablet is administered orally with an additional drug: The non-nucleoside polymerase (NS5B) inhibitor dasabuvir[109,110]. This regimen is given with and without RBV in genotype 1 infection.
The combination ombitasvir-paritaprevir-ritonavir plus dasabuvir is generally well tolerated, and mild adverse effects are nausea, pruritus, insomnia, diarrhea, and asthenia[111,112]. Some of these symptoms may be attributable to RBV[113,114].
The most important studies that evaluated treatment duration of ledipasvir/sofosbuvir treatment and its safety and efficacy (SVR12) in naive and treatment-experienced patients are ION-1, LONESTAR, and ION-2[115-117] (Figure 3).
Figure 3.
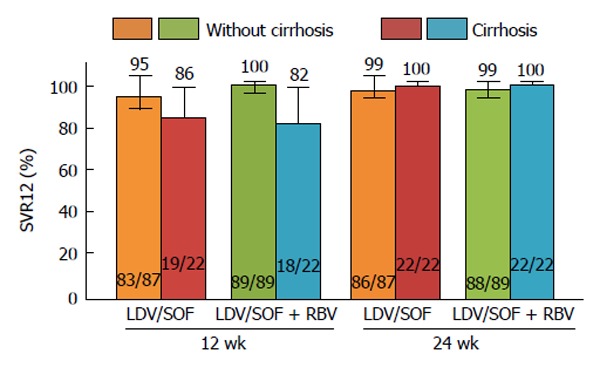
ION-2 sub-analysis of cirrhosis vs without cirrhosis. Error bars represent 95%CIs. LDV: Ledipasvir; RBV: Ribavirin; SOF: Sofosbuvir; SVR12: Sustained virologic response at 12 wk post-treatment.
NS5B RNA-DEPENDENT RNA POLYMERASE INHIBITORS
NS5B is an RNA-dependent RNA polymerase involved in post-translational processing that is necessary for replication of HCV. The structure of NS5B is highly conserved across all HCV genotypes, so the drugs that inhibit NS5B have efficacy against all six genotypes.
There are two classes of polymerase inhibitors: NPIs and NNPIs. These two classes generally differ in specificity, according to their mode of action.
The NPIs mimic natural components and thus are incorporated into the nascent RNA chain, causing premature chain termination[118]. NNPIs act as allosteric inhibitors, and in fact they bind to one of four allosteric sites on the surface of NS5B.
NPIS
NPIs have high antiviral efficacy across all genotypes, although they have a very high barrier to resistance.
Sofosbuvir: Sofosbuvir is the first NS5B NPI available in the United States.
Sofosbuvir is a pangenotypic NS5B polymerase inhibitor with a high barrier to resistance and favorable clinical pharmacology profile. It is administered orally as a 400 mg pill once a day, and has no food effect. Sofosbuvir is well tolerated, and the most commonly reported side effects of sofosbuvir and RBV, with or without PEG-IFN, are fatigue, headache, nausea, insomnia, and anemia[74,119].
Although renal clearance is the major form of elimination, in patients with mild or moderate renal impairment (glomerular filtration rate greater than 30 mL/min)[120], any adjustment dose is not required.
No dose adjustment has been needed in patients with moderate (Child Pugh class B) or severe (Child Pugh class C) hepatic impairment.
Sofosbuvir has substantially less drug interactions than those observed with the HCV PIs. Sofosbuvir is a substrate of P-glycoprotein (P-gp), a drug transporter, so drugs that are potent intestinal P-gp inducers may decrease sofosbuvir levels. Thus, coadminstration of sofosbuvir is not recommended with rifampin, rifabutin, rifapentine, St. John’s wort, carbamazepine, phenytoin, phenobarbital, oxcarbazepine, or tipranavir/ritonavir.
Sofosbuvir was approved by FDA for genotype 1 in combination with PR, and in genotypes 2 and 3 in IFN free regimens in December 2013, in Canada during the same month and in Europe in January 2014 (European Medical Agency approval).
In the NEUTRINO study (an open-label, single-arm phase III registration trial) 327 treatment-naive patients were treated with a regimen comprising sofosbuvir plus PR for 12 wk[119]. The overall patient population included mainly those infected with genotype 1 (89%) as well as a few patients infected with genotypes 4, 5 and 6; 17% of patients in this trial had cirrhosis. This sofosbuvir-based triple-therapy regimen resulted in a very high RVR, with the 4-wk RVR rate approaching 99%. The SVR rate for the entire trial population remained high at 90%, 12 wk after the end of treatment (with 99% of patients achieving virologic response at the end of treatment). Analyzing the groups based on viral genotype, patients with genotype 1 had an SVR rate of 89%, and the small number of patients with genotype 4, 5 and 6 had SVR rates between 96% and 100%. Overall, this sofosbuvir-based triple therapy regimen resulted in very high SVR rates across all genotypes that were evaluated. One important point from the NEUTRINO trial was the relative decrease in the overall response rate for patients with cirrhosis (SVR, 80%) compared with non-cirrhotics (SVR, 92%)[121].
Other representative studies on genotype 1 are ELECTRON, QUANTUM, VALENCE and LONESTAR-2[122-125].
Genotypes 2 and 3 have been studied together in three sofosbuvir phase III trials (FISSION, POSITRON, and FUSION)[119,122,126].
Therapy with sofosbuvir-RBV for 12 wk in patients with HCV genotype 2 infection and for 24 wk in patients with HCV genotype 3 infection resulted in high rates of SVR[127].
To date there are very few data on genotype 4 patients treated with sofosbuvir without PEG-IFN[128]. There are no data currently on treatment-experienced populations or any patients with genotypes 5 and 6[129].
Sofosbuvir is used in various combinations with other antivirals for different indications: (1) with ledipasvir for HCV GT-1; (2) with SMV (± RBV) for HCV GT-1; (3) with RBV for HCV GT-2, -3, -4, -5, and -6 infection (and among patients with any genotype awaiting liver transplant); and (4) with PEG-IFN and RBV for genotypes HCV GT-1 and -4.
NNPIs
NNPIs bind to one of four allosteric sites on the surface of NS5B and cause a conformational change, making the enzyme ineffective. Despite the active site of NS5B is well conserved across all genotypes, and they should have a pan-genotype antiviral activity, NNIs have a more limited spectrum of activity specifically targeting against GT-1 (all NNPIs in clinical development have been optimized for GT-1). They have a low to moderate barrier to resistance variable toxicity profiles[130]. Consequently, this class of drug has been studied primarily as an adjunct to more potent compounds with higher barriers to resistance.
Dasabuvir: Dasabuvir is a non-nucleoside polymerase (NS5B) inhibitor administered with the fixed-dose combination ombitasvir-paritaprevir-ritonavir (12.5 mg ombitasvir/75 mg paritaprevir/50 mg ritonavir).
ABT-450/ritonavir with ombitasvir (ABT-267) and dasabuvir (ABT-333): TURQUOISE-II is a global, multi-center, randomized, open-label study evaluating the efficacy and safety of 12 or 24 wk of treatment with ABT-450/ritonavir (150/100 mg) co-formulated with ombitasvir (ABT-267) 25 mg, dosed once daily, and dasabuvir (ABT-333) 250 mg with RBV in adult patients with GT-1 HCV infection with compensated liver cirrhosis. Patients achieved SVR12 rates of 91.8% and 95.9% in the 12 and 24-wk treatment arms, respectively[131]. In TURQUOISE-II, both cirrhotic non-responders and treatment-naive cirrhotic subjects achieved higher SVR rates if they were genotype 1b-infected vs genotype 1a-infected. According to Asselah et al[132] we support the efficacy and safety profile in GT-1 HCV cirrhotic patients, and in some cases the efficacy was demonstrated also in borderline compensated cirrhosis. However, current data in patients with cirrhosis and other HCV genotypes, such as genotype 3 and 4, are clearly an unfulfilled need. Another significant study is the PEARL-II[133].
New drugs: Cyclophilin A inhibitors
Cyclophilins (Cyp) are host proteins involved in the HCV lifecycle. CypA binds to the non-structural protein NS5A of HCV to promote replication of viral RNA, so molecules that are CypA antagonists, such as cyclosporines, are potent inhibitors of HCV replication. NS2, a non-structural protein of HCV involved in virus assembly, also plays an important role in the inhibitory effect of CypA inhibitors; NS2 modulates HCV sensitivity to cyclosporines and so NS2 may increase the inhibitory effect of cyclosporines on HCV replication[134,135].
Alisporivir, is the first Cyp A inhibitor in clinical development. It is a cyclosporine analog without immunosuppressive properties, and due to its mechanism of action, alisporivir is a pangenotypic antiviral, provides a high barrier for development of viral resistance, and does not permit cross-resistance to direct-acting antivirals.
This drug is also well tolerated. This drug has been used alone or in combination with PEG-IFN and RBV with very promising results[136,137].
GUIDELINES TREATMENTS HEPATITIS C
The treatment of CHC is performed following the American, European and Italian guidelines (AASLD, EASL, and AISF guidelines); this allows to optimize the therapy and customize it for various patient characteristics. Priority should be given to patients with advanced disease, patients with extrahepatic manifestations, HIV coinfection, post-liver transplantation recurrence and non-hepatic solid organ transplant recipients. Patients with mild disease can be treated with regimens containing PEG-IFN or deferred up to a worsening of the disease and the degree of liver fibrosis[138,139].
DISCUSSION AND CONCLUSION
Today, it can be anticipated that the future of HCV infection treatment seems very bright after the addition of first-generation HCV PIs as well as SMV and the first-in-kind HCV RNA polymerase inhibitor, ‘‘sofosbuvir’’, in the standard of care (i.e., PEG-IFN/RBV). However, the real success of these drugs is very much dependent on careful monitoring of viral load and resistance, patterns of response to previous treatment, side effects and drug-drug interactions. Moreover, the logical meaning of novel emerging therapies must be to achieve high SVR and thorough clearance of the virus from treated patients. Nevertheless, the triple therapeutic regimens have several limitations. First, concomitant use of PEG-IFN plus RBV is essential to prevent the emergence of viral escape mutants and viral breakthrough during triple therapy. Second, triple therapy becomes less effective in prior null responders to PEG-IFN plus RBV and cannot be administered to patients who are contraindicated for PEG-IFN or RBV. To overcome these limitations, in the near future, many patients will be treated with two or more DAAs with or without IFN-α plus RBV based combination therapies. Currently, the approval of sofosbuvir- and SMV-based IFN-free regimens is an indication in this way. Triple and quadruple treatment regimens including multiple DAAs with or without PEG-IFN and RBV will likely be a suitable option for difficult-to-treat populations and for the prior null responders. All-oral IFN free regimens including drugs with a high genetic barrier to antiviral resistance (e.g., NS5B inhibitors) and high antiviral efficacy (e.g., NS3/4A PIs or NS5A inhibitors) may be a potent option for numerous patients contraindicated for PEG-IFN plus RBV. All oral regimens consisting of daclatasvir plus sofosbuvir once daily presented higher rates of SVR in untreated HCV GT-1, -2 and -3 infected patients and in HCV GT-1 infected patients who had failed previous treatment with PIs. We hope that such combinational treatment strategies will become ‘‘the weapon’’ to treat the majority of HCV infected patients who represent the difficult population (i.e., IL-28 polymorphism, HCV genotypes 1 and 4 subtypes, receipt of RBV, and the emergence of resistant variants) and will be more efficient to access the treatment in the near future. The testing of adenovirus vector based vaccines, which escalate the innate and acquired immune response against the most conserved regions of HCV genome in chimpanzees and humans, may be a promising therapeutic approach against HCV in the near future, although its fate still needs to be exploited fully in diverse HCV populations. One thing must be of special concern is whether the newly developed or being developed DAAs added in triple or quadruple therapies are safer or not than antiretroviral and traditional IFNs. Overall, the achievements in the field of HCV medicines may predict that we are near to complete elimination of HCV disease in the world[140]. The real challenges that our efforts must be directed are: (1) the effectiveness of IFN-free regimens in HCV-3, especially in cirrhotic non-responders; in this setting, combination with PEG-IFN is still possible; (2) the effectiveness of IFN-free regimens in decompensated cirrhosis are scarce in relation to the current correlation data between SVR and clinical outcome (literature confirms that the results of IFN-free regimens are good in compensated cirrhosis even if further clinical development is necessary in certain groups to improve SVR rates); (3) the development of new treatment strategies for patients who show resistance to new drugs; and (4) free-access to care[141]. In fact, many patients with CHC have mild disease and are currently excluded from the interferon-free treatment. In the near future we will inevitably prioritize this category in order to prevent progression to cirrhosis, decompensation and HCC.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 24, 2015
First decision: August 10, 2015
Article in press: December 18, 2015
P- Reviewer: Abenavoli L, Han SY, Rodriguez-Frias F, Tovo CV S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Liu SQ
References
- 1.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521, 521.e1-6. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 2.Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, Vogel W, Mendes Correa MC, Hézode C, Lázaro P, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21 Suppl 1:34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 3.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 5.Malaguarnera M, Scuderi L, Ardiri Al, Malaguarnera G, Bertino N, Ruggeri IM, Carmela Greco G, Ozyalcn E, Bertino E, Bertino G. Type II Mixed Cryoglobulinemia in patients with Hepatitis C Virus: treatment with Pegylated-interferon and ribavirin. Acta Medica Mediterr. 2015;31:431. [Google Scholar]
- 6.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 7.Ly KN, Xing J, Klevens RM, Jiles RB, Holmberg SD. Causes of death and characteristics of decedents with viral hepatitis, United States, 2010. Clin Infect Dis. 2014;58:40–49. doi: 10.1093/cid/cit642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowie BC, Allard N, MacLachlan JH. O86 European responses in focus: comparing viral hepatitis and hiv related deaths in europe 1990-2010 in the global burden of disease study 2010. J Hepatol. 2014;60(1 Supplement):35–36. [Google Scholar]
- 9.Mahajan R, Xing J, Liu SJ, Ly KN, Moorman AC, Rupp L, Xu F, Holmberg SD. Mortality among persons in care with hepatitis C virus infection: the Chronic Hepatitis Cohort Study (CHeCS), 2006-2010. Clin Infect Dis. 2014;58:1055–1061. doi: 10.1093/cid/ciu077. [DOI] [PubMed] [Google Scholar]
- 10.Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, Wu MS, Liu YY, Wu CY. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CS, Kao JH, Chao YC, Lin HH, Fan YC, Huang CJ, Tsai PS. Interferon-based therapy reduces risk of stroke in chronic hepatitis C patients: a population-based cohort study in Taiwan. Aliment Pharmacol Ther. 2013;38:415–423. doi: 10.1111/apt.12391. [DOI] [PubMed] [Google Scholar]
- 12.Zabala V, Tong M, Yu R, Ramirez T, Yalcin EB, Balbo S, Silbermann E, Deochand C, Nunez K, Hecht S, et al. Potential contributions of the tobacco nicotine-derived nitrosamine ketone (NNK) in the pathogenesis of steatohepatitis in a chronic plus binge rat model of alcoholic liver disease. Alcohol Alcohol. 2015;50:118–131. doi: 10.1093/alcalc/agu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caponnetto P, Russo C, Di Maria A, Morjaria JB, Barton S, Guarino F, Basile E, Proiti M, Bertino G, Cacciola RR, et al. Circulating endothelial-coagulative activation markers after smoking cessation: a 12-month observational study. Eur J Clin Invest. 2011;41:616–626. doi: 10.1111/j.1365-2362.2010.02449.x. [DOI] [PubMed] [Google Scholar]
- 14.Bertino G, Ardiri AM, Alì FT, Boemi PM, Cilio D, Di Prima P, Fisichella A, Ierna D, Neri S, Pulvirenti D, et al. Obesity and related diseases: an epidemiologic study in eastern Sicily. Minerva Gastroenterol Dietol. 2006;52:379–385. [PubMed] [Google Scholar]
- 15.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58–S68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Bertino G, Di Carlo I, Ardiri A, Calvagno GS, Demma S, Malaguarnera G, Bertino N, Malaguarnera M, Toro A, Malaguarnera M. Systemic therapies in hepatocellular carcinoma: present and future. Future Oncol. 2013;9:1533–1548. doi: 10.2217/fon.13.171. [DOI] [PubMed] [Google Scholar]
- 17.Bertino G, Demma S, Ardiri A, Proiti M, Gruttadauria S, Toro A, Malaguarnera G, Bertino N, Malaguarnera M, Malaguarnera M, et al. Hepatocellular carcinoma: novel molecular targets in carcinogenesis for future therapies. Biomed Res Int. 2014;2014:203693. doi: 10.1155/2014/203693. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Bertino G, Demma S, Ardiri A, Proiti M, Mangia A, Gruttadauria S, Toro A, Di Carlo I, Malaguarnera G, Bertino N, et al. The immune system in hepatocellular carcinoma and potential new immunotherapeutic strategies. Biomed Res Int. 2015;2015:731469. doi: 10.1155/2015/731469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Biondi A, Malaguarnera G, Vacante M, Berretta M, D’Agata V, Malaguarnera M, Basile F, Drago F, Bertino G. Elevated serum levels of Chromogranin A in hepatocellular carcinoma. BMC Surg. 2012;12 Suppl 1:S7. doi: 10.1186/1471-2482-12-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS. Hepatocellualar carcinoma serum markers. Semin Oncol. 2012;39:410–433. doi: 10.1053/j.seminoncol.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Bertino G, Neri S, Bruno CM, Ardiri AM, Calvagno GS, Malaguarnera M, Toro A, Malaguarnera M, Clementi S, Bertino N, et al. Diagnostic and prognostic value of alpha-fetoprotein, des-γ-carboxy prothrombin and squamous cell carcinoma antigen immunoglobulin M complexes in hepatocellular carcinoma. Minerva Med. 2011;102:363–371. [PubMed] [Google Scholar]
- 22.Bertino G, Ardiri AM, Calvagno GS, Bertino N, Boemi PM. Prognostic and diagnostic value of des-γ-carboxy prothrombin in liver cancer. Drug News Perspect. 2010;23:498–508. doi: 10.1358/dnp.2010.23.8.1444236. [DOI] [PubMed] [Google Scholar]
- 23.Bertino G, Ardiri AM, Calvagno GS, Boemi PM. In chronic viral hepatitis without malignancy, abnormal serum carbohydrate 19-9 antigen levels are associated with liver disease severity and are related to different viral aetiology. Dig Liver Dis. 2010;42:458–459. doi: 10.1016/j.dld.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Bertino G, Ardiri AM, Santonocito MM, Boemi PM. Some patients with HCC haven’t abnornormal des-gamma-carboxy prothrombin and alpha-fetoprotein levels. Panminerva Med. 2009;51:133–134. [PubMed] [Google Scholar]
- 25.Bertino G, Ardiri AM, Boemi PM, Ierna D, Interlandi D, Caruso L, Minona E, Trovato MA, Vicari S, Li Destri G, et al. A study about mechanisms of des-gamma-carboxy prothrombin’s production in hepatocellular carcinoma. Panminerva Med. 2008;50:221–226. [PubMed] [Google Scholar]
- 26.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 27.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 28.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 29.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, Ascione A, Santantonio T, Piccinino F, Andreone P, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 30.Zeuzem S. Heterogeneous virologic response rates to interferon-based therapy in patients with chronic hepatitis C: who responds less well? Ann Intern Med. 2004;140:370–381. doi: 10.7326/0003-4819-140-5-200403020-00033. [DOI] [PubMed] [Google Scholar]
- 31.Marcellin P, Boyer N, Gervais A, Martinot M, Pouteau M, Castelnau C, Kilani A, Areias J, Auperin A, Benhamou JP, et al. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-alpha therapy. Ann Intern Med. 1997;127:875–881. doi: 10.7326/0003-4819-127-10-199711150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Poynard T, Moussalli J, Munteanu M, Thabut D, Lebray P, Rudler M, Ngo Y, Thibault V, Mkada H, Charlotte F, et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol. 2013;59:675–683. doi: 10.1016/j.jhep.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Maylin S, Martinot-Peignoux M, Moucari R, Boyer N, Ripault MP, Cazals-Hatem D, Giuily N, Castelnau C, Cardoso AC, Asselah T, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology. 2008;135:821–829. doi: 10.1053/j.gastro.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 34.Toccaceli F, Laghi V, Capurso L, Koch M, Sereno S, Scuderi M. Long-term liver histology improvement in patients with chronic hepatitis C and sustained response to interferon. J Viral Hepat. 2003;10:126–133. doi: 10.1046/j.1365-2893.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 35.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 36.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 37.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516.e1. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Fontana RJ, Sanyal AJ, Ghany MG, Lee WM, Reid AE, Naishadham D, Everson GT, Kahn JA, Di Bisceglie AM, Szabo G, et al. Factors that determine the development and progression of gastroesophageal varices in patients with chronic hepatitis C. Gastroenterology. 2010;138:2321–2331, 2331.e1-2. doi: 10.1053/j.gastro.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, Manns MP, Hansen BE, Schalm SW, Janssen HL. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–684. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 40.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 41.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 42.Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 44.Innes HA, Hutchinson SJ, Allen S, Bhattacharyya D, Bramley P, Carman B, Delahooke TE, Dillon JF, Goldberg DJ, Kennedy N, et al. Ranking predictors of a sustained viral response for patients with chronic hepatitis C treated with pegylated interferon and ribavirin in Scotland. Eur J Gastroenterol Hepatol. 2012;24:646–655. doi: 10.1097/MEG.0b013e32835201a4. [DOI] [PubMed] [Google Scholar]
- 45.Bräu N. Evaluation of the hepatitis C virus-infected patient: the initial encounter. Clin Infect Dis. 2013;56:853–860. doi: 10.1093/cid/cis957. [DOI] [PubMed] [Google Scholar]
- 46.Trembling PM, Tanwar S, Rosenberg WM, Dusheiko GM. Treatment decisions and contemporary versus pending treatments for hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10:713–728. doi: 10.1038/nrgastro.2013.163. [DOI] [PubMed] [Google Scholar]
- 47.Dore GJ. The changing therapeutic landscape for hepatitis C. Med J Aust. 2012;196:629–632. doi: 10.5694/mja11.11531. [DOI] [PubMed] [Google Scholar]
- 48.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 49.Bertino G, Ardiri A, Boemi PM, Calvagno GS, Ruggeri IM, Speranza A, Santonocito MM, Ierna D, Bruno CM, Valenti M, et al. Epoetin alpha improves the response to antiviral treatment in HCV-related chronic hepatitis. Eur J Clin Pharmacol. 2010;66:1055–1063. doi: 10.1007/s00228-010-0868-4. [DOI] [PubMed] [Google Scholar]
- 50.Neri S, Bertino G, Petralia A, Giancarlo C, Rizzotto A, Calvagno GS, Mauceri B, Abate G, Boemi P, Di Pino A, et al. A multidisciplinary therapeutic approach for reducing the risk of psychiatric side effects in patients with chronic hepatitis C treated with pegylated interferon α and ribavirin. J Clin Gastroenterol. 2010;44:e210–e217. doi: 10.1097/MCG.0b013e3181d88af5. [DOI] [PubMed] [Google Scholar]
- 51.Neri S, Pulvirenti D, Bertino G. Psychiatric symptoms induced by antiviral therapy in chronic hepatitis C: comparison between interferon-alpha-2a and interferon-alpha-2b. Clin Drug Investig. 2006;26:655–662. doi: 10.2165/00044011-200626110-00005. [DOI] [PubMed] [Google Scholar]
- 52.Malaguarnera M, Vacante M, Bertino G, Neri S, Malaguarnera M, Gargante MP, Motta M, Lupo L, Chisari G, Bruno CM, et al. The supplementation of acetyl-L-carnitine decreases fatigue and increases quality of life in patients with hepatitis C treated with pegylated interferon-α 2b plus ribavirin. J Interferon Cytokine Res. 2011;31:653–659. doi: 10.1089/jir.2011.0010. [DOI] [PubMed] [Google Scholar]
- 53.Malaguarnera M, Vacante M, Giordano M, Motta M, Bertino G, Pennisi M, Neri S, Malaguarnera M, Li Volti G, Galvano F. L-carnitine supplementation improves hematological pattern in patients affected by HCV treated with Peg interferon-α 2b plus ribavirin. World J Gastroenterol. 2011;17:4414–4420. doi: 10.3748/wjg.v17.i39.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malaguarnera G, Pennisi M, Gagliano C, Vacante M, Malaguarnera M, Salomone S, Drago F, Bertino G, Caraci F, Nunnari G, et al. Acetyl-L-Carnitine Supplementation During HCV Therapy With Pegylated Interferon-α 2b Plus Ribavirin: Effect on Work Performance; A Randomized Clinical Trial. Hepat Mon. 2014;14:e11608. doi: 10.5812/hepatmon.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Chassey B, Navratil V, Tafforeau L, Hiet MS, Aublin-Gex A, Agaugué S, Meiffren G, Pradezynski F, Faria BF, Chantier T, et al. Hepatitis C virus infection protein network. Mol Syst Biol. 2008;4:230. doi: 10.1038/msb.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q, Brass AL, Ng A, Hu Z, Xavier RJ, Liang TJ, Elledge SJ. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc Natl Acad Sci USA. 2009;106:16410–16415. doi: 10.1073/pnas.0907439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, Landthaler M, Landgraf P, Kan S, Lindenbach BD, et al. Cellular cofactors affecting hepatitis C virus infection and replication. Proc Natl Acad Sci USA. 2007;104:12884–12889. doi: 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tai AW, Benita Y, Peng LF, Kim SS, Sakamoto N, Xavier RJ, Chung RT. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe. 2009;5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 61.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 62.Ploss A, Evans MJ. Hepatitis C virus host cell entry. Curr Opin Virol. 2012;2:14–19. doi: 10.1016/j.coviro.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartenschlager R, Penin F, Lohmann V, André P. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 2011;19:95–103. doi: 10.1016/j.tim.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Friedel CC, Haas J. Virus-host interactomes and global models of virus-infected cells. Trends Microbiol. 2011;19:501–508. doi: 10.1016/j.tim.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Katze MG, Fornek JL, Palermo RE, Walters KA, Korth MJ. Innate immune modulation by RNA viruses: emerging insights from functional genomics. Nat Rev Immunol. 2008;8:644–654. doi: 10.1038/nri2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panda D, Cherry S. Cell-based genomic screening: elucidating virus-host interactions. Curr Opin Virol. 2012;2:784–792. doi: 10.1016/j.coviro.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berger KL, Cooper JD, Heaton NS, Yoon R, Oakland TE, Jordan TX, Mateu G, Grakoui A, Randall G. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc Natl Acad Sci USA. 2009;106:7577–7582. doi: 10.1073/pnas.0902693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reiss S, Rebhan I, Backes P, Romero-Brey I, Erfle H, Matula P, Kaderali L, Poenisch M, Blankenburg H, Hiet MS, et al. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe. 2011;9:32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 71.Mangia A, Cenderello G, Orlandini A, Piazzolla V, Picciotto A, Zuin M, Ciancio A, Brancaccio G, Forte P, Carretta V, et al. Individualized treatment of genotype 1 naïve patients: an Italian multicenter field practice experience. PLoS One. 2014;9:e110284. doi: 10.1371/journal.pone.0110284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asselah T, Marcellin P. Interferon free therapy with direct acting antivirals for HCV. Liver Int. 2013;33 Suppl 1:93–104. doi: 10.1111/liv.12076. [DOI] [PubMed] [Google Scholar]
- 73.Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, Bernstein DE, Afdhal N, Vierling JM, Gordon SC, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100–2107. doi: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed] [Google Scholar]
- 74.Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, Marcellin P, Manns M, Nikitin I, Poordad F, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918–1929. doi: 10.1002/hep.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poordad F, Dieterich D. Treating hepatitis C: current standard of care and emerging direct-acting antiviral agents. J Viral Hepat. 2012;19:449–464. doi: 10.1111/j.1365-2893.2012.01617.x. [DOI] [PubMed] [Google Scholar]
- 76.Pockros PJ. New direct-acting antivirals in the development for hepatitis C virus infection. Therap Adv Gastroenterol. 2010;3:191–202. doi: 10.1177/1756283X10363055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manzano-Robleda Mdel C, Ornelas-Arroyo V, Barrientos-Gutiérrez T, Méndez-Sánchez N, Uribe M, Chávez-Tapia NC. Boceprevir and telaprevir for chronic genotype 1 hepatitis C virus infection. A systematic review and meta-analysis. Ann Hepatol. 2015;14:46–57. [PubMed] [Google Scholar]
- 78.Pawlotsky JM. Treatment failure and resistance with direct-acting antiviral drugs against hepatitis C virus. Hepatology. 2011;53:1742–1751. doi: 10.1002/hep.24262. [DOI] [PubMed] [Google Scholar]
- 79.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 82.Kiser JJ, Burton JR, Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10:596–606. doi: 10.1038/nrgastro.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hunt D, Pockros P. What are the promising new therapies in the field of chronic hepatitis C after the first-generation direct-acting antivirals? Curr Gastroenterol Rep. 2013;15:303. doi: 10.1007/s11894-012-0303-3. [DOI] [PubMed] [Google Scholar]
- 84.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 85.Sanford M. Simeprevir: a review of its use in patients with chronic hepatitis C virus infection. Drugs. 2015;75:183–196. doi: 10.1007/s40265-014-0341-2. [DOI] [PubMed] [Google Scholar]
- 86.Williams JA, Ring BJ, Cantrell VE, Jones DR, Eckstein J, Ruterbories K, Hamman MA, Hall SD, Wrighton SA. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos. 2002;30:883–891. doi: 10.1124/dmd.30.8.883. [DOI] [PubMed] [Google Scholar]
- 87.Perumpail RB, Wong RJ, Ha LD, Pham EA, Wang U, Luong H, Kumari R, Daugherty TJ, Higgins JP, Younossi ZM, et al. Sofosbuvir and simeprevir combination therapy in the setting of liver transplantation and hemodialysis. Transpl Infect Dis. 2015;17:275–278. doi: 10.1111/tid.12348. [DOI] [PubMed] [Google Scholar]
- 88.Sulkowski M, Jacobson IM, Ghalib R, Rodriguez-Torres M, Younossi Z, Corregidor A, Fevery B, Callewaert K, Symonds W, De La Rosa G, et al. O7 Once-daily simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in HCV genotype 1 prior null responders with Metavir F0-2: COSMOS study subgroup analysis. J Hepatol. 2014;60:S4. [Google Scholar]
- 89.Lawitz E, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, Sulkowski MS, DeJesus E, Pearlman B, Rabinovitz M, Gitlin M, et al. Simeprevir plus sofosbuvir with/without ribavirin in HCV genotype 1 prior null-responder/treatment-naive patients (COSMOS study): primary endpoint (SVR12) results in patients with METAVIR F3-4 (Cohort 2) Abstract presented at: EASL - The International Liver Congress. 49th Annual Meeting of the European Association for the Study of the Liver. London (UK), 2014. [Accessed; 2014. p. Jun 25]. Available from: http://www.natap.org/2014/EASL/EASL_26.htm. [Google Scholar]
- 90.Foster GR, Strasser S, Christensen C, Ma J, Bekele BN, Brainard DM, Symonds WT, McHutchison JG, Conway B, Crespo I, et al. O66 Sofosbuvir-based regimens are associated with high SVR rates across genotypes and among patients with multiple negative predictive factors. J Hepatol. 2014;60:S27. [Google Scholar]
- 91.Pearlman BL, Ehleben C, Perrys M. The combination of simeprevir and sofosbuvir is more effective than that of peginterferon, ribavirin, and sofosbuvir for patients with hepatitis C-related Child’s class A cirrhosis. Gastroenterology. 2015;148:762–770.e2; quiz e11-12. doi: 10.1053/j.gastro.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 92.Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–413. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 93.Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414–426. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 94.Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, Horban A, Brown A, Peeters M, Lenz O, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014;146:1669–79.e3. doi: 10.1053/j.gastro.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 95.Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C, et al. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219–227. doi: 10.1016/j.jhep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Izumi N, Hayashi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C, et al. Once-daily simeprevir with peginterferon and ribavirin for treatment-experienced HCV genotype 1-infected patients in Japan: the CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 2014;49:941–953. doi: 10.1007/s00535-014-0949-8. [DOI] [PubMed] [Google Scholar]
- 97.Kumada H, Hayashi N, Izumi N, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Rito K, Komada Y, Seto C, et al. Simeprevir (TMC435) once daily with peginterferon-α-2b and ribavirin in patients with genotype 1 hepatitis C virus infection: The CONCERTO-4 study. Hepatol Res. 2015;45:501–513. doi: 10.1111/hepr.12375. [DOI] [PubMed] [Google Scholar]
- 98.Sulkowski MS, Asselah T, Lalezari J, Ferenci P, Fainboim H, Leggett B, Bessone F, Mauss S, Heo J, Datsenko Y, et al. Faldaprevir combined with pegylated interferon alfa-2a and ribavirin in treatment-naïve patients with chronic genotype 1 HCV: SILEN-C1 trial. Hepatology. 2013;57:2143–2154. doi: 10.1002/hep.26276. [DOI] [PubMed] [Google Scholar]
- 99.Nishiguchi S, Sakai Y, Kuboki M, Tsunematsu S, Urano Y, Sakamoto W, Tsuda Y, Steinmann G, Omata M. Safety and efficacy of faldaprevir with pegylated interferon alfa-2a and ribavirin in Japanese patients with chronic genotype-1 hepatitis C infection. Liver Int. 2014;34:78–88. doi: 10.1111/liv.12254. [DOI] [PubMed] [Google Scholar]
- 100.Zeuzem S, Asselah T, Angus P, Zarski JP, Larrey D, Müllhaupt B, Gane E, Schuchmann M, Lohse A, Pol S, et al. Efficacy of the protease inhibitor BI 201335, polymerase inhibitor BI 207127, and ribavirin in patients with chronic HCV infection. Gastroenterology. 2011;141:2047–2055; quiz e14. doi: 10.1053/j.gastro.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 101.Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse AW, Müllhaupt B, Schuchmann M, Bourlière M, Buti M, Roberts SK, et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369:630–639. doi: 10.1056/NEJMoa1213557. [DOI] [PubMed] [Google Scholar]
- 102.Kanda T, Yokosuka O, Omata M. Antiviral therapy for “difficult-to-treat” hepatitis C virus-infected patients. Chin Med J (Engl) 2013;126:4568–4574. [PubMed] [Google Scholar]
- 103.Hézode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer-Stepniewska K, Marcellin P, Hall C, Schnell G, Pilot-Matias T, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet. 2015;385:2502–2509. doi: 10.1016/S0140-6736(15)60159-3. [DOI] [PubMed] [Google Scholar]
- 104.Tellinghuisen TL, Foss KL, Treadaway J. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 2008;4:e1000032. doi: 10.1371/journal.ppat.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ivachtchenko AV, Mitkin OD, Yamanushkin PM, Kuznetsova IV, Bulanova EA, Shevkun NA, Koryakova AG, Karapetian RN, Bichko VV, Trifelenkov AS, et al. Discovery of novel highly potent hepatitis C virus NS5A inhibitor (AV4025) J Med Chem. 2014;57:7716–7730. doi: 10.1021/jm500951r. [DOI] [PubMed] [Google Scholar]
- 106.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dore GJ, Lawitz E, H’ezode C, Shafran S, Ramji A, Tatum H, Taliani G, Tran A, Brunetto M, Zaltron S, et al. Daclatasvir combined with peginterferon alfa-2A and ribavirin for 12 or 16 weeks in patients with HCV genotype 2 or 3 infection: COMMAND GT2/3 STUDY. J Hepatol. 2013;58(suppl 1):S570–571. [Google Scholar]
- 108.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 109.Lawitz EJ, Gruener D, Hill JM, Marbury T, Moorehead L, Mathias A, Cheng G, Link JO, Wong KA, Mo H, et al. A phase 1, randomized, placebo-controlled, 3-day, dose-ranging study of GS-5885, an NS5A inhibitor, in patients with genotype 1 hepatitis C. J Hepatol. 2012;57:24–31. doi: 10.1016/j.jhep.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 110.Poordad F, Lawitz E, DeJesus E, Kowdley KN, Gaultier I, Cohen DE, Xie W, Larsen L, Pilot-Matias T, Koev G, et al. 1206 ABT-072 or ABT-333 combined with pegylated interferon/ribavirin after 3-day monotherapy in HCV genotype 1 (GT1)- infected treatment-naive subjects: 12-week sustained virologic response (SVR12) and safety results. J Hepatol. 2012;56 Suppl 2:S478. [Google Scholar]
- 111.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 112.Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, Sulkowski MS, Wedemeyer H, Tam E, Desmond P, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604–1614. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 113.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 114.Khatri A, Menon RM, Marbury TC, Lawitz EJ, Podsadecki TJ, Mullally VM, Ding B, Awni WM, Bernstein BM, Dutta S. Pharmacokinetics and safety of co-administered paritaprevir plus ritonavir, ombitasvir, and dasabuvir in hepatic impairment. J Hepatol. 2015;63:805–812. doi: 10.1016/j.jhep.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 115.Jacobson IM, Marcellin P, Mangia A, Kwo PY, Foster G, Buti M, Brau N, Muir AJ, Yang J, Mo H, Ding X, Pang P, Symonds WT, McHutchison JG, Zeuzem S, Afdhal NH. Muir AJ, Yang JC, Mo H, Ding X, Pang P, Symonds WT, McHutchisonJG, Zeuzem S, Afdhal NH. Tu2038 All Oral Fixed-dose Combination Sofosbuvir/Ledipasvir With or Without Ribavirin for 12 or 24 Weeks in Treatment-Naive Genotype 1 HCV-Infected Patients: The Phase 3 ION-1 Study. J Hepatol. 2014;Supplement 60:S523–S524. [Google Scholar]
- 116.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 117.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 118.Koch U, Narjes F. Recent progress in the development of inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. Curr Top Med Chem. 2007;7:1302–1329. doi: 10.2174/156802607781212211. [DOI] [PubMed] [Google Scholar]
- 119.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 120.Kirby BJ, Symonds WT, Kearney BP, Mathias AA. Pharmacokinetic, Pharmacodynamic, and Drug-Interaction Profile of the Hepatitis C Virus NS5B Polymerase Inhibitor Sofosbuvir. Clin Pharmacokinet. 2015;54:677–690. doi: 10.1007/s40262-015-0261-7. [DOI] [PubMed] [Google Scholar]
- 121.Alexopoulou A, Karayiannis P. Interferon-based combination treatment for chronic hepatitis C in the era of direct acting antivirals. Ann Gastroenterol. 2015;28:55–65. [PMC free article] [PubMed] [Google Scholar]
- 122.Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, Hindes RG, Berrey MM. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 123.Rodriguez-Torres M, Lawitz E, Kowdley KV, Nelson DR, Dejesus E, McHutchison JG, Cornpropst MT, Mader M, Albanis E, Jiang D, et al. Sofosbuvir (GS-7977) plus peginterferon/ribavirin in treatment-naïve patients with HCV genotype 1: a randomized, 28-day, dose-ranging trial. J Hepatol. 2013;58:663–668. doi: 10.1016/j.jhep.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 124.Lawitz E, Poordad F, Brainard DM, Hyland RH, An D, Dvory-Sobol H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis. Hepatology. 2015;61:769–775. doi: 10.1002/hep.27567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 126.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 127.Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 128.Ruane PJ, Ain D, Stryker R, Meshrekey R, Soliman M, Wolfe PR, Riad J, Mikhail S, Kersey K, Jiang D, et al. Sofosbuvir plus ribavirin for the treatment of chronic genotype 4 hepatitis C virus infection in patients of Egyptian ancestry. J Hepatol. 2015;62:1040–1046. doi: 10.1016/j.jhep.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 129.Feld JJ. Interferon-free strategies with a nucleoside/nucleotide analogue. Semin Liver Dis. 2014;34:37–46. doi: 10.1055/s-0034-1371009. [DOI] [PubMed] [Google Scholar]
- 130.Au JS, Pockros PJ. Novel therapeutic approaches for hepatitis C. Clin Pharmacol Ther. 2014;95:78–88. doi: 10.1038/clpt.2013.206. [DOI] [PubMed] [Google Scholar]
- 131.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 132.Asselah T, Bruno S, Craxi A. HCV cirrhosis at the edge of decompensation: will paritaprevir with ritonavir, ombitasvir, dasabuvir, and ribavirin solve the need for treatment? J Hepatol. 2014;61:1430–1433. doi: 10.1016/j.jhep.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 133.Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, Müllhaupt B, Horsmans Y, Weiland O, Reesink HW, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359–365.e1. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 134.Binder M, Quinkert D, Bochkarova O, Klein R, Kezmic N, Bartenschlager R, Lohmann V. Identification of determinants involved in initiation of hepatitis C virus RNA synthesis by using intergenotypic replicase chimeras. J Virol. 2007;81:5270–5283. doi: 10.1128/JVI.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paul D, Romero-Brey I, Gouttenoire J, Stoitsova S, Krijnse-Locker J, Moradpour D, Bartenschlager R. NS4B self-interaction through conserved C-terminal elements is required for the establishment of functional hepatitis C virus replication complexes. J Virol. 2011;85:6963–6976. doi: 10.1128/JVI.00502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Flisiak R, Feinman SV, Jablkowski M, Horban A, Kryczka W, Pawlowska M, Heathcote JE, Mazzella G, Vandelli C, Nicolas-Métral V, et al. The cyclophilin inhibitor Debio 025 combined with PEG IFNalpha2a significantly reduces viral load in treatment-naïve hepatitis C patients. Hepatology. 2009;49:1460–1468. doi: 10.1002/hep.22835. [DOI] [PubMed] [Google Scholar]
- 137.Zeuzem S, Flisiak R, Vierling JM, Mazur W, Mazzella G, Thongsawat S, Abdurakhmanov D, Van Kính N, Calistru P, Heo J, et al. Randomised clinical trial: alisporivir combined with peginterferon and ribavirin in treatment-naïve patients with chronic HCV genotype 1 infection (ESSENTIAL II) Aliment Pharmacol Ther. 2015;42:829–844. doi: 10.1111/apt.13342. [DOI] [PubMed] [Google Scholar]
- 138.European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 139.AISF Guidelines e Position Papers. 2015. Available from: http://www.webaisf.org/pubblicazioni/guidelines-e-position-papers.aspx.
- 140.Shahid I, ALMalki WH, Hafeez MH, Hassan S. Hepatitis C virus infection treatment: An era of game changer direct acting antivirals and novel treatment strategies. Crit Rev Microbiol. 2014:1–13. doi: 10.3109/1040841X.2014.970123. [DOI] [PubMed] [Google Scholar]
- 141.Petta S, Craxì A. Current and future HCV therapy: do we still need other anti-HCV drugs? Liver Int. 2015;35 Suppl 1:4–10. doi: 10.1111/liv.12714. [DOI] [PubMed] [Google Scholar]


