Abstract
When the genes encoding NF-κB subunits were first isolated, their homology to the previously identified c-Rel proto-oncogene and its viral homologue v-Rel was clear. This provided the first indication that these transcription factors also had a role in cancer. Because of its homology to v-Rel, which transforms chicken B cells together with the important role c-Rel can have as a regulator of B- and T-cell proliferation, most attention has focussed on its role in B-cell lymphomas, where the REL gene is frequently amplified. However, a growing number of reports now indicate that c-Rel has important functions in many solid tumours, although studies in mice suggest it may not always function as an oncogene. Moreover, c-Rel is a critical regulator of fibrosis, which provides an environment for tumour development in many settings. Overall, c-Rel is emerging as a complex regulator of tumorigenesis, and there is still much to learn about its functions in human malignancies and the response to cancer therapies.
Keywords: c-Rel, NF-κB, cancer, lymphoma, fibrosis
The NF-κB subunits RelA/p65, RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2) comprise a family of dimeric transcription factors with both common and distinct biological functions. NF-κB complexes are present in all cells but are generally held in an inactive form bound to a variety of inhibitory proteins, termed inhibitors of NF-κB (IκBs) (Perkins, 2012). IκBs possess a series of ankyrin repeat motifs that are also found in p100 and p105, the precursor forms of p52 and p50, which allows them to function as IκB-like NF-κB inhibitors. A wide range of NF-κB inducers, including inflammatory cytokines, cell stresses such as DNA damage, immune receptor engagement, bacterial products and viral proteins, can activate the IκB kinase (IKK) complex, resulting in IκB phosphorylation, degradation and the release of active NF-κB dimers (Perkins, 2012).
NF-κB activity is usually kept under tight control, with a variety of negative feedback loops, such as resynthesis of IκBα or expression of the ubiquitin editor A20, acting to limit the magnitude and duration of a typical NF-κB response (Perkins, 2012). However, in many human illnesses, NF-κB is aberrantly active and either causes or contributes to the pathology of the disease (Karin, 2009; Perkins, 2012; Bradford and Baldwin, 2014). This is particularly true with inflammatory diseases, where NF-κB-driven expression of genes encoding inflammatory cytokines such as tumour necrosis factor (TNF) α or interleukin 6 (IL-6), drives disease progression (Karin, 2009). Its critical role in the inflammatory phenotype allows NF-κB to act as a promoter of inflammation-associated cancers (Karin, 2009). However, NF-κB can also contribute to cancer in many others ways. Aberrant NF-κB activity in cancer only rarely results from direct mutation of the NF-κB subunits but arises either through mutation of upstream regulators (e.g., Ras, Myd88 or the B-cell receptor) leading to constitutive IKK activity or via effects of the tumour microenvironment (Bradford and Baldwin, 2014). Constitutive activation of NF-κB in tumour cells can activate many genes that regulate cancer-related cellular processes, including apoptosis, proliferation, angiogenesis and metastasis (Bradford and Baldwin, 2014). Thus NF-κB actively contributes to many of the ‘hallmarks of cancer', resulting in more rapid disease progression, increased metastatic potential, a higher proportion of tumour recurrence and therapeutic resistance (Bradford and Baldwin, 2014).
The NF-κB subunit c-Rel
The NF-κB subunit c-Rel was first identified as the cellular homologue of the avian Rev-T retroviral oncoprotein v-Rel (Gilmore and Gerondakis, 2011). v-Rel causes lymphoma in birds and c-Rel is the only NF-κB family member that can also transform chicken lymphoid cells in vitro (Gilmore and Gerondakis, 2011).
c-Rel, which is encoded by the REL gene in humans, has important roles in many aspects of lymphoid cell function (Gilmore and Gerondakis, 2011). c-Rel is expressed in mammalian B cells regardless of developmental stage, although c-Rel levels increase during B-cell development (Grumont and Gerondakis, 1994; Liou et al, 1994). c-rel knockout mice develop normally with no effects on haematopoietic cell development but do display several immunological defects, which include reduced mature B- and T-cell proliferation and activation in response to mitogenic stimuli, abnormal germinal centre formation and reduced numbers of marginal zone B cells (Gilmore and Gerondakis, 2011). In addition to cancer (see below), c-Rel has a key role in a number of human diseases, such as inflammatory bowel disease and rheumatoid arthritis together with cardiac and skin fibrosis (Wang et al, 2008; Gilmore and Gerondakis, 2011; Gaspar-Pereira et al, 2012; Fullard et al, 2013).
The most common isoform of human c-Rel is 587 amino acids. Overall, c-Rel has a similar structure to the RelA and RelB members of the NF-κB family, with an N-terminal DNA-binding and dimerisation domain termed the Rel homology domain and a C-terminal transcriptional activation domain (Figure 1A). c-Rel is most commonly found as a dimer with the p50 or RelA NF-κB subunits but other combinations can occur (Gilmore and Gerondakis, 2011). c-Rel has a slightly different DNA-binding specificity compared with other NF-κB subunits (Sanjabi et al, 2005), but ChIP-Seq analysis did not reveal any significant differences in DNA-binding site preference in EBV-transformed B cells (Zhao et al, 2014). Although posttranslational modifications can have profound regulatory effects on other NF-κB subunits, relatively little is known about how such modifications contribute to c-Rel activity and function (Gilmore and Gerondakis, 2011). c-Rel is generally described as an activator of transcription that can function to establish a permissive chromatin environment at NF-κB-regulated promoters (van Essen et al, 2010), but whether this varies in different cellular contexts has not been thoroughly explored. Similar to RelA, c-Rel is a regulator of antiapoptotic genes such as Bcl-xL (Gilmore and Gerondakis, 2011). However c-Rel also regulates other cellular functions. For example, it can induce the expression of CLSPN, a component of the checkpoint kinase Chk1 signalling pathway in the human U2OS osteosarcoma cell line (Kenneth et al, 2010). c-Rel can also regulate the expression of EZH2, a histone methyl transferase frequently upregulated in many cancers, in both primary murine B and T cells as well as human leukaemia and multiple myeloma cell lines (Neo et al, 2014).
Figure 1.
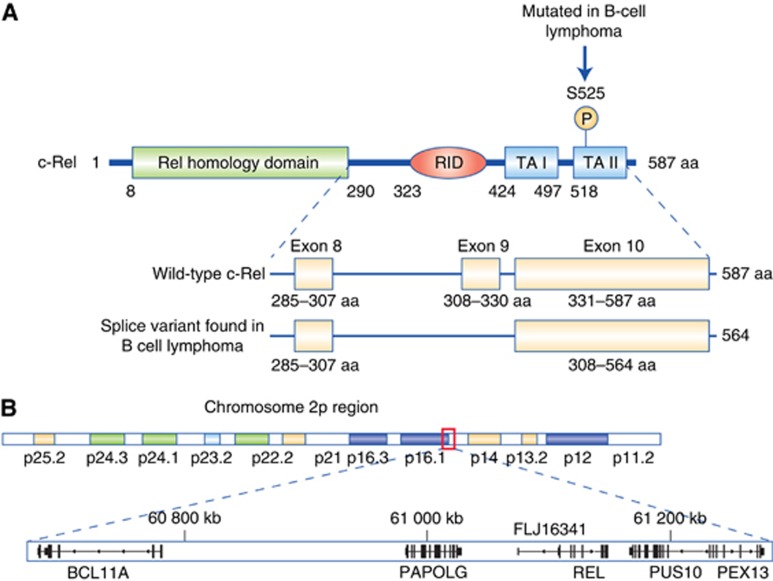
Structure and genomic location of human c-Rel. (A) Schematic diagram showing the structure of c-Rel and amino-acid positions of different regulatory motifs. A putative IKK phosphorylation site found mutated in some B-cell lymphoma patient samples together with a splice variant that removes 23 amino acids from the REL inhibitory domain (RID) also found in some B-cell lymphoma cell lines and patient samples are shown. TA I and TA II are c-Rel transcriptional activation domains. Adapted from (Leeman et al, 2008). (B) Diagram demonstrating the close proximity of the REL gene to the BCL11A proto-oncogene and the pseudouridine kinase PUS10 on human chromosome 2. Both genes therefore have the potential to be co-amplified in human cancers. Figure compiled using the Integrated Genomics Viewer and the hg19 build of the human genome.
The role of c-Rel in B-cell lymphoma
NF-κB has a key role in several types of lymphoma, with many B-cell lymphomas dependent on mutations that activate the NF-κB pathway (Lim et al, 2012). Activation of NF-κB can arise in B-cell lymphoma owing to mutations in upstream regulatory genes such as TNFAIP3, CARD11, MYD88, NFKBIA and CD79A/B, chromosomal translocations such as t(11;18)(q21;q21)/API-MALT1 or to signalling through cell surface receptors, such as CD40 and the EBV latent membrane protein 1 (Compagno et al, 2009; Hamoudi et al, 2010; Lim et al, 2012). Many diffuse large B-cell lymphomas (DLBCL), including almost all activated B-cell-like (ABC-DLBCL), primary mediastinal large B-cell lymphomas (PMBL) and a subset of germinal centre B-cell-like DLBCL (GCB-DLBCL), in addition to classical Hodgkin lymphoma (CHL) and MALT lymphomas, possess distinct NF-κB target gene signatures thought to promote lymphoma progression and survival (Compagno et al, 2009; Hamoudi et al, 2010; Lim et al, 2012).
Despite this, the contribution of individual NF-κB proteins to lymphomagenesis is poorly understood. However, evidence suggests an important role for c-Rel. Genomic and cytogenetic studies of human lymphomas have identified amplification of the region of chromosome 2p13 where the REL gene is located (Table 1). Amplifications and gains of REL, often associated with elevated levels of nuclear c-Rel protein, have been detected in approximately 50% of CHL and at lower levels in other types of B-cell lymphoma, while c-Rel nuclear localisation is associated with both ABC- and GCB-DLBCL (Barth et al, 2001, 2003; Martin-Subero et al, 2002; Weniger et al, 2007; Lenz et al, 2008; Curry et al, 2009; Enciso-Mora et al, 2010; Gilmore and Gerondakis, 2011; Lim et al, 2012; Li et al, 2015). Interestingly, in a recent study of 460 DLBCL patients, the 26% scoring positive for nuclear c-Rel exhibited higher levels of clinical features, such as extra nodal disease (Li et al, 2015). In this study, nuclear c-Rel did not correlate with overall survival in the whole population of either ABC- or GCB-DLBCL patients but was a significant indicator of negative outcome in distinct subsets of patients. For example, in all patients with mutant p53 tumour suppressor or in ABC-DLBCL patients with low levels of the antiapoptotic protein Bcl-2, c-Rel positivity was associated with poor overall survival (Li et al, 2015).
Table 1. List of different cancers where a role for c-Rel is known or implied.
| Disease | Alteration | Biological effect | References |
|---|---|---|---|
|
c-Rel in lymphoid cancers | |||
| Primary mediastinal B-cell lymphoma (PMBL) | Amplification of REL locus | Correlates with increased REL mRNA, nuclear c-Rel and NF-κB activity. Use of IKKβ inhibitor induced cell death in cell lines | Weniger et al, 2007 |
| Classical Hodgkins lymphoma (CHL) | Gain of 2p | Correlates with nuclear c-Rel staining and constitutive NF-κB activity | Joos et al, 2002; Martin-Subero et al, 2002; Barth et al, 2003; Enciso-Mora et al, 2010 |
| Germinal centre B-cell diffuse large B-cell lymphoma (GCB-DLBCL) | Amplification of REL locus and nuclear localised c-Rel | Not clear. Some studies indicate poor overall survival associated with c-Rel positivity but others do not | Lenz et al, 2008; Curry et al, 2009; Li et al, 2015 |
| Activated B-cell diffuse large B-cell lymphoma (ABC-DLBCL) | Distinct NF-κB gene signature and nuclear localised c-Rel | Lymphomas are dependent on this gene signature for proliferation and survival. c-Rel positivity associated with poor overall survival in some disease subtypes | Lenz et al, 2008; Campagno et al, 2009; Curry et al, 2009; Li et al, 2015 |
| Marginal zone lymphoma | Increased REL mRNA expression | Shorter overall survival correlates with increased REL and other NF-κB gene expression | Barth et al, 2001 |
| Adult T-cell leukaemia/lymphoma (ATLL) | Increased c-Rel expression | Increased expression confers resistance to therapy | Ramos et al, 2007 |
|
c-Rel in solid tumours | |||
| Breast cancer | Increased REL mRNA, high nuclear c-Rel expression | c-Rel expression shown to induce mammary tumours in murine breast cancer models | Cogswell et al, 2000; Romieu-Mourez et al, 2003 |
| Colitis-associated adenoma | Loss of c-Rel in mice | Increased disease susceptibility and tumour burden | Burkitt et al, 2015 |
| Gastric cancer | Loss of c-Rel in mice | c-Rel−/− mice developed lesions similar to low-grade MALT lymphomas when exposed to pathogens | Burkitt et al, 2013 |
| Pancreatic cancer | Increased c-Rel expression in cell lines | c-Rel acts to mediate TRAIL-induced apoptosis by controlling tumour-promoting genes, such as NFATc2 | Geismann et al, 2014 |
| Head and neck cancer | Amplification and nuclear localisation of c-Rel | Role for c-Rel in cancers expressing mutant p53 where it inactivates p73 | Lu et al, 2011 |
|
Other c-Rel-regulated pathways affecting tumorigenesis | |||
| Graft versus host disease (GVHD) | c-Rel expression drives T-cell response | Homing to GVHD organs reduced in c-Rel−/− T-cells. c-Rel inhibition reduced T-cell activation without compromising antitumour activity | Yu et al, 2013 |
| Fibrosis | Loss of c-Rel in mice | Potentiates fibrosis in multiple organs via the regulation of gene expression | Gieling et al, 2010; Gaspar-Pereira et al, 2012; Fullard et al, 2013 |
Abbreviations: MALT=mucosa-associated lymphoid tissue; IKKβ=IκB kinase β; NFATc2=nuclear factor of activated t-cells, cytoplasmic, calcineurin-dependent 2; NF-κB=nuclear factor κB; TRAIL=tumour necrosis factor-related apoptosis-inducing ligand. Please note that it was not possible to list all the primary literature here and a more comprehensive list of references, together with haematological malignancies associated with c-Rel, can be found in Gilmore and Gerondakis (2011) (n=112) and Lim et al. (2012) (n=153).
Although the REL gene is frequently amplified in PBML and GCB-DLBCL, the association of this event with c-Rel activity and an NF-κB gene signature has been questioned (Feuerhake et al, 2005; Lenz et al, 2008; Li et al, 2015). For example, in 460 DLBCL patients, there was a poor correlation between c-Rel nuclear localisation and mRNA levels in both ABC and GCB forms of the disease (Li et al, 2015). Furthermore, a potential complication to our understanding of REL gene amplification is the close proximity of another myeloid and B-cell proto-oncogene, BCL11A, on human chromosome 2 (Figure 1B). Indeed, both the REL and BCL11A genes have been shown to be co-amplified in CHL (Martin-Subero et al, 2002), while the pseudouridine kinase PUS10 that is also in close proximity to REL (Figure 1B) is the most significantly upregulated gene in this region in GCB-DLBCL (Lenz et al, 2008). However, whether BCL11A or PUS10 contribute to any of the pathological phenotypes associated with c-Rel is not known.
An additional potential complication in interpreting this data that has not generally been considered is the identification of an alternatively spliced hyperactive form of c-Rel (Leeman et al, 2008). This isoform lacks a central inhibitory domain and is preferentially expressed in a variety of lymphoma cell lines and DLBCL patient cells (Leeman et al, 2008; Figure 1A). Furthermore, a transactivation domain mutation (S525P) in c-Rel was identified in B-cell lymphomas from two patients, and this mutation enhances the ability of c-Rel to transform cells in vitro (Figure 1A; Starczynowski et al, 2007). However, the significance and frequency of these findings to disease progression and treatment has yet to be established.
c-Rel in solid tumours
c-Rel is also associated with the malignant progression of solid tumours (Table 1). Unlike the situation with lymphoma or other haematological malignancies, these studies have used animal models to assess c-Rel's contribution to the disease. For example, in the azoxymethane/dextran sulphate model of colitis-associated colon adenocarcinoma, c-Rel−/− mice show greater susceptibility to disease (Burkitt et al, 2015). The number of polyps formed in c-Rel−/− animals was not only significantly greater but were larger with higher proliferation indices, suggesting that loss of c-Rel drives a more aggressive form of the disease. Similarly, in a Helicobacter felis (H. felis)-induced model of gastric cancer, unlike NF-κB1−/− mice, c-Rel−/− animals did not develop spontaneous gastric atrophy after either acute or chronic exposure. However, after 1 year, half of the c-Rel−/− mice exposed to H. felis developed lesions similar to low-grade MALT lymphomas (Burkitt et al, 2013). These inflammatory gastric lesions were characterised as being highly proliferative and comprised of predominately B cells, while also partially affecting the mucosa and surrounding gastric glands. These studies suggest that, in contrast to its more commonly characterised tumour-promoting activities, c-Rel can also act to suppress tumorigenesis.
An interaction between the p53 family member ΔNp63α and c-Rel has been reported following TNF-α stimulation in a subset of head and neck carcinoma cell lines with mutant p53 (Lu et al, 2011). This interaction decreases the interaction of ΔNp63α with the tumour-suppressor TAp73 and alters the latter's effects on gene expression. For example, in cell lines with mutant p53, depletion of c-Rel by siRNA treatment was shown to increase the expression of the CDK inhibitor p21WAF1 gene and the two pro-apoptotic genes PUMA and NOXA, indicating that c-Rel mediates cell survival in head and neck cancer by inactivating TAp73. Another correlation between c-Rel and p63 emerged from the DLBCL study discussed above (Li et al, 2015). Here, in ABC-DLBCL, c-Rel nuclear positivity was associated with poor overall survival in patients with low p63 expression (Li et al, 2015).
It has also been suggested that c-Rel has a role in breast cancer. Expression of mRNA for c-Rel, as well as for other NF-κB family members, was shown to be upregulated in 35 primary inflammatory breast cancers (Cogswell et al, 2000). Moreover, in a study using transgenic mice in which c-Rel was expressed in breast tissue under the control of the mouse mammary tumour virus, approximately one-third of these mice developed tumours, albeit with a long latency of approximately 20 months (Romieu-Mourez et al, 2003).
Recently, a novel role for c-Rel in highly aggressive pancreatic ductal adenocarcinoma (PDAC) cell lines has been reported. In this case, c-Rel was found to be a key mediator of TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in PDAC and that the tumour promoter, NFATc2, was under the control of c-Rel and TRAIL, resulting in a resistance to TRAIL-mediated apoptosis (Geismann et al, 2014).
The inflammation, fibrosis and cancer axis
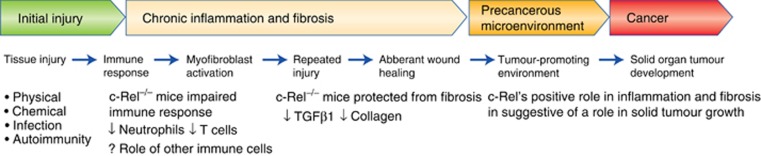
c-Rel can potentially promote cancer by driving organ fibrosis (see Figure 2). Organ fibrosis is a pathological condition characterised by non-physiological wound healing leading to the excess deposition of extracellular matrix. The progression of chronic diseases in parenchymal organs such as the liver, kidney and lung are associated with fibrosis and extensive tissue remodelling. This fibrosis eventually leads to loss of organ function, and it can also act as a precancerous state in which the development of solid tumours is favoured (Elsharkawy and Mann, 2007). A key component of fibrosis is the recruitment and trans-differentiation of precursor cells to activated myofibroblasts. Activated myofibroblasts secrete a plethora of proinflammatory cytokines and chemokines, such as IL-6, IL-8, the growth factor VEGFA and the matrix metalloproteinase MMP9. These molecules are important for normal wound healing but can also drive tumour cell growth and metastasis (Coulouarn and Clement, 2014).
Figure 2.
Schematic diagram showing how fibrosis can lead to cancer development and the role of c-Rel in this process.
Importantly, c-Rel activity has recently been identified as a common potentiator of fibrosis in multiple organs. A role for c-Rel in myofibroblast activation is implied by analysis of c-Rel−/− mouse hepatic stellate cells, which display reduced levels of classical profibrogenic genes such as collagen I and alpha smooth muscle actin (Gieling et al, 2010). c-Rel has also been shown to be an important regulator of epidermal homeostasis and skin fibrosis in a mouse model of bleomycin-induced skin fibrosis. Here c-Rel−/− mice display reduced keratinocyte proliferation as well as reduced levels of fibrosis (Fullard et al, 2013). Similarly c-Rel is important for the development of stress-induced cardiac hypertrophy and fibrosis, with c-Rel−/− mice showing reduced levels of fibrosis in the heart and reduced cardiac growth (Gaspar-Pereira et al, 2012). The reduction of cardiac fibrosis and growth was attributed to the downregulation of two key regulators of cardiac hypertrophy, myocyte enhancer factor 2A and GATA4 (Gaspar-Pereira et al, 2012).
Hepatocellular carcinoma (HCC) is one of the most common forms of liver cancer, and 80% of these tumours arise in a setting of established fibrosis and/or cirrhosis (Coulouarn and Clement, 2014). In the liver, c-Rel has been implicated in modulating both fibrosis and regeneration. The livers of c-Rel−/− mice, following chronic treatment with hepatotoxic carbon tetrachloride or bile duct ligation, show impaired wound healing and reduced fibrosis, characterised by reduced levels of both collagen and hepatic myofibroblasts (Gieling et al, 2010).
Taken together, these reports indicate that by targeting the pathways regulating c-Rel, c-Rel itself or the gene products that c-Rel activates to induce fibrosis development may provide new strategies for the treatment of HCC and other cancers driven by a fibrotic microenvironment.
Effects of c-Rel on cancer therapy
NF-κB is known to affect the cellular response to many common cancer therapies and c-Rel can also affect the treatment of haematological malignancies. For example, adult T-cell leukaemia/lymphoma (ATLL) is caused by the human T-cell leukaemia virus type 1 and is treated with antiviral therapy, zidovudine (AZT) in combination with interferon alpha, resulting in good rates of remission. However, resistance to AZT in cells from ATLL patients has been associated with high expression of c-Rel and IRF-4 (Ramos et al, 2007).
c-Rel also regulates graft vs host disease (GVHD), a problem affecting patients following allogeneic haematopoietic stem cell transplantation (allo-HSCT) for the treatment of a variety of haematological malignancies, such as acute myeloid leukaemia. In mice, bone marrow transfer of c-Rel-deficient donor T cells significantly reduces GVHD compared with normal T cells (Yu et al, 2013). Moreover, these c-Rel−/− T cells also exhibit reduced homing to GVHD organs, such as the lung, liver and spleen. It has therefore been proposed that targeting c-Rel would provide a therapeutic strategy for preventing GVHD in patients undergoing allo-HSCT. Indeed, a small-molecule inhibitor of c-Rel, IT-603, which reportedly acts by directly binding to c-Rel and inhibiting its DNA-binding activity, was shown to reduce the c-Rel-induced activation of T cells without affecting the antitumour activity of allo-HSCT in mice (Shono et al, 2014).
Conclusions
Descriptions of the NF-κB pathway, in common with other highly investigated research areas, often contain many assumptions and simplifications regarding the role of pathway components. This is especially true of the NF-κB subunits and c-Rel in particular (Perkins, 2012). NF-κB subunits are subject to extensive regulation, that can involve their level of expression, interactions with heterologous transcriptional regulators and posttranslational modifications. This can determine their ability to regulate specific gene targets and thereby affect their functions in different physiological or pathological contexts. This review has highlighted not only the well-established association of c-Rel with B-cell lymphoma but also discussed evidence of a role for c-Rel in solid tumours. It is apparent from reports in these areas that c-Rel function is complex, can vary in different cell types or contexts and potentially contributes to tumorigenesis in tissues where it is not mutated through, for example, regulating the fibrosis–cancer axis. However, in mouse models of colitis-associated adenoma and gastric cancer (Burkitt et al, 2013; Burkitt et al, 2015), deletion of c-Rel had the opposite effect and resulted in increased susceptibility to disease. This underlines the importance of a thorough understanding of NF-κB subunit function in different cancer types if therapeutic intervention is to avoid unanticipated, negative consequences.
Acknowledgments
We apologise to the many researchers whose articles we could not cite in this review owing to space limitations. We thank Tom Gilmore, Fiona Oakley and all members of the NDP laboratory for helpful advice and assistance. JEH is funded by Leukemia Lymphoma Research grant 11022 and Cancer Research UK grant C1443/A12750. JL is the recipient of a PhD studentship jointly funded by the MRC and NIHR Newcastle Biomedical Research Centre.
The authors declare no conflict of interest.
References
- Barth TF, Bentz M, Leithauser F, Stilgenbauer S, Siebert R, Schlotter M, Schlenk RF, Dohner H, Moller P (2001) Molecular-cytogenetic comparison of mucosa-associated marginal zone B-cell lymphoma and large B-cell lymphoma arising in the gastro-intestinal tract. Gene Chromosome Cancer 31(4): 316–325. [DOI] [PubMed] [Google Scholar]
- Barth TF, Martin-Subero JI, Joos S, Menz CK, Hasel C, Mechtersheimer G, Parwaresch RM, Lichter P, Siebert R, Mooller P (2003) Gains of 2p involving the REL locus correlate with nuclear c-Rel protein accumulation in neoplastic cells of classical Hodgkin lymphoma. Blood 101(9): 3681–3686. [DOI] [PubMed] [Google Scholar]
- Bradford JW, Baldwin AS (2014) IKK/nuclear factor-κB and oncogenesis: roles in tumor-initiating cells and in the tumor microenvironment. Adv Cancer Res 121: 125–145. [DOI] [PubMed] [Google Scholar]
- Burkitt MD, Hanedi AF, Duckworth CA, Williams JM, Tang JM, O'Reilly LA, Putoczki TL, Gerondakis S, Dimaline R, Caamano JH, Pritchard DM (2015) NF-κB1, NF-κB2 and c-Rel differentially regulate susceptibility to colitis-associated adenoma development in C57BL/6 mice. J Pathol 236(3): 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt MD, Williams JM, Duckworth CA, O'Hara A, Hanedi A, Varro A, Caamano JH, Pritchard DM (2013) Signaling mediated by the NF-κB sub-units NF-κB1, NF-κB2 and c-Rel differentially regulate Helicobacter felis-induced gastric carcinogenesis in C57BL/6 mice. Oncogene 32(50): 5563–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS Jr (2000) Selective activation of NF-κB subunits in human breast cancer: potential roles for NF-κB2/p52 and for Bcl-3. Oncogene 19(9): 1123–1131. [DOI] [PubMed] [Google Scholar]
- Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, Bertoni F, Ponzoni M, Scandurra M, Califano A, Bhagat G, Chadburn A, Dalla-Favera R, Pasqualucci L (2009) Mutations of multiple genes cause deregulation of NF-κB in diffuse large B-cell lymphoma. Nature 459(7247): 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulouarn C, Clement B (2014) Stellate cells and the development of liver cancer: therapeutic potential of targeting the stroma. J Hepatol 60(6): 1306–1309. [DOI] [PubMed] [Google Scholar]
- Curry CV, Ewton AA, Olsen RJ, Logan BR, Preti HA, Liu YC, Perkins SL, Chang CC (2009) Prognostic impact of C-REL expression in diffuse large B-cell lymphoma. J Hematop 2(1): 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsharkawy AM, Mann DA (2007) Nuclear factor-κB and the hepatic inflammation-fibrosis-cancer axis. Hepatology 46(2): 590–597. [DOI] [PubMed] [Google Scholar]
- Enciso-Mora V, Broderick P, Ma Y, Jarrett RF, Hjalgrim H, Hemminki K, van den Berg A, Olver B, Lloyd A, Dobbins SE, Lightfoot T, van Leeuwen FE, Forsti A, Diepstra A, Broeks A, Vijayakrishnan J, Shield L, Lake A, Montgomery D, Roman E, Engert A, von Strandmann EP, Reiners KS, Nolte IM, Smedby KE, Adami HO, Russell NS, Glimelius B, Hamilton-Dutoit S, de Bruin M, Ryder LP, Molin D, Sorensen KM, Chang ET, Taylor M, Cooke R, Hofstra R, Westers H, van Wezel T, van Eijk R, Ashworth A, Rostgaard K, Melbye M, Swerdlow AJ, Houlston RS (2010) A genome-wide association study of Hodgkin's lymphoma identifies new susceptibility loci at 2p16.1 (REL), 8q24.21 and 10p14 (GATA3). Nat Genet 42(12): 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerhake F, Kutok JL, Monti S, Chen W, LaCasce AS, Cattoretti G, Kurtin P, Pinkus GS, de Leval L, Harris NL, Savage KJ, Neuberg D, Habermann TM, Dalla-Favera R, Golub TR, Aster JC, Shipp MA (2005) NFκB activity, function, and target-gene signatures in primary mediastinal large B-cell lymphoma and diffuse large B-cell lymphoma subtypes. Blood 106(4): 1392–1399. [DOI] [PubMed] [Google Scholar]
- Fullard N, Moles A, O'Reilly S, van Laar JM, Faini D, Diboll J, Reynolds NJ, Mann DA, Reichelt J, Oakley F (2013) The c-Rel subunit of NF-κB regulates epidermal homeostasis and promotes skin fibrosis in mice. Am J Pathol 182(6): 2109–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Pereira S, Fullard N, Townsend PA, Banks PS, Ellis EL, Fox C, Maxwell AG, Murphy LB, Kirk A, Bauer R, Caamano JH, Figg N, Foo RS, Mann J, Mann DA, Oakley F (2012) The NF-κB subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am J Pathol 180(3): 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geismann C, Grohmann F, Sebens S, Wirths G, Dreher A, Hasler R, Rosenstiel P, Hauser C, Egberts JH, Trauzold A, Schneider G, Sipos B, Zeissig S, Schreiber S, Schafer H, Arlt A (2014) c-Rel is a critical mediator of NF-κB -dependent TRAIL resistance of pancreatic cancer cells. Cell Death Dis 5: e1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieling RG, Elsharkawy AM, Caamano JH, Cowie DE, Wright MC, Ebrahimkhani MR, Burt AD, Mann J, Raychaudhuri P, Liou HC, Oakley F, Mann DA (2010) The c-Rel subunit of nuclear factor-κB regulates murine liver inflammation, wound-healing, and hepatocyte proliferation. Hepatology 51(3): 922–931. [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Gerondakis S (2011) The c-Rel transcription factor in development and disease. Genes Cancer 2(7): 695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumont RJ, Gerondakis S (1994) The subunit composition of NF-κB complexes changes during B-cell development. Cell Growth Differ 5(12): 1321–1331. [PubMed] [Google Scholar]
- Hamoudi RA, Appert A, Ye H, Ruskone-Fourmestraux A, Streubel B, Chott A, Raderer M, Gong L, Wlodarska I, De Wolf-Peeters C, MacLennan KA, de Leval L, Isaacson PG, Du MQ (2010) Differential expression of NF-κB target genes in MALT lymphoma with and without chromosome translocation: insights into molecular mechanism. Leukemia 24(8): 1487–1497. [DOI] [PubMed] [Google Scholar]
- Joos S, Menz CK, Wrobel G, Siebert R, Gesk S, Ohl S, Mechtersheimer G, Trümper L, Möller P, Lichter P, Barth TF (2002) Classical Hodgkin lymphoma is characterized by recurrent copy number gains of the short arm of chromosome 2. Blood 99(4): 1381–1387. [DOI] [PubMed] [Google Scholar]
- Karin M (2009) NF-κB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 1(5): a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneth NS, Mudie S, Rocha S (2010) IKK and NF-κB -mediated regulation of Claspin impacts on ATR checkpoint function. EMBO J 29(17): 2966–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman JR, Weniger MA, Barth TF, Gilmore TD (2008) Deletion analysis and alternative splicing define a transactivation inhibitory domain in human oncoprotein REL. Oncogene 27(53): 6770–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, Carty S, Lam LT, Shaffer AL, Xiao W, Powell J, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Connors JM, Campo E, Jaffe ES, Delabie J, Smeland EB, Rimsza LM, Fisher RI, Weisenburger DD, Chan WC, Staudt LM (2008) Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA 105(36): 13520–13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xu-Monette ZY, Ok CY, Tzankov A, Manyam GC, Sun R, Visco C, Zhang M, Montes-Moreno S, Dybkaer K, Chiu A, Orazi A, Zu Y, Bhagat G, Richards KL, Hsi ED, Choi WW, Krieken JH, Huh J, Ponzoni M, Ferreri AJ, Moller MB, Wang J, Parsons BM, Winter JN, Piris MA, Pham LV, Medeiros JL, Young KH (2015) Prognostic impact of c-Rel nuclear expression and REL amplification and crosstalk between c-Rel and the p53 pathway in diffuse large B-cell lymphoma. Oncotarget 6(27): 23157–23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KH, Yang Y, Staudt LM (2012) Pathogenetic importance and therapeutic implications of NF-κB in lymphoid malignancies. Immunol Rev 246(1): 359–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou HC, Sha WC, Scott ML, Baltimore D (1994) Sequential induction of NF-κB /Rel family proteins during B-cell terminal differentiation. Mol Cell Biol 14(8): 5349–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Yang X, Duggal P, Allen CT, Yan B, Cohen J, Nottingham L, Romano RA, Sinha S, King KE, Weinberg WC, Chen Z, Van Waes C (2011) TNF-α promotes c-REL/ΔNp63α interaction and TAp73 dissociation from key genes that mediate growth arrest and apoptosis in head and neck cancer. Cancer Res 71(21): 6867–6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Subero JI, Gesk S, Harder L, Sonoki T, Tucker PW, Schlegelberger B, Grote W, Novo FJ, Calasanz MJ, Hansmann ML, Dyer MJ, Siebert R (2002) Recurrent involvement of the REL and BCL11A loci in classical Hodgkin lymphoma. Blood 99(4): 1474–1477. [DOI] [PubMed] [Google Scholar]
- Neo WH, Lim JF, Grumont R, Gerondakis S, Su IH (2014) c-Rel regulates Ezh2 expression in activated lymphocytes and malignant lymphoid cells. J Biol Chem 289(46): 31693–31707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins ND (2012) The diverse and complex roles of NF-κB subunits in cancer. Nat Rev Cancer 12(2): 121–132. [DOI] [PubMed] [Google Scholar]
- Ramos JC, Ruiz P Jr, Ratner L, Reis IM, Brites C, Pedroso C, Byrne GE Jr, Toomey NL, Andela V, Harhaj EW, Lossos IS, Harrington WJ Jr (2007) IRF-4 and c-Rel expression in antiviral-resistant adult T-cell leukemia/lymphoma. Blood 109(7): 3060–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu-Mourez R, Kim DW, Shin SM, Demicco EG, Landesman-Bollag E, Seldin DC, Cardiff RD, Sonenshein GE (2003) Mouse mammary tumor virus c-rel transgenic mice develop mammary tumors. Mol Cell Biol 23(16): 5738–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjabi S, Williams KJ, Saccani S, Zhou L, Hoffmann A, Ghosh G, Gerondakis S, Natoli G, Smale ST (2005) A c-Rel subdomain responsible for enhanced DNA-binding affinity and selective gene activation. Genes Dev 19(18): 2138–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shono Y, Tuckett AZ, Ouk S, Liou HC, Altan-Bonnet G, Tsai JJ, Oyler JE, Smith OM, West ML, Singer NV, Doubrovina E, Pankov D, Undhad CV, Murphy GF, Lezcano C, Liu C, O'Reilly RJ, van den Brink MR, Zakrzewski JL (2014) A small-molecule c-Rel inhibitor reduces alloactivation of T cells without compromising antitumor activity. Cancer Disc 4(5): 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starczynowski DT, Trautmann H, Pott C, Harder L, Arnold N, Africa JA, Leeman JR, Siebert R, Gilmore TD (2007) Mutation of an IKK phosphorylation site within the transactivation domain of REL in two patients with B-cell lymphoma enhances REL's in vitro transforming activity. Oncogene 26(19): 2685–2694. [DOI] [PubMed] [Google Scholar]
- van Essen D, Zhu Y, Saccani S (2010) A feed-forward circuit controlling inducible NF-kappaB target gene activation by promoter histone demethylation. Mol Cell 39(5): 750–760. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rickman BH, Poutahidis T, Schlieper K, Jackson EA, Erdman SE, Fox JG, Horwitz BH (2008) c-Rel is essential for the development of innate and T cell-induced colitis. J Immunol 180(12): 8118–8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger MA, Gesk S, Ehrlich S, Martin-Subero JI, Dyer MJ, Siebert R, Moller P, Barth TF (2007) Gains of REL in primary mediastinal B-cell lymphoma coincide with nuclear accumulation of REL protein. Gene Chromosome Cancer 46(4): 406–415. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang D, Kaosaard K, Liu C, Fu J, Haarberg K, Anasetti C, Beg AA, Yu XZ (2013) c-Rel is an essential transcription factor for the development of acute graft-versus-host disease in mice. Eur J Immunol 43(9): 2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Barrera LA, Ersing I, Willox B, Schmidt SC, Greenfeld H, Zhou H, Mollo SB, Shi TT, Takasaki K, Jiang S, Cahir-McFarland E, Kellis M, Bulyk ML, Kieff E, Gewurz BE (2014) The NF-κB genomic landscape in lymphoblastoid B cells. Cell Rep 8(5): 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]