Abstract
Background:
Trastuzumab was introduced a decade ago and has improved outcomes for HER2-positive breast cancer. We investigated the factors predictive of pathological complete response (pCR), prognostic factors for disease-free survival (DFS), and interactions between pCR and DFS after neoadjuvant treatment.
Methods:
We identified 287 patients with primary HER2-positive breast cancers given neoadjuvant chemotherapy (NAC) between 2002 and 2011. Univariate and multivariate analyses of clinical and pathological factors associated with pCR and DFS were performed.
Results:
pCR rates differed between patients receiving neoadjuvant trastuzumab treatment or not (47.7% versus 19.3%, P<0.0001). DFS also differed significantly between patients receiving adjuvant trastuzumab or not (hazard ratio=4.84, 95% CI (2.52; 9.31), P<0.001). We analysed 199 patients given neoadjuvant and adjuvant trastuzumab. Multivariate analysis identified older age and hormone receptor-negative tumours as independent predictors of pCR. T stage (hazard ratio=2.55, 95% CI (1.01; 6.48), P=0.05) and strict pCR (hazard ratio=9.15, 95% CI (1.22; 68.83), P=0.03) were independent predictors of DFS. The latter association was significant in the HR-negative subgroup (P=0.02) but not in the HR-positive subgroup (P=0.12).
Conclusions:
Major pCR and DFS gains in HER2-positive BC were observed since ‘trastuzumab' era. Further improvements rely on the enrollment of accurately selected patients into clinical trials.
Keywords: breast cancer, HER2-positive, neoadjuvant chemotherapy, pathological complete response, prognostic factors, trastuzumab
Breast cancer (BC) is the most frequently diagnosed cancer and the leading cause of cancer-related death in women. HER2-positive breast carcinomas display amplification and overexpression of the HER2 tyrosine kinase receptor gene (17q12). This subgroup is defined by aggressive pathological features and a high rate of early distant metastatic events. Trastuzumab-based treatments have been used for the past decade and have improved outcomes in patients with early or metastatic HER2-positive breast cancer.
Neoadjuvant treatment is currently being used in patients with early-stage and advanced disease. Its clinical benefits are: (a) higher rates of breast-conserving surgery, (b) similar prognoses for breast cancer patients receiving a neoadjuvant and for those receiving an adjuvant therapy regimen, and (c) a body of evidence showing that the achievement of a pathological complete response (pCR) after neoadjuvant chemotherapy (NAC) is associated with a good prognosis in specific subgroups (triple-negative, HER2-positive). Furthermore, it may serve as a test of in vivo chemosensitivity, making it possible to evaluate the efficacy of systemic therapy early and to discontinue ineffective treatment.
In parallel, interest has increased in the use of pCR as a surrogate marker for long-term outcome to accelerate the approval process for new drugs since the publication by the Food and Drug Administration (FDA) of a set of guidelines entitled ‘Guidance for Industry. Pathologic Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval'.
In the past few years, the combination of trastuzumab with NAC has become standard, as two phase III trials comparing a regimen in which trastuzumab was added to NAC and NAC alone reported higher pCR rates (MD Anderson Cancer Center trial: pCR rates: 26.3% versus 65.2% with and without trastuzumab, respectively (Buzdar et al, 2007); NOAH trial: pCR rates: 19% versus 38%, respectively) and longer disease-free survival (DFS) for the combined treatment (NOAH trial: 3-year EFS, 71% versus 56% with and without trastuzumab, respectively (Gianni et al, 2010)). In patients with HER2-positive breast tumours for whom neoadjuvant treatment is indicated, trastuzumab is generally added to chemotherapy, and the patient then receives 1 year of adjuvant trastuzumab treatment.
However, factors predictive of pCR and prognostic factors for survival have yet to be identified, and there is still no robust demonstration of the correlation between pCR and outcome in patients treated with optimal therapy. The aim of this study was to identify factors predictive of pCR and prognostic factors in a large cohort of HER2-positive breast cancer patients treated by NAC plus trastuzumab.
Materials and methods
Patients
We analysed a cohort of 287 T1–3NxM0 patients with HER2-positive invasive breast carcinoma (NEOREP Cohort, CNIL declaration number 1547270) treated at Institut Curie between 2002 and 2012. We included only unilateral, non-recurrent, non-inflammatory, non-metastatic tumours, excluding T4 and lobular tumours. All patients received NAC, followed by surgery and radiotherapy. The study was approved by the Breast Cancer Study Group of Institut Curie and was conducted according to institutional and ethical rules concerning research on tissue specimens and patients. Informed consent from the patients was not required.
Tumour samples
The following histological features were retrieved: tumour type, initial tumour size and nodal status, grade (Elston and Ellis), oestrogen receptor (ER) and progesterone receptor (PR) status, HER2 status, number of metastatic nodes, and total sentinel and non-sentinel nodes. ER and PR status were determined as follows. Tissue sections were rehydrated and antigen retrieval was carried out in citrate buffer (10 mM, pH 6.1). The sections were then incubated with antibodies against for ER (clone 6F11, Novocastra, Leica Biosystems, Newcastle, UK; 1/200) and PR (clone 1A6, Novocastra, 1/200). The antibodies were then detected with the Vectastain Elite ABC peroxidase-conjugated mouse IgG kit (Vector, Burlingame, CA, USA), with diaminobenzidine (Dako A/S, Glostrup, Denmark) as the chromogen. Positive and negative controls were included in each run. Cases were considered positive for ER and PR if at least 10% of the tumour nuclei were stained, in accordance with standard guidelines used in France (Harvey et al, 1999; Gligorov and Namer, 2007). Tumours were considered to be hormone receptor (HR)-positive if they were positive for either ER or PR, and HR-negative if they were negative for both ER and PR. HER2 overexpression status was determined according to the American Society of Clinical Oncology (ASCO) guidelines (Wolff et al, 2007).
Treatments
Patients were treated according to national guidelines. NAC regimens changed over time (anthracycline-based regimen or sequential anthracycline–taxane regimen), with trastuzumab used in an adjuvant and/or neoadjuvant setting since the middle of the past decade. Endocrine therapy (tamoxifen, aromatase inhibitor, or GnRH agonists) was prescribed when indicated. Surgery was performed 4–6 weeks after the end of chemotherapy. All patients received adjuvant radiotherapy. Trastuzumab treatments changed over time, and the whole cohort was split into three distinct groups according to trastuzumab use. Patients who did not receive any trastuzumab were indicated as cohort A (n=35); patients who received only adjuvant trastuzumab were indicated as cohort B (n=53); and patients who received both neoadjuvant and adjuvant trastuzumab were indicated as cohort C (n=199).
Pathology assessment at NAC completion
A pCR was defined as the absence of residual invasive cancer cells in the breast and axillary lymph nodes (ypT0/is+/ypN0). Strict pCR (spCR) was defined as an absence of invasive and non-invasive residuals in the breast and invasive disease in the axillary nodes (ypT0 ypN0).
DSF and overall survival (OS)
DFS was defined as the time from surgery to death, loco-regional recurrence, or distant recurrence, whichever occurred first, and OS was defined as the time from surgery to death. Patients for whom none of these events were recorded were censored at the date of their last known contact. Survival probabilities were estimated by the Kaplan–Meier method, and survival curves were compared with log-rank tests.
Descriptive analysis of pCR and DFS rates according to the three cohorts
For the pCR rate descriptive analysis, because of the known major impact on trastuzumab use on pCR rates, we chose to pool cohorts A and B (in both of which patients did not receive neaoadjuvant trastuzumab) and compared the resulting pooled cohort with cohort C (in which patients received neoadjuvant trastuzumab).
For the DFS and OS descriptive analysis, because of the known major impact on trastuzumab on DFS rates, we chose to pool cohorts B and C (in both of which patients received trastuzumab) and compared the resulting pooled cohort with cohort A (in which patients did not receive any trastuzumab).
Statistical analysis
The study population was described in terms of frequencies for qualitative variables or medians and associated ranges for quantitative variables. The cutoff date for the analysis was 13 March 2013.
The statistical analyses of the factors predictive of pCR and prognostic for DFS were performed in the cohort C only, as neoadjuvant trastuzumab in association with chemotherapy followed by adjuvant trastuzumab represents the gold standard treatment in 2015.
Factors predictive of pCR were introduced into a univariate logistic regression model. A multivariate logistic model was then implemented. The covariates selected for the multivariate analysis were those with a likelihood ratio test P-value <0.10 in univariate analysis. A backward stepwise selection procedure was used.
Hazard ratios and their associated 95% confidence intervals were calculated with the Cox proportional hazard model. Variables with a P-value for the likelihood ratio test <0.10 in univariate analysis were included in the multivariate model. Backward selection was used to establish the final multivariate model. The proportional hazards hypothesis was tested for each factor, with Schoenfeld's residuals test and plotting. The significance threshold was 5%. Analyses were performed with the R software, version 2.13.2 (R Development Core Team, 2011).
Results
Overall, 287 patients were identified in our database. The baseline characteristics of these patients are summarised in Table 1. All 287 patients received NAC and underwent surgery followed by radiotherapy. The median age of the patients was 48 years (27–79); 193 patients had T2 tumours (67.2%), and 169 had clinically involved nodes (58.9%). In total, 129 patients had HR-negative breast cancer (44.9%). Trastuzumab treatments changed over time and the characteristics of the patients are presented by treatment (cohort A, n=35, no trastuzumab at all; cohort B, n=53, adjuvant trastuzumab only; cohort C, n=199, both neoadjuvant and adjuvant trastuzumab) in Table 1. There were significant differences between cohorts A, B, and C for treatment period, number of nodes involved (patients in cohort C were less likely to have nodes involved), and median follow-up. Strict pCR (spCR) and pCR rates differed significantly between the three cohorts (P<0.0001). These rates were higher in patients given neoadjuvant trastuzumab (cohort C, pCR rate: 47.7%) than in patients who did not receive this treatment (cohorts A and B, pCR rate: 19.3% P<0.0001) and in the HR-negative subgroups than in the HR-positive subgroups (pCR rate: 48.8% versus 31.2%, P=0.003) (Table 2).
Table 1. Patients, tumours, and treatment characteristics, by cohort (cohort A, n=35, no trastuzumab; cohort B, n=53, adjuvant trastuzumab only; cohort C, n=199, both neadjuvant and adjuvant trastuzumab).
|
Whole population (n=287) |
Cohort A (n=35), no trastuzumab |
Cohort B (n=53), adjuvant tz* only |
Cohort C (n=199), neoadjuvant and adjuvant tz* |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | P-value | |
|
Time period | |||||||||
| 2002–2005 | 70 | 24.4% | 34 | 97.1% | 25 | 47.2% | 11 | 5.5% | <0.01 |
| 2006–2011 | 217 | 75.6% | 1 | 2.9% | 28 | 52.8% | 188 | 94.5% | |
|
Age | |||||||||
| <45 y.o. | 115 | 40.1% | 12 | 34.3% | 24 | 45.3% | 79 | 39.7% | 0.70 |
| 45–55 y.o | 94 | 32.8% | 11 | 31.4% | 18 | 34.0% | 65 | 32.7% | |
| >55 y.o. | 78 | 27.2% | 12 | 34.3% | 11 | 20.8% | 55 | 27.6% | |
|
Menopausal status | |||||||||
| Premenopausal | 176 | 61.3% | 20 | 57.1% | 36 | 67.9% | 120 | 60.3% | 0.42 |
| Postmenopausal | 110 | 38.3% | 15 | 42.9% | 16 | 30.2% | 79 | 39.7% | |
|
T stage | |||||||||
| T1 | 18 | 6.3% | 3 | 8.6% | 2 | 3.8% | 13 | 6.5% | 0.89 |
| T2 | 193 | 67.2% | 24 | 68.6% | 35 | 66.0% | 134 | 67.3% | |
| T3 | 75 | 26.1% | 8 | 22.9% | 15 | 28.3% | 52 | 26.1% | |
|
N stage | |||||||||
| N0 | 118 | 41.1% | 20 | 57.1% | 17 | 32.1% | 81 | 40.7% | 0.06 |
| N1, N2, N3 | 169 | 58.9% | 15 | 42.9% | 36 | 67.9% | 118 | 59.3% | |
|
BMI | |||||||||
| BMI⩽30 | 253 | 88.2% | 31 | 88.6% | 49 | 92.5% | 173 | 86.9% | 0.59 |
| BMI>30 | 33 | 11.5% | 4 | 11.4% | 4 | 7.5% | 25 | 12.6% | |
|
Histological type | |||||||||
| Ductal | 271 | 94.4% | 29 | 82.9% | 50 | 94.3% | 192 | 96.5% | 0.84 |
| Others | 7 | 2.4% | 1 | 2.9% | 1 | 1.9% | 5 | 2.5% | |
|
Grade (EE) | |||||||||
| Grade I | 2 | 0.7% | 1 | 2.9% | 0 | 0.0% | 1 | 0.5% | 0.23 |
| Grade II | 93 | 32.4% | 12 | 34.3% | 20 | 37.7% | 61 | 30.7% | |
| Grade III | 179 | 62.4% | 17 | 48.6% | 29 | 54.7% | 133 | 66.8% | |
|
Hormone receptor (HR) | |||||||||
| Positive | 157 | 54.7% | 18 | 51.4% | 28 | 52.8% | 111 | 55.8% | 0.88 |
| Negative | 129 | 44.9% | 17 | 48.6% | 24 | 45.3% | 88 | 44.2% | |
|
Oestrogen receptor (ER) | |||||||||
| Negative | 138 | 48.1% | 17 | 48.6% | 27 | 50.9% | 94 | 47.2% | 0.89 |
| Positive | 149 | 51.9% | 18 | 51.4% | 26 | 49.1% | 105 | 52.8% | |
|
Progesterone receptor (PR) | |||||||||
| Negative | 183 | 63.8% | 25 | 71.4% | 33 | 62.3% | 125 | 62.8% | 0.51 |
| Positive | 100 | 34.8% | 9 | 25.7% | 19 | 35.8% | 72 | 36.2% | |
|
Surgery | |||||||||
| Lumpectomy | 199 | 69.3% | 26 | 74.3% | 34 | 64.2% | 139 | 69.8% | 0.61 |
| Mastectomy | 88 | 30.7% | 9 | 25.7% | 19 | 35.8% | 60 | 30.2% | |
| Sentinel node Biopsy | 8 | 2.8% | 0 | 0.0% | 0 | 0.0% | 8 | 4.0% | 0.32 |
| Axillary node dissection | 279 | 97.2% | 35 | 100.0% | 53 | 100.0% | 191 | 96.0% | |
|
Number of nodes involved | |||||||||
| None | 200 | 69.7% | 21 | 60.0% | 25 | 47.2% | 154 | 77.4% | <0.01 |
| 1–3 | 67 | 23.3% | 11 | 31.4% | 21 | 39.6% | 35 | 17.6% | |
| ⩾4 | 20 | 7.0% | 3 | 8.6% | 7 | 13.2% | 10 | 5.0% | |
|
Strict pCR | |||||||||
| No | 211 | 73.5% | 29 | 82.9% | 49 | 92.5% | 133 | 66.8% | <0.01 |
| Yes | 76 | 26.5% | 6 | 17.1% | 4 | 7.5% | 66 | 33.2% | |
|
pCR | |||||||||
| No | 175 | 61.0% | 26 | 74.3% | 45 | 84.9% | 104 | 52.3% | <0.01 |
| Yes | 112 | 39.0% | 9 | 25.7% | 8 | 15.1% | 95 | 47.7% | |
|
Adjuvant chemotherapy | |||||||||
| No | 254 | 88.5% | 33 | 94.3% | 37 | 69.8% | 184 | 92.5% | <0.01 |
| Yes | 33 | 11.5% | 2 | 5.7% | 16 | 30.2% | 15 | 7.5% | |
|
Trastuzumab | |||||||||
| No | 35 | 12.2% | 35 | 100.0% | 0 | 0.0% | 0 | 0.0% | |
| Adjuvant only | 53 | 18.5% | 0 | 0.0% | 53 | 100.0% | 0 | 0.0% | |
| Neoadjuvant and adjuvant | 199 | 69.3% | 0 | 0.0% | 0 | 0.0% | 199 | 100.0% | |
|
Endocrine therapy | |||||||||
| No | 150 | 52.3% | 19 | 54.3% | 32 | 60.4% | 99 | 49.7% | 0.38 |
| Yes | 137 | 47.7% | 16 | 45.7% | 21 | 39.6% | 100 | 50.3% | |
| Tamoxifen | 65 | 22.6% | 14 | 40.0% | 7 | 13.2% | 44 | 22.1% | |
| Aromatase inhibitor | 50 | 17.4% | 1 | 2.9% | 5 | 9.4% | 44 | 22.1% | |
| Others | 22 | 7.7% | 1 | 2.9% | 9 | 17.0% | 12 | 6.0% | |
|
Follow-up | |||||||||
| Median (range) | 46 | (6–122) | 96 | (40–122) | 67 | (25–95) | 33 | (6–92) | <0.01 |
Abbreviations: EE=Elston Ellis; pCR=pathological complete response; tz*=trastuzumab; y.o.=years old.
Missing values: menopausal status n=1; T stage n=1; BMI n=1; histological type n=9; grade n=13; HR, n=1; PR: n=4; radiotherapy n=2; adjuvant chemotherapy n=34.
Table 2. Pathological response rates by definition, by cohort, and by hormone receptor status.
| Whole population (n=287) | P | Cohort A (n=35) | Cohort B (n=53) | Cohort C (n=199) | P | |
|---|---|---|---|---|---|---|
|
pCR | ||||||
| No | 175/287 (60.9%) | 26/35 (74.3%) | 45/53 (84.9%) | 104/199 (52.3%) | ||
| Yes | 112/287 (39.0%) | 9/35 (25.7%) | 8/53 (15.1%) | 95/199 (47.7%) | <0.0001 | |
|
17/88 (19.3%) |
95/199 (47.7%) | <0.0001 | ||||
| HR positive | 49/157 (31.2%) | 2/18 (11.1%) | 5/28 (17.9%) | 42/111 (37.8%) | ||
| HR negative | 63/129 (48.8%) | 0.0035 | 7/17 (41.2%) | 3/24 (12.5%) | 53/88 (60.2%) | |
|
Strict pCR | ||||||
| No | 211/287 (73.5%) | 29/35 (82.8%) | 49/53 (92.5%) | 133/199 (66.8%) | ||
| Yes | 76/287 (26.5%) | 6/35 (17.1%) | 4/53 (7.5%) | 66/199 (33.1%) | 0.0002 | |
|
10/88 (11.4%) |
66/199 (33.1%) | <0.00001 | ||||
| HR positive | 29/157 (18.5%) | 1/18 (5.6%) | 2/28 (7.1%)a | 26/111 (23.4%) | ||
| HR negative | 47/129 (36.4%) | 0.001 | 5/17 (29.4%) | 2/24 (8.3%) | 40/88 (45.5%) | |
Abbreviations: HR=hormone receptor; pCR=pathological complete response.
HR not available, n=1.
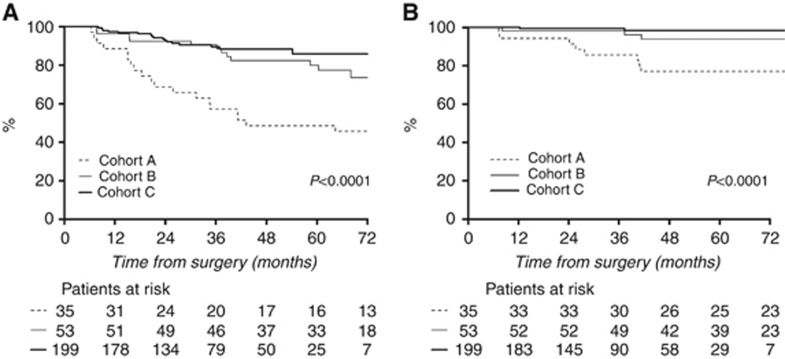
DFS (Figure 1A) also differed significantly between cohorts (P<0.001). Patients treated without trastuzumab (cohort A) had a higher risk of relapse (hazard ratio=4.84 95% CI (2.52; 9.31)) than patients receiving adjuvant trastuzumab with or without neoadjuvant trastuzumab (cohorts B and C pooled). Five-year DFS rates were 48.6% (95% CI (34.5–68.3), cohort A) versus 83.5% (95% CI (77.6–89.9), cohorts B and C pooled) and were not different between cohorts B and C (cohort B: 80.0%, 95% CI (69.5–92.0) versus cohort C 85.8%, 95% CI (79.0–93.3)).
Figure 1.
(A) DFS and (B) OS by cohort (cohort A: no trastuzumab; cohort B: adjuvant trastuzumab only; cohort C: neoadjuvant and adjuvant trastuzumab).
OS (Figure 1B) was also significantly lower in cohort A (hazard ratio=9.01, 95% CI (2.95–27.52)) than in cohorts B and C pooled (P<0.001; 5-year OS rates: 76.9%, 95% CI (64.1–92.3)) versus 96.9; 95% CI (94.2–99.7) respectively).
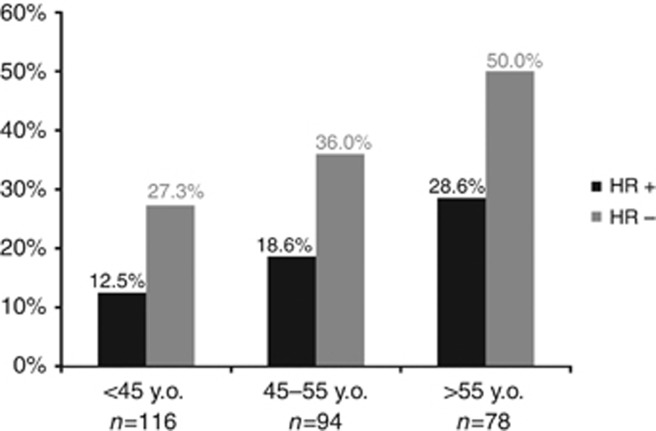
pCR was predicted and prognostic analysis was performed for cohort C only (patients who received optimal neoadjuvant and adjuvant treatment, n=199). After neoadjuvant treatment, 66 patients had no residual disease on the surgical specimen, and 29 patients had residual carcinoma in situ only (spCR rate: 33.2% (66 out of 199); pCR: 47.7% (95 out of 199)). The following results are given for spCR. Univariate logistic regression analysis identified two factors correlated with spCR: age at diagnosis and HR expression. Both factors remained significant in the multivariate logistic regression model (Table 3). spCR rates increased with age in both HR-positive tumours (12.5, 18.6, and 28.6% for patients <45 years, 45–55, and >55 years, respectively), and in HR-negative ones (27.3, 36.0 and 50.0%, respectively) (Figure 2).
Table 3. Odds ratios for predicting strict pCR (univariate and multivariate analyses).
|
Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| pCR (%) | OR | 95% CI | P-value | OR | 95% CI | P-value | |
|
BMI, kg m−2 | |||||||
| BMI⩽30 | 33.5 | 1 | 0.88 | ||||
| BMI>30 | 32.0 | 0.93 | (0.36; 2.23) | ||||
|
Age | |||||||
| <45 y.o. | 24.1 | 1 | 0.02 | 1 | — | 0.03 | |
| 45–55 y.o | 35.4 | 1.73 | (0.84; 3.60) | 1.44 | (0.68; 3.05) | ||
| >55 y.o. | 43.6 | 2.44 | (1.17; 5.19) | 2.74 | (1.27; 5.93) | ||
|
Menopausal status | |||||||
| Premenopausal | 29.2 | 1 | 0.14 | ||||
| Postmenopausal | 39.2 | 1.57 | (0.86; 2.86) | ||||
|
T stage | |||||||
| T1–T2 | 34.0 | 1 | 0.67 | ||||
| T3 | 30.8 | 0.86 | (0.43; 1.68) | ||||
|
N stage | |||||||
| N0 | 37.0 | 1 | 0.34 | ||||
| N1, N2, N3 | 30.5 | 0.75 | (0.41; 1.36) | ||||
|
Grade (EE) | |||||||
| Grade I–II | 35.5 | 1 | 0.66 | ||||
| Grade III | 32.3 | 0.87 | (0.46; 1.65) | ||||
|
ER | |||||||
| Positive | 21.9 | 1 | <0.01 | ||||
| Negative | 45.7 | 3.01 | (1.64; 5.56) | ||||
|
PR | |||||||
| Positive | 19.4 | 1 | <0.01 | ||||
| Negative | 41.6 | 2.95 | (1.51; 5.88) | ||||
|
HR | |||||||
| Positive | 23.4 | 1 | <0.01 | 1 | — | <0.01 | |
| Negative | 45.5 | 2.72 | (1.49; 5.05) | 2.97 | (1.57; 5.63) | ||
Abbreviations: BMI=body mass index; CI=confidence interval; EE=Elston Ellis; ER=oestrogen receptor; HR=hormone receptor; OR=odds ratio; pCR=pathological complete response; PR=progesterone receptor; y.o.=years old.
Figure 2.
pCR rates by age and HR status.
After a median of 33 months of follow-up (range: 6–92), 18 patients experienced relapses (8 local, 3 regional, 7 distant). Two of these patients died. In univariate analysis, the factors associated with DFS were age at diagnosis, spCR and pCR, menopausal status and initial tumour stage. Tumour stage (T3: HR=2.55, 95% CI (1.01–6.48) versus T1–T2: HR=1, reference class) and spCR (No pCR: HR=9.15, 95% CI (1.22–68.83) versus spCR (reference class), P=0.03) remained significantly associated with DFS in multivariate analysis (Table 4), though the number of events was very low in patients whose tumour achieved pCR after NAC. Five-year DFS rates were 78% (95% CI (66.9–90.9); no pCR group) versus 95% (95% CI (89.4–100); spCR group).
Table 4. Hazard ratios for predicting DFS (univariate and multivariate analyses).
|
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
|
BMI, kg m−2 | ||||||
| BMI⩽30 | 1 | — | 0.24 | |||
| BMI>30 | 2.05 | (0.67; 6.26) | ||||
|
Age | ||||||
| <45 y.o. | 1 | — | 0.04 | |||
| 45–55 y.o | 1.32 | (0.51; 3.43) | ||||
| >55 y.o. | 0.17 | (0.02; 1.36) | ||||
|
Menopausal status | ||||||
| Premenopausal | 1 | — | 0.08 | |||
| Postmenopausal | 0.40 | (0.13; 1.21) | ||||
|
T stage | ||||||
| T1–T2 | 1 | — | 0.07 | 1 | — | 0.05 |
| T3 | 2.44 | (0.96; 6.19) | 2.55 | (1.01–6.48) | ||
|
N stage | ||||||
| N0 | 1 | — | 0.45 | |||
| N1, N2, N3 | 1.45 | (0.55; 3.87) | ||||
|
Grade (EE) | ||||||
| Grade I–II | 1 | — | 0.39 | |||
| Grade III | 0.65 | (0.25; 1.71) | ||||
|
HR | ||||||
| Positive | 1 | — | 0.41 | |||
| Negative | 1.48 | (0.59; 3.76) | ||||
|
pCR | ||||||
| Yes | 1 | — | <0.01 | |||
| No | 4.68 | (1.36–16.18) | ||||
|
Strict pCR | ||||||
| Yes | 1 | — | <0.01 | 1 | — | 0.03 |
| No | 8.81 | (1.17–66.25) | 9.15 | (1.22; 68.83) | ||
Abbreviations: BMI=body mass index; CI=confidence interval; DFS=disease-free survival; EE=Elston Ellis; HR=hazard ratio; HR=hormone receptor; pCR=pathological complete response; y.o.=years old.
The persistence of in situ carcinoma after chemotherapy was not associated with shorter DFS than the absence of any residual disease (P=0.17) or invasive disease only (P=0.32).
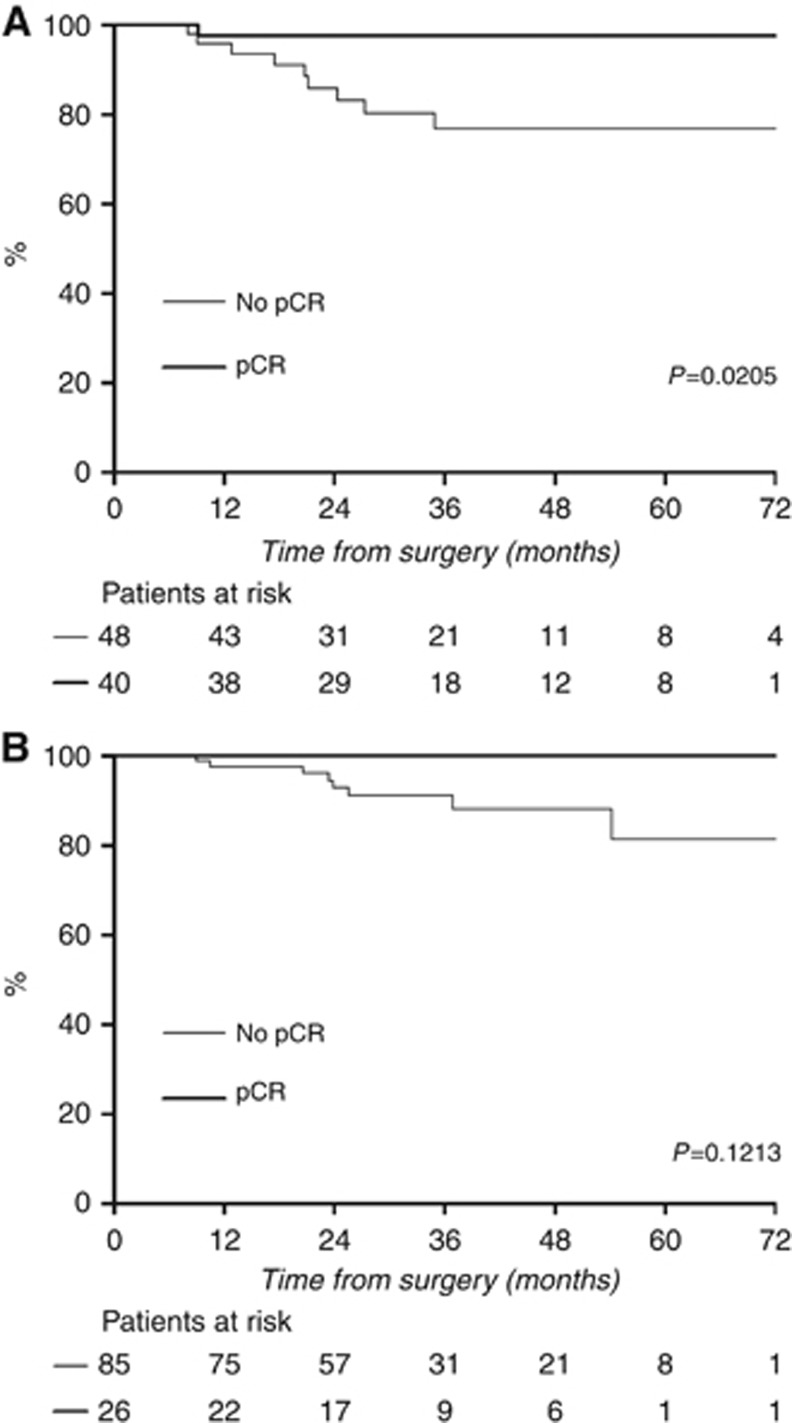
pCR was positively associated with DFS in patients with HR-negative tumours (Figure 3A) but not in those with HR-positive tumours (Figure 3B).
Figure 3.
Association of pCR and DFS in (A) patients with HR-negative tumours and (B) patients with HR-positive tumours.
Discussion
Our retrospective longitudinal study highlights the major impact of the introduction of trastuzumab on HER2-positive tumours, with a dramatic improvement in pCR (19.3 to 47.7%), DFS (5-year DFS: 48.6 to 83.5%), and OS rates (5-year OS: 76.9 to 96.9%) between ‘pre-trastuzumab' and ‘trastuzumab' eras. In patients treated by NAC plus trastuzumab, we identified age at diagnosis and HR status as predictive factors for spCR and pCR and tumour stage at diagnosis as prognostic factors for DFS.
Our study confirms that patients with pCR have excellent DFS and OS. Several studies documented trastuzumab benefits in real-world practice in the adjuvant (Vici et al, 2014; Matos et al, 2014; Inwald et al, 2014; Bonifazi et al, 2014; Seferina et al, 2015; Jackisch et al, 2014) and in the metastatic setting (Olson et al, 2013; Karam et al, 2013; Park et al, 2009; Jackisch et al, 2014). Most of these authors found that the magnitude of trastuzumab benefits was equivalent to what was observed in clinical trials (improvement the relative risk for DFS by approximately 50% and OS by 30%). Few – if any – observational studies focussed on the neoadjuvant setting. Our results suggest an even higher magnitude of trastuzumab benefits in a population of HER2-positive breast tumours treated by NAC. Because of the retrospective, non-randomised design of the study, we cannot conclude to the single role of trastuzumab effect. Indeed, there were significant differences in the three cohorts in the number of nodes involved as node-negative patients represented 77.4% of cohort C, versus 60% and 47.2% of cohorts A and B respectively. As it is known that the prognostic of breast carcinoma following NAC is largely driven by nodal status (Hennessy et al, 2005), we can assume that the dramatic differences in DFS between the three cohorts are not only explained by the trastuzumab treatment but also by post-NAC nodal status.
As expected from previous studies of neoadjuvant treatment (Untch et al, 2012; Baselga et al, 2012; Gianni et al, 2012), the absence of HR expression was an important predictor of pCR. This relationship may be quantitative, as some authors have reported an inverse correlation between the level of HR expression and pCR (Bhargava et al, 2011). Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate composed of the cytotoxic agent DM1 and trastuzumab, connected by a stable thioether linker. The ADAPT trial (NCT01745965) is currently investigating if the concomitant adjunction of endocrine therapy to T-DM1 neoadjuvant therapy would increase pCR rates in HER2+/HR+ operable breast cancers.
In our cohort, older age was significantly associated with spCR. These finding are consistent with those of a retrospective study of 229 HER2-positive tumours treated by NAC plus trastuzumab, in which both being young and premenopausal status were significantly associated with lower pCR rates (Kim et al, 2013). By contrast, Huober et al (2010) found no difference in pCR rates between two age groups (<40 years versus ⩾40 years) for 475 HER2-positive tumours. However, none of these patients were treated with neoadjuvant trastuzumab. Similarly, the German Breast Group (GBG) and the AGO-B study group published a meta-analysis focussing on the impact of age on NAC outcomes. In 1820 patients with HER2-positive tumours, pCR rates did not differ significantly with age in either HR-positive or HR-negative tumours (Loibl et al, 2015). Patients with HER2-positive disease received anti-HER2 treatment as part of the neoadjuvant treatment in three of the eight trials of this meta-analysis.
Initial T stage remained a significant prognostic factor. This finding is consistent with those of several other studies (Kim et al, 2013; Takada et al, 2014; Tanioka et al, 2014), although similar results were obtained only for the HR-negative subgroup in the study by Takada et al (2014).
In a sub-study of EORTC 10994/BIG 1-00 phase III trial (Fei et al, 2015) on 283 patients with pCR achievement after NAC, only clinical tumour size independently predicted relapse. Several hypotheses can be drawn to explain the independent impact of tumoral size. The first one is that large tumours may be more likely to present intrinsic or acquired chemoresistance. Causal factors may first include a variety of physical and mechanical effects (e.g., inefficient distribution of the drug, central necrosis and hypoxia, anarchic neoangiogenesis). Second, the immune reaction appears to evolve with tumour progression, and it is known that immune subpopulations densities change with increasing stage (Bindea et al, 2013; Fridman et al, 2012) potentially impairing the response to chemotherapy. Third, tumoral heterogeneity increases with tumour size, leading to the potential emergence of drug-multiresistant clones.
A second hypothesis considers the kinetics of the tumour growth. Mathematical modellings (Hartung et al, 2014) validate the link between primary tumour size and emission rate, that is, metastatic spreading. In clinical practice, this relation between a large tumour size and the presence of circulating tumour cells in peripheral blood has also been identified (Liao et al, 2014). Considering initial exponential growth phase of the Gompertz model (Benzekry et al, 2014) and the high proliferation rate of HER2-positive breast cancers, it seems plausible that these tumours may rapidly toggle from localised breast cancers to a micrometastatic disease. The subsequent pivotal transition between micrometastases and macrometastases (namely the metastatic colonisation) is still poorly understood. It remains unknown if tumoral size may impact this process. Both phenomena (chemoresistance and micrometastic spreading) may coexist and share pathways by complex homing interactions.
It remains a matter of debate whether pCR can be used as a surrogate for DFS in HER2-positive breast carcinomas, particularly those that are HR-positive. In our cohort, residual disease was associated with a hazard ratio for relapse of 9 relative to patients with spCR. This effect was limited to HR-negative tumours. In a large meta-analysis of 6377 patients with primary breast cancer receiving neoadjuvant anthracycline-taxane-based chemotherapy in seven randomised trials, Von Minckwitz et al (2012) identified pCR as a surrogate marker for both DFS and OS in HER2-positive subgroups. In patients with HER2-positive tumours treated with trastuzumab (n=662), pCR was associated with a hazard ratio of 2.85 ((1.69–4.83), P<0.001) for DFS and of 14.11 ((1.93–103.03), P<0.009) for OS. However, the prognostic impact of pCR was restricted to HR-negative tumours. It was not observed in the luminal B/HER2-positive subgroup. In a recent pooled analysis of 12 international trials and 11955 patients (CTNeoBC), Cortazar et al (2014) found a significant association between pCR and event-free survival in both the HR-positive and HR-negative subgroups, although the magnitude of this effect was greater in HR-negative tumours (HR-positive, 0.58 (0.42–0.82); HR-negative: 0.25 (0.18–0.34)). However, a subset of HER2-positive breast cancer did not receive adjuvant trastuzumab. When the analysis was restricted to patients who received trastuzumab, the association between pCR and OS was not significant in HR-positive tumours (0.56 (0.23–1.37)). In addition, three multicenter retrospective studies on HER2-positive breast tumours treated with NAC and trastuzumab identified pCR as a surrogate marker for DFS in HR-negative disease (Tanioka et al, 2014; Takada et al, 2014; Kim et al, 2013), but the results for the HR-positive group were discordant, with a positive association retrieved by some authors (Kim et al, 2013) but not by others (Takada et al, 2014; Tanioka et al, 2014).
Our study adds weight to the findings of previous investigations, because it focusses on a particular breast cancer subtype and reports results for a large population treated with NAC and trastuzumab, the gold standard treatment in 2015. This study provides a better representation of real-life experience than previous meta-analyses of clinical trials, because, although meta-analysis provide an effective means of acquiring large amounts of data, the patients included in clinical trials differ from the general population. Our data confirm the association of pCR with DFS in HER2-positive, HR-negative breast cancers and provide new insight that could improve prognostic prediction. The absence of a significant effect in the HR-positive subgroup might be due to biological differences though we cannot exclude a lack of statistical power.
The confirmation of a quantitative correlation between increments in pCR and gains in survival in large data sets is of paramount importance for accelerated drug approval for the neoadjuvant model. It is particularly important because the HER2-targeting drug pipeline contains many candidates. The novel anti-HER2 antibody pertuzumab has obtained accelerated approval from the US FDA (Prowell and Pazdur, 2012) for use in the neoadjuvant setting for HER2-positive breast cancer, based on the results of the NEOSPHERE trial (Gianni et al, 2012). Definitive approval for pertuzumab will depend on the results of the APHINITY trial evaluating the addition of pertuzumab to adjuvant trastuzumab-based chemotherapy. Controversy concerning the legitimacy of pCR as a surrogate re-emerged with the results of the ALTTO trial in ASCO 2014 (Piccart-Gebhart et al, 2014). In this study, addition of lapatinib to standard trastuzumab adjuvant therapy was not found to improve survival in women with HER2-positive early breast cancer. This result was unexpected, because the combination of lapatinib and trastuzumab was associated with higher rates of pCR rates in the neoALTTO trial (Baselga et al, 2012). Improving pCR rates may theoretically: (i) increase conservative treatment probabilities; and (ii) identify a population at higher risk of relapse and thus help selecting patients likely to benefit from new therapies. Accurate and sharp patient selection may avoid failure of all-comers trials such as ALTTO and MARIANNE (NCT01120184). The KATHERINE trial (NCT01772472) is currently investigating TDM-1 as alternative adjuvant treatment to trastuzumab in HER2-positive patients with residual disease following NAC. Our study supports this design for new drug testing, bearing in mind that even in patients with residual disease DFS rates were high in our cohort.
Conclusion
Trastuzumab considerably modifies the prognosis of HER2-positive breast carcinomas. These tumours have an excellent prognosis when pCR is achieved. However, it remains unclear whether second-line HER2-targeted treatments with pertuzumab, lapatinib or TDM1 following NAC improve survival in selected patients. Our findings suggest that patients with HER2-positive tumours of a large initial size, for which pCR is not achieved at the end of NAC, remain at risk of relapse despite adjuvant trastuzumab treatments. Such patients could be studied in second-line treatment trials. However, there is a need to rethink future clinical trial designs bearing in mind several pitfalls: (i) sufficient recruitment of patients despite the scarcity of trastuzumab-resistant patients; (ii) consider a different disease setting with possibly already micrometastatic populations and thus consider new therapeutic targets to investigate (Mina and Sledge, 2011); and (iii) finally, expected events may be low, and only international collaborative works will allow sufficient population size. The challenge still needs to be overcome.
Acknowledgments
We thank Roche, France for financial support for construction of the Institut Curie neoadjuvant database (NEOREP). Funding was also obtained from the Site de Recherche Integrée en Cancérologie/Institut National du Cancer (Grant No. INCa-DGOS-4654). A-S Hamy-Petit was supported by an ITMO-INSERM-AVIESAN cancer translational research grant.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang T-W, Horváth Z, Coccia-Portugal M, Domont J, Tseng L-M, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M NeoALTTO Study Team (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379: 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzekry S, Lamont C, Beheshti A, Tracz A, Ebos JML, Hlatky L, Hahnfeldt P (2014) Classical mathematical models for description and prediction of experimental tumor growth. PLoS Comput Biol 10: e1003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava R, Dabbs DJ, Beriwal S, Yildiz IA, Badve P, Soran A, Johnson RR, Brufsky AM, Lembersky BC, McGuire KP, Ahrendt GM (2011) Semiquantitative hormone receptor level influences response to trastuzumab-containing neoadjuvant chemotherapy in HER2-positive breast cancer. Mod Pathol 24: 367–374. [DOI] [PubMed] [Google Scholar]
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pagès F, Speicher MR, Trajanoski Z, Galon J (2013) Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39: 782–795. [DOI] [PubMed] [Google Scholar]
- Bonifazi M, Franchi M, Rossi M, Zambelli A, Moja L, Zambon A, Corrao G, La Vecchia C, Zocchetti C, Negri E (2014) Long term survival of HER2-positive early breast cancer treated with trastuzumab-based adjuvant regimen: a large cohort study from clinical practice. Breast 23: 573–578. [DOI] [PubMed] [Google Scholar]
- Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, Pusztai L, Green MC, Singletary SE, Hunt KK, Sahin AA, Esteva F, Symmans WF, Ewer MS, Buchholz TA, Hortobagyi GN (2007) Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res 13: 228–233. [DOI] [PubMed] [Google Scholar]
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384: 164–172. [DOI] [PubMed] [Google Scholar]
- Fei F, Messina C, Slaets L, Chakiba C, Cameron D, Bogaerts J, Bonnefoi H (2015) Tumour size is the only predictive factor of distant recurrence after pathological complete response to neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancers: a sub-study of EORTC 10994/BIG 1-00 phase III trial. Eur J Cancer 51: 301–309. [DOI] [PubMed] [Google Scholar]
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12: 298–306. [DOI] [PubMed] [Google Scholar]
- Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375: 377–384. [DOI] [PubMed] [Google Scholar]
- Gianni L, Pienkowski T, Im Y-H, Roman L, Tseng L-M, Liu M-C, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im S-A, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13: 25–32. [DOI] [PubMed] [Google Scholar]
- Gligorov J, Namer M (2007) Recommandations pour la Pratique Clinique: Saint Paul de Vence. Oncologie 9: 593–644. [Google Scholar]
- Hartung N, Mollard S, Barbolosi D, Benabdallah A, Chapuisat G, Henry G, Giacometti S, Iliadis A, Ciccolini J, Faivre C, Hubert F (2014) Mathematical modeling of tumor growth and metastatic spreading: validation in tumor-bearing mice. Cancer Res 74: 6397–6407. [DOI] [PubMed] [Google Scholar]
- Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17: 1474–1481. [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Hortobagyi GN, Rouzier R, Kuerer H, Sneige N, Buzdar AU, Kau SW, Fornage B, Sahin A, Broglio K, Singletary SE, Valero V (2005) Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol 23: 9304–9311. [DOI] [PubMed] [Google Scholar]
- Huober J, von Minckwitz G, Denkert C, Tesch H, Weiss E, Zahm DM, Belau A, Khandan F, Hauschild M, Thomssen C, Högel B, Darb-Esfahani S, Mehta K, Loibl S (2010) Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat 124: 133–140. [DOI] [PubMed] [Google Scholar]
- Inwald EC, Ortmann O, Zeman F, Koller M, Hofstädter F, Gerstenhauer M, Klinkhammer-Schalke M (2014) Guideline concordant therapy prolongs survival in HER2-positive breast cancer patients: results from a large population-based cohort of a cancer registry. BioMed Res Int 2014: 137304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackisch C, Schoenegg W, Reichert D, Welslau M, Selbach J, Harich H-D, Tesch H, Wohlfarth T, Eustermann H, Hinke A (2014) Trastuzumab in advanced breast cancer—a decade of experience in Germany. BMC Cancer 14: 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam I, Hamilton S, Nichol A, Woods R, Speers C, Kennecke H, Tyldesley S (2013) Population-based outcomes after brain radiotherapy in patients with brain metastases from breast cancer in the pre-Trastuzumab and Trastuzumab eras. Radiat Oncol 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MM, Allen P, Gonzalez-Angulo AM, Woodward WA, Meric-Bernstam F, Buzdar AU, Hunt KK, Kuerer HM, Litton JK, Hortobagyi GN, Buchholz TA, Mittendorf EA (2013) Pathologic complete response to neoadjuvant chemotherapy with trastuzumab predicts for improved survival in women with HER2-overexpressing breast cancer. Ann Oncol 24: 1999–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Wang S-Y, Meng X-Y, Yang J, Shi M-J, Liu H-L, Chen F-F, Xiong B (2014) Circulating tumor cells in breast cancer and its association with tumor clinicopathological characteristics: a meta-analysis. Med Oncol 31: 343. [DOI] [PubMed] [Google Scholar]
- Loibl S, Jackisch C, Lederer B, Untch M, Paepke S, Kümmel S, Schneeweiss A, Huober J, Hilfrich J, Hanusch C, Gerber B, Eidtmann H, Denkert C, Costa SD, Blohmer J-U, Nekljudova V, Mehta K, von Minckwitz G (2015) Outcome after neoadjuvant chemotherapy in young breast cancer patients: a pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res Treat 152: 377–387. [DOI] [PubMed] [Google Scholar]
- Matos E, Zakotnik B, Kuhar CG (2014) Effectiveness of adjuvant trastuzumab in daily clinical practice. Radiol Oncol 48: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina LA, Sledge GW (2011) Rethinking the metastatic cascade as a therapeutic target. Nat Rev Clin Oncol 8: 325–332. [DOI] [PubMed] [Google Scholar]
- Olson EM, Najita JS, Sohl J, Arnaout A, Burstein HJ, Winer EP, Lin NU (2013) Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast 22: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Park MJ, Ji SH, Yi SY, Lim DH, Nam DH, Lee J-I, Park W, Choi DH, Huh SJ, Ahn JS, Kang WK, Park K, Im Y-H (2009) Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer 100: 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Holmes AP, Baselga J, Azambuja ED, Dueck AC, Viale G, Zujewski JA, Goldhirsch A, Santillana S, Pritchard KI, Wolff AC, Jackisch C, Lang I, Untch M, Smith IE, Boyle F, Xu B, Gomez HL, Gelber RD, Perez EA (2014) First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L), or their combination (T+L) in the adjuvant treatment of HER2-positive early breast cancer (EBC). J Clin Oncol 32: 5s. [Google Scholar]
- Prowell TM, Pazdur R (2012) Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 366: 2438–2441. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2011) R: A Language and Environment for Statistical Computing. The R Foundation for Statistical Computing: Vienna, Austria, Available from http://www.R-project.org/. [Google Scholar]
- Seferina SC, Lobbezoo DJA, de Boer M, Dercksen MW, van den Berkmortel F, van Kampen RJW, van de Wouw AJ, de Vries B, Joore MA, Peer PGM, Voogd AC, Tjan-Heijnen VCG (2015) Real-life use and effectiveness of adjuvant trastuzumab in early breast cancer patients: a study of the Southeast Netherlands Breast Cancer Consortium. Oncologist 20: 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Ishiguro H, Nagai S, Ohtani S, Kawabata H, Yanagita Y, Hozumi Y, Shimizu C, Takao S, Sato N, Kosaka Y, Sagara Y, Iwata H, Ohno S, Kuroi K, Masuda N, Yamashiro H, Sugimoto M, Kondo M, Naito Y, Sasano H, Inamoto T, Morita S, Toi M (2014) Survival of HER2-positive primary breast cancer patients treated by neoadjuvant chemotherapy plus trastuzumab: a multicenter retrospective observational study (JBCRG-C03 study). Breast Cancer Res Treat 145: 143–153. [DOI] [PubMed] [Google Scholar]
- Tanioka M, Sasaki M, Shimomura A, Fujishima M, Doi M, Matsuura K, Sakuma T, Yoshimura K, Saeki T, Ohara M, Tsurutani J, Watatani M, Takano T, Kawabata H, Mukai H, Naito Y, Hirokaga K, Takao S, Minami H (2014) Pathologic complete response after neoadjuvant chemotherapy in HER2-overexpressing breast cancer according to hormonal receptor status. Breast 23: 466–472. [DOI] [PubMed] [Google Scholar]
- Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer J-U, Hilfrich J, Strumberg D, Fasching PA, Kreienberg R, Tesch H, Hanusch C, Gerber B, Rezai M, Jackisch C, Huober J, Kühn T, Nekljudova V, von Minckwitz G German Breast Group (GBG)Arbeitsgemeinschaft Gynäkologische Onkologie-Breast (AGO-B) Study Group (2012) Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol 13: 135–144. [DOI] [PubMed] [Google Scholar]
- Vici P, Pizzuti L, Natoli C, Moscetti L, Mentuccia L, Vaccaro A, Sergi D, Di Lauro L, Trenta P, Seminara P, Santini D, Iezzi L, Tinari N, Bertolini I, Sini V, Mottolese M, Giannarelli D, Giotta F, Maugeri-Saccà M, Barba M, Marchetti P, Michelotti A, Sperduti I, Gamucci T (2014) Outcomes of HER2-positive early breast cancer patients in the pre-trastuzumab and trastuzumab eras: a real-world multicenter observational analysis. The RETROHER study. Breast Cancer Res Treat 147: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Minckwitz G, Untch M, Blohmer J-U, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30: 1796–1804. [DOI] [PubMed] [Google Scholar]
- Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical OncologyCollege of American Pathologists (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25: 118–145. [DOI] [PubMed] [Google Scholar]