Abstract
Leptospirosis causes significant economic loss within the cattle industry worldwide. Current diagnostic methods are generally inadequate for dealing with large numbers of samples, are outdated, and provide little useful diagnostic and epidemiological information. This aim of this study was to apply a microsphere immunoassay (MIA), utilising Luminex xMap technology, to 200 bovine serum samples to determine this method's usefulness in leptospirosis diagnosis in comparison with the current gold standard, the microscopic agglutination test (MAT). Although MAT is the most widely used laboratory test for the diagnosis of leptospirosis, its reliance on live cultures, subjective interpretation of results and an inability to differentiate between antibody classes, suggest MAT is no longer the best method for the diagnosis of leptospirosis. The results presented in this paper show that MIA was able to determine reactive from non-reactive samples when compared with MAT, and was able to differentiate IgG and IgM classes of antibody. The results suggest increased sensitivity in MIA and the ability to multiplex up to 500 antigens at one time allows for significant improvements in cost-effectiveness as well as a reduced dependency on live cultures. The relatively low cost, high throughput platform and differentiation of antibody class, as shown in previous research, make this assay worthy of consideration for the diagnosis of leptospirosis in small-scale or large-scale bovine populations.
Keywords: Leptospira, Leptospira hardjo, Cattle, Dairy cattle, Diagnostics
Leptospirosis infections are widely recognised as a common cause of reproductive failure and economic loss in cattle. Leptospira borgpetersenii serovar Hardjo and L. interrogans serovar Pomona have been shown to be the most important pathogenic serovars in cattle, responsible for systemic illness, abortion, neonatal death, weak calves and various production losses throughout the world (Bolin and Alt 2001). Serovars causing infection in cattle have been split into two groups; those adapted to and maintained by cattle (serovar Hardjo) and incidental infections caused by serovars maintained by other domestic and wild animals (Aslantas and Ozdemir 2005). Serovars for the latter group are dependent on the country and area in which cattle are located. In Australia, serovars Hardjobovis, Pomona and L. borgpetersenii serovar Tarassovi are the most commonly serologically diagnosed serovars (Cousins and others 1985) with infection rates varying from state to state. Leptospira weilii serovar Topaz has also become a commonly diagnosed infecting serovar in Australia since its isolation in cattle in 1994 (Corney and others 2008). Leptospiral infections in cattle are generally subclinical, produce low antibody titres, and can affect animals with rapid transmission rates. Infections in cattle can occur through mucous membranes or through abrasions in the skin with direct transmission occurring among animals through exposure to infected urine, postabortion uterine discharge or through milk (Hairgrove 2004). Serovar Hardjo is associated with a prolonged renal carrier state and may also be associated with chronic renal disease (Hairgrove 2004) suggesting that leptospires are present in the urine for long periods of time. Leptospires within the proximal renal tubules, genital tract and mammary glands of cattle have been shown to be protected from circulating antibodies (Ellis 1994) which allows persistence and multiplication in these areas. Serum antibody levels often decline to undetectable levels in chronic leptospirosis infections in cattle, making diagnosis extremely difficult in many cases.
Serological diagnosis of leptospirosis in cattle is most often performed by microscopic agglutination testing (MAT) but can also be performed by ELISA. These methods have been shown to have some disadvantages. In a review of laboratory techniques for diagnosing leptospiral infections in cattle, Smith and others (1994) highlighted several issues with the current diagnostic methods, in particular MAT. Leptospiral antibodies can persist in cattle with titres of >1:400 for up to 12 months and in some cases for up to two years (Smith and others 1994). However, agglutinating antibodies commonly wane over time and the sensitivity of MAT in detecting these antibodies in animals infected for more than two years is low (Allen and others 1982). Previous serology studies (Prescott and others 1988) have shown that due to the low sensitivity of MAT, seroprevalence may in fact be double the reported figures for specific serovars in cattle. It has been suggested that increasing the sensitivity by decreasing the initial serum dilution (Blackmore 1985) may resolve the under-reporting of seroprevalence however this may also increase the rate of false positives. Smith and others (1994) also point out that a major concern with MAT testing of cattle samples is that there is no differentiation between IgG and IgM antibodies and therefore vaccination status and efficacy cannot be determined. Paired serum samples are currently required to estimate the stage of infection. With MAT however, determining the stage of infection can be difficult in cases of bovine abortion or stillbirth, as infection most commonly occurs one week to four weeks before the expulsion of the fetus and by this time MAT titres have stabilised (Cousins and others 1985). Paired sample collection can also be difficult due to the costs involved in sample collection and testing. Few false-positive results occur in MAT as the surface antigens of leptospires are not shared with any other organism; however cross-reactions do occur within and between some leptospiral serogroups. The use of live leptospires as antigens in MAT is also a disadvantage of this test. Problems associated with this include the lack of antigen standardisation and quality control and that culture maintenance is both time-consuming and costly. The MAT utilises a specific panel of antigens for each test depending on the region of sample collection, availability of antigens and cost-effectiveness. Therefore, another disadvantage of this assay is that, in areas where endemic strains are unknown, or poorly characterised, the diagnosis of leptospirosis may be excluded due to a lack of utilising the correct panel of antigens.
The use of ELISA for testing cattle samples has some advantages over MAT including the use of inactivated antigens, objective analysis and determination of antibody class. However, there are still some issues with this type of test. ELISA has been shown to be more sensitive than MAT (Cousins and others 1985) but in human samples it is often too sensitive and produces large numbers of false-positive results. Although ELISA has some advantages over MAT, it is not used routinely as a diagnostic test for leptospirosis as it is unable to provide serovar-specific results for large numbers of samples. Bercovich and others (1990) evaluated the ELISA method for the specific detection of Hardjo infections and found it to be an advantageous alternative to MAT. However, cross-reactions are often seen with leptospirosis and ELISA cannot determine the source of cross-reactions in one test. The need for more sensitive, specific and high-throughput diagnostic testing for leptospiral infections in cattle has been highlighted previously (Gardner and others 1996).
The aim of this study was to apply a microsphere immunoassay (MIA), utilising Luminex xMap technology to bovine serum samples to determine the usefulness of leptospirosis diagnosis by MIA in comparison with the current gold standard, MAT.
Materials and methods
Ethics
The study was approved by the Public and Environmental Health Research Committee and the Humans Ethics Committee, Queensland Health Forensic and Scientific Services. The ethics approval number for this project is HEC 13-17.
Samples
Bovine serum samples for routine leptospirosis serology were submitted to the WHO/Food and Agriculture Organization/OIE Collaborating Centre for Leptospirosis Reference and Research during 2013 from veterinary laboratories around Australia. There was no associated information available for the samples indicating vaccination status, clinical signs and symptoms or reasons for testing. A subsample was selected from MAT reactive and non-reactive test results, de-identified and re-tested by the gold standard MAT method (Faine 1982), using a panel of 12 endemic serovars (Table 1). Briefly, serum was diluted to 1:25 in phosphate buffered saline (PBS) and dispensed into a 96-well microtitre plate followed by the addition of a pure leptospiral culture. Agglutination was observed under dark field microscopy and reported as a titre, the end point being the final dilution of serum at which 50 per cent or more of the leptospires are agglutinated.
TABLE 1:
Leptospira cultures (antigens) used in MAT and bovine microsphere immunoassay with associated bead-set numbers
| Serovar | COOH bioplex magnetic bead-set number |
|---|---|
| Leptospira Interrogans serovar Pomona | 45 |
| Leptospira Borgpetersenii serovar Hardjobovis | 27 |
| L. Borgpetersenii serovar Tarassovi | 35 |
| L. Interrogans serovar Australis | 26 |
| L. Interrogans serovar Zanoni | 28 |
| L. Interrogans serovar Robinsoni | 34 |
| L. Interrogans serovar Canicola | 52 |
| L. Interrogans serovar Szwajizak | 44 |
| L. Interrogans serovar Medanensis | 43 |
| Leptospira Kirschneri serovar Grippotyphosa | 54 |
| L. Borgpetersenii serovar Arborea | 20 |
| Leptospira Weilii serovar Topaz | 29 |
MAT, microscopic agglutination test
IgG and IgM MIA for leptospirosis diagnosis in human beings (Wynwood and others 2015) were adapted for use with bovine serum samples. This assay has the ability to test up to 88 samples per plate at one time (excluding any control samples). Two MIAs (IgG and IgM) were performed on the 200 bovine serum samples.
Antigen preparation
Antigen preparation for use in MIA utilised pure leptospiral cultures. Whole cell cultures (antigens) were centrifuged, washed with PBS and then diluted in PBS to give a cell density of 1.8×109/ml and further diluted 1:4 in Triton-X 100 detergent. Then 25 μl of each antigen was suspended in 1 ml Ellinghausen-McCullough-Johnson-Harris broth to ensure that cellular inactivation had occurred. The broths were checked under dark field microscopy every 3 days for 21 days. An absence of leptospires in the broths indicated that any live leptospires had been inactivated.
Antigen coupling
Leptospiral antigen preparations (listed in Table 1) were covalently coupled to individual Bio-Plex Pro Magnetic COOH bead-sets using the Bio-Rad Amine Coupling Kit, producing 160 μl of each coupled bead-set. The bead yield per coupling reaction was approximately 2500 beads per well (in a 96-well microtitre plate). Each individual coupled bead-set was further diluted in PBS to a working dilution, just before performing the assay, to give a reading of approximately 100 beads per bead-set per well as per the manufacturer's instructions (Biorad Instruction Manual 2005). Coupled beads were then checked for sensitivity and specificity using rabbit anti-sera of known serovar and titre, obtained from MAT results (Wynwood and others 2015).
Microsphere immunoassays
Samples for the IgG immunoassay were diluted 1:200 in PBS and conjugated with biotinylated Sigma Protein A. Samples for the IgM immunoassay were diluted to 1:400 in PBS and conjugated with KPL Biotin-Labelled Bovine IgM antibody.
Working dilutions (100 μl) of coupled beads were added to each required well of a 96-well microtitre filter plate and a vacuum applied. The diluted samples (100 μl) were then added to the plate and incubated for 45 minutes on a shaker at room temperature (18–24°C).
The plates were then vacuum-washed three times with 150 μl PBS per well. Biotinylated secondary antibodies were then added followed by a second 45 minute incubation and a vacuum wash step applied as described above. One hundred microliters of diluted streptavidin R-PE, a biotin-binding protein, was added to each well followed by a final incubation and wash step as per previous steps. For resuspension of beads 150 μl of PBS per well was added to the plate which was placed on the shaker at room temperature (18–24°C) for 10 minutes. All plate wells were then analysed using Luminex xMap technology on a BioPlex 200 platform. The MIA results are reported as mean fluorescent intensity and were deemed congruent or incongruent relative to the standard of comparison (MAT) and based on the cut-off points. Cut-off points for determination of reactive versus non-reactive results were based on previous human sample assays (Wynwood 2015) and established using known reactive and known non-reactive serum samples. Cut-off values for reactive samples were determined using five reactive sera for each MAT titre ranging from 1:100 to 1:6400 and developing a standard curve (Wynwood and others 2015) using the titres obtained from MAT testing and comparing them with the mean fluorescent intensities from the MIA titrations. These cut-off points are shown in Table 2.
TABLE 2:
Cut-off points for reactivity equivalents of samples (Wynwood and others 2015)
| MAT Titre | MIA IgG and IgM MFI | |
|---|---|---|
| Non-reactive | <1:50 | <1200 |
| Equivocal | 1:50–1:200 | 1201–3999 |
| Reactive low | 1:400–1:1600 | 4000–9999 |
| Reactive high | 1:3200+ | 10000+ |
MAT, microscopic agglutination test; MIA, microsphere immunoassay
Results
A total of 200 bovine serum samples were tested. Table 3 shows that the results revealed a greater number of reactive samples using MIA compared with MAT (see also Figs 1 and 2). The majority of the MIA reactive samples (75) were IgM reactive only, 13 were IgG reactive only and 22 were reactive for IgM and IgG.
TABLE 3:
Comparison of leptospirosis serology results for validation samples
| MIA IgG and IgM |
||
|---|---|---|
| REACT | NR | |
| MAT (Total Ab) | ||
| Reactive N=64 | 53 | 11 |
| Non-reactive N=136 | 46 | 90 |
MAT, microscopic agglutination test; MIA, microsphere immunoassay
FIG 1.

A comparison of the microsphere immunoassay (MIA) and microscopic agglutination test (MAT) reactive samples
FIG 2.

Analysis of reactive samples by antibody type measured by microsphere immunoassay (MIA)
Serovar Hardjo was the dominant infecting serovar in the reactive MAT (32 per cent) and reactive MIA IgG (54 per cent) samples. Fig 3 shows the MIA IgG and MAT results for one bovine sample. These results show the typical antibody pattern seen in serovar Hardjo infections in cattle in Australia when tested against all 12 serovars. Typical cross reactions are seen with members of the same serogroup (Sejroe) including serovar Medanensis as well as serovar Szwajizak (Mini serogroup) and serovar Pomona (Pomona serogroup) as expected (Corney, and others 1993).
FIG 3.
Serovar analysis of a typical Hardjo reactive bovine sample. MAT, microscopic agglutination test; MIA, microsphere immunoassay
Table 4 shows the breakdown of the serovar results for this assay. The majority of the IgM reactive serum samples, 74 (76 per cent), resulted in non-specific reactions as expected with serology samples at an unknown stage.
TABLE 4:
Reactive samples results
| Serovar | ||
|---|---|---|
| IgG | IgM | |
| Arborea | 6 | |
| Australis | 14 | |
| Hardjo | 19 | |
| Zanoni | ||
| Topaz | ||
| Robinsoni | ||
| Tarassovi | 5 | 3 |
| Medanensis | 3 | |
| Szwajizak | ||
| Pomona | 3 | |
| Canicola | ||
| Grippotyphosa | 3 | |
| Non-specific | 2 | 74 |
| Total: | 35 | 97 |
Discussion
Diagnostic testing plays an important role in monitoring and maintaining the health of livestock, detecting exposure to and identifying infectious agents in an individual infection, or a herd epidemic (Gardner and others 1996). The results of this study suggest that using MIA for diagnostic testing of bovine samples has better quality control and is capable of providing more valuable diagnostic information when compared with the current routine diagnostic method, MAT. In particular, as there are currently more than 300 known leptospiral serovars, a multiplexing assay, such as MIA, potentially allows for the comprehensive screening and serovar differentiation for large numbers of samples in a short amount of time, reducing labour, time and costs when compared with MAT. Wynwood and others (2015) suggest that MIA reduces testing costs by almost 30 per cent when compared with MAT as well as reducing the amount of serum required to perform the testing.
There are a number of potential logistic and other advantages of employing MIA rather than MAT. As MIA utilises inactivated, quantitated antigens which are stable in the long term (Biorad Instruction Manual 2005), the ability to manage and monitor quality control of the antigens provides a stable basis for rigorous method validation and quality assurance, and allows the generation and maintenance of a large panel of serovars. MIA also eliminates the need for continuous subculturing of many tubes of live leptospires as the antigens prepared for bead coupling are stable for at least six months at −20°C (Biorad 2005). Further studies will determine whether this shelf life may be extended. In contrast to MIA, MAT utilises live leptospires as antigens which causes problems in antigen standardisation, is a risk to staff performing the test, and maintenance stocks require continual subculturing. Of the 64 MAT reactive samples, only 53 (83 per cent) were also reactive on MIA. The remaining 11 (17 per cent) had low-level MAT titres (1:50–1:100) and were non-reactive on MIA. False-positive samples in MAT, when compared with MIA results, may be due to the effects of autoagglutination if the age of MAT cultures is not optimal, if there is a heavy culture density or as a result of contamination in the microtitre plate. MAT also relies heavily on the training and skill of the operator. Results are determined as the titre with the end point being the final dilution of serum at which 50 per cent or more of the leptospires can be seen to be agglutinated. An advantage of MIA is that the level of antibody present determines the intensity of fluorescence, making the analysis of the results, in this sense at least, objective, by defining fluorescence levels for determination of reactive versus non-reactive results.
Although the MIA can utilise up to 500 antigens, in this study, a selected panel of 12 Australian endemic leptospiral serovars was used to detect leptospiral IgG and IgM antibodies in bovine serum samples (Table 1). These serovars were chosen to represent current known circulating serovars in Australia. In contrast, the current MAT panel utilised for routine bovine sample testing at the WHO/FAO/OIE Collaborating Centre for Leptospirosis Reference and Research, Queensland, Australia, consists of only three serovars – Hardjo, Pomona and Tarassovi. Serovar Topaz is also included for completeness since its isolation in a bovine urine sample in Australia in 1994 (Corney and others 2008).
MIA can be utilised to test large numbers of cattle samples in a small space of time making it an ideal test for vaccine efficacy studies, management of large cattle populations and disease monitoring. Previous studies have indicated that the ability to increase numbers of bovine samples tested will improve epidemiological information (Hathaway and others 1986) and provide better diagnostic information relating to prevalence of the disease and specific serovars than what is currently available with MAT.
Although ELISA has been shown to be specific for serovars when coated with the desired serovar antigen (Smith and others 1994) the multiplexing ability of MIA allows potentially up to 500 pathogens in one well at a time. The major disadvantage of ELISA is that to achieve the same results, each sample would need to be tested on individually coated plates for each antigen.
Leptospiral antibody was detected in an extra 46 samples in MIA when compared with MAT. This may be due to MIA being more sensitive (that is, detecting lower antibody titres), or due to false-positive results. Previous work (Wynwood and others 2015) strongly suggests the former, rather than the latter: In this previous work, both acute and convalescent samples were available and antibodies that were detected in the acute samples (correspondingly lower titres) by MIA, were then detected in convalescent sera (correspondingly higher titres) by MIA and also by MAT, suggesting that MIA is more sensitive than MAT. Nevertheless, for this current study, whilst it is likely that MIA produced a larger number of reactive samples than MAT due to increased sensitivity rather due to an increase in false-positive results, more work needs to be done to confirm that this is the case. This is of particular interest since it has also been shown that low level IgM can persist for up to two years in bovine samples (Worthington 1982) and these titres are not always at a level high enough for MAT to detect. Using MIA, these persistent low-level antibodies could be detected and monitored, giving a more thorough indication of the progression of leptospirosis in cattle.
Although a reactive serology result does not always indicate current infection (Milner and others 1980) a diagnostic assay with the ability to determine antibody class does make the determination of the stage or progress of infection possible. Agglutinating antibody titres after vaccination are generally lower than those following a natural infection (Kingscote and Proulx 1986). Given the potential higher sensitivity of MIA, it may be possible to pick up postvaccination IgG antibody as postvaccination antibodies decrease rapidly (Marshall and others 1979). Allen and others (1982) showed that 95 per cent of vaccinated cattle did not show antibody on MAT 20 weeks postvaccination. The absence of te reactive MAT does not mean that protection has waned—it may be that the antibody is at a level below the detectable limit of MAT. Infections with serovar Hardjo, in particular, can be difficult to diagnose serologically. In one study of abortion caused by infection with serovar Hardjo, 22.8 per cent of aborting cattle had no detectable antibodies (by MAT) at the time of abortion (Ellis and others 1982). A similar study (Ellis and others 1981) found that 19.6 per cent of Hardjo renal carriers had no detectable agglutinating antibodies by MAT. In these types of cases, a more sensitive test, such as MIA, would be beneficial.
Maintenance host disease is often difficult to diagnose as it is generally subclinical (Hairgrove 2004), producing low antibody titres and has rapid transmission rates. In maintenance hosts, leptospirosis is generally characterised by a high prevalence of infection, relatively mild clinical signs, and persistent infection in the kidney and sometimes the genital tract. The serovar Hardjo IgG reactive results in this study are possibly the result of previous vaccinations, which, in Australia include serovars Hardjo and Pomona (Virbac 2012), but could also be the result of past infections. In this study, there was no information available on the clinical or vaccination status of each animal therefore it is difficult to confirm the cause of the reactive IgG antibody. However, in the absence of the IgM antibody, it is plausible that there was no current infection at the time of sample collection. The high number of samples showing low level IgM may occur as a result of an acute infection but may also be due to a chronic infection. Chronically infected cattle are difficult to diagnose with MAT as the titres often fall below detectable levels (Smith and others 1994) and no differentiation of antibody class is possible. It is often assumed that a static antibody level in MAT is indicative of a past infection only, however this may also be the result of chronic infection with persistent IgM. Seventy-five per cent of IgM reactive samples in this study showed non-specific IgM, reflecting the constant challenge within the herd and incidental transmission from other domestic and wild animals. Non-specific reactions are those where a sample has reactive serology for more than one serovar where there is a difference of less than 1.5 times. The most commonly seen serovars in the non-specific IgM reactions were L. interrogans serovar Australis, L. interrogans serovar Canicola, serovars Topaz and Tarassovi (Fig 4) which are all serovars which are associated with Australian domestic animals and wildlife. In Australia, serovars Hardjo, Pomona and L. interrogans serovar Zanoni (McClintock and others 1993) and serovar Topaz (Corney and others 2008) have been isolated in cattle while common serological reactions include serovars Hardjo, Pomona, Tarassovi, L. interrogans serovar Australis, L. interrogans serovar Szwajizak, L. interrogans serovar Medanensis and L. interrogans serovar Kremastos (Corney and others 1993). Leptospirosis has been found in many wild animal species in Australia including wallabies, possums, rats (Milner and others 1981), Eastern grey kangaroos (Roberts and others 2010) and feral pigs (Mason and others 1998). The most common serovars noted in these cases are Pomona, Tarassovi and Australis (Roberts and others 2010) which is consistent with what is being seen in cattle with low level cross-reacting IgM antibody reactions. These reactions may also suggest early immune-phase infections before maturation of antibodies.
FIG 4.
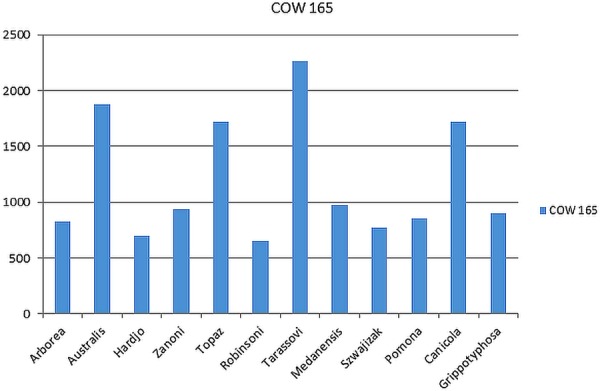
Non-specific cross-reacting IgM sample
In Australia, leptospirosis research in bovine populations has halted over the last decade. Thorough serological investigations will provide a better picture of current circulating serovars and the physiological and epidemiological effects of leptospirosis in Australian cattle. In summary, the results from this study suggest that MIA is a beneficial diagnostic tool able to differentiate between IgG and IgM antibody classes, reduce costs and test large numbers of samples against many serovars at one time. Further work will need to be performed on this assay for validation purposes and in other animal species. Future sero-survey data utilising MIA would assist in determining the seroprevalence and variations of disease pattern and impact of leptospirosis serovars in cattle in Australia.
Acknowledgments
Technical advice: C.T. Taylor, Queensland Health Public and Environmental Health Virology Laboratory (Serology), and M.F. Dohnt, WHO/OIE/FAO Collaborating Centre for Reference and Research on Leptospirosis, Queensland Health Forensic and Scientific Services.
Footnotes
Funding: FSS Operational Funding, Queensland Health Communicable Diseases Department.
Ethics approval: Forensic and Scientific Services (FSS) Research and Development Advisory Committee, Queensland Health.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: Data is available on request to the corresponding author.
References
- Allen J. D., Meney C. L., Wilks C. R. (1982) Evaluation of a hardjo-pomona vaccine to prevent leptospuria in cattle exposed to a natural challenge with Leptospira Interrogans serovar hardjo. Australian Veterinary Journal 58, 93–96 doi:10.1111/j.1751-0813.1982.tb00598.x [DOI] [PubMed] [Google Scholar]
- Aslantas O., Ozdemir V (2005) Determination of the seroprevalence of leptospirosis in cattle by MAT and ELISA in Hatay, Turkey. Turkish Journal Veterinary Science 29, 1019–1024 [Google Scholar]
- Bercovich Z., Taaijke R., Bokhout B. A. (1990) Evaluation of an ELISA for the diagnosis of experimentally induced and naturally occurring Leptospira hardjo infections in cattle. Veterinary Microbiology 21, 255–262 doi:10.1016/0378-1135(90)90036-U [DOI] [PubMed] [Google Scholar]
- Biorad Instruction Manual. (2005) Bio-Plex Pro Magnetic COOH Beads, Bio-Plex COOH Beads, Amine Coupling Kit – Instruction Manual
- Blackmore D. K. (1985) Dairy Cattle Production. Proceedings of the Post Graduate Committee in Veterinary Science University of Sydney, Vol. 78, 425 [Google Scholar]
- Bolin C. A., Alt D. P. (2001) Use of a monovalent leptospiral vaccine to prevent renal colonization and urinary shedding in cattle exposed to Leptospira borgpetersenii serovar hardjo. American Journal Veterinary Research 62, 995–1000 doi:10.2460/ajvr.2001.62.995 [DOI] [PubMed] [Google Scholar]
- Corney B. G., Slack A. T., Symonds M. L., Dohnt M. F., McClintock C. S., McGowan M. R., Smythe L. D. (2008) Leptospira weilii serovar Topaz, a new member of the Tarassovi serogroup isolated from a bovine source in Queensland, Australia. International Journal of Systematic and Evolutionary Microbiology 58, 2249–2252 doi:10.1099/ijs.0.64884-0 [DOI] [PubMed] [Google Scholar]
- Cousins D. V., Robertson G. M., Hustas L. (1985) The use of enzyme-linked immunosorbent assay (ELISA) to detect the IgM and IgM antibody response to Leptospira Interrogans serovars Hardjo, Pomona and Tarassovi in cattle. Veterinary Microbiology 10, 439–450 doi:10.1016/0378-1135(85)90026-4 [DOI] [PubMed] [Google Scholar]
- Ellis W. A. (1994) Leptospirosis as a cause of reproductive failure. Veterinary Clinics North America Food Animal Practice 10, 463–478 [DOI] [PubMed] [Google Scholar]
- Ellis W. A., O'Brien J. J., Cassells J. (1981) Role of cattle in the maintenance of Leptospira interrogans serotype hardjo infection in Northern Ireland. Veterinary Record 108, 555–557 doi:10.1136/vr.108.26.555 [DOI] [PubMed] [Google Scholar]
- Ellis W. A., O'Brien J. J., Neill S. D., Hanna J. (1982) Bovine Leptospirosis: microbiological and serological findings in aborted foetuses. Veterinary Record 110, 147–150 doi:10.1136/vr.110.7.147 [DOI] [PubMed] [Google Scholar]
- Faine S. Guidelines for the Control of Leptospirosis. Geneva, Switzerland: World Health Organization, 1982. [Google Scholar]
- Gardner I. A., Hietala S., Boyce W. M. (1996) Validity of using serological tests for diagnosis of diseases in wild animals. Revue Scientifique Technique 15, 323–335 [DOI] [PubMed] [Google Scholar]
- Hairgrove T. (2004) Leptospirosis in cattle. AABP Proceedings Vol. 37 [Google Scholar]
- Hathaway S. C., Little T. W. A., Pritchard D. G. (1986) Problems associated with the serological diagnosis of Leptospira interrogans serovar hardjo infection in bovine populations. The Veterinary Record 119, 84–86 doi:10.1136/vr.119.4.84 [DOI] [PubMed] [Google Scholar]
- Kingscote B. F., Proulx J. (1986) The successful management of Leptospira hardjo infection in a beef herd in Northern Ontario. Canadian Veterinary Journal 27, 435–439 [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1080/00480169.1979.34620. doi:10.7589/0090-3558-34.4.738 Marshall, R.B., Broughton, E.S. and Hellstrom, J.S. (1979). Protection of cattle against natural challenge with Leptospira interrogans serovar hardjo using a hardjo‑pomona vaccine. New Zealand Veterinary Journal 27: 114–116. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Fleming P. J. S., Smythe L. D., Dohnt M. D. Norris M. A., Symonds M. L. (1998) Leptospira Interrogans antibodies in feral pigs from New South Wales. Journal of Wildlife Diseases 34, 787–743 doi:10.7589/0090-3558-34.4.738 [DOI] [PubMed] [Google Scholar]
- McClintock C. S., McGowan M. R., Corney B. G., Colley J., Smythe L., Dohnt M., Woodrow M. (1993) Isolation of Leptospira interrogans serovars hardjo and zanoni from a dairy (cattle) herd in north Queensland. Australian Veterinary Journal 70, 393 doi:10.1111/j.1751-0813.1993.tb00826.x [DOI] [PubMed] [Google Scholar]
- Milner A. R., Wilks C. R., Calvert K (1980). The prevalence of antibodies to members of Leptospira Interrogans in cattle. Australian Veterinary Journal 56, 327–330 doi:10.1111/j.1751-0813.1980.tb05741.x [DOI] [PubMed] [Google Scholar]
- doi: 10.7589/0090-3558-17.2.197. doi:10.7589/0090-3558-34.4.738 Milner, A.R., Wilks, C.R., Spratt, D.M. and Presidente, P.J.A. (1981). The prevalence of antileptospiral agglutinins in sera of wildlife in southeastern Australia. Journal of Wildlife Diseases 17: 197–202. [DOI] [PubMed] [Google Scholar]
- Prescott J. F., Miller R. B., Nicholson V. M., Martin S. W., Lesnick T. (1988) Seroprevalence and association with abortion of leptospirosis of cattle in Ontario. Canadian Journal Veterinary Research 52, 210–215 [PMC free article] [PubMed] [Google Scholar]
- Roberts M. W., Smythe L., Dohnt M., Symonds M., Slack A. (2010) Serologic-based investigation of leptospirosis in a population of free-ranging Eastern grey kangaroos (Macropus giganteus) indicating the presence of Leptospira weilii Serovar Topaz. Journal of Wildlife Diseases 46, 564–569 doi:10.7589/0090-3558-46.2.564 [DOI] [PubMed] [Google Scholar]
- Smith C. R., Ketterer P. J., McGowan M. R., Coreny B. G (1994). A review of laboratory techniques and their use in the diagnosis of Leptospira interrogans serovar hardjo infection in cattle. Australian Veterinary Journal 71, 290–294 doi:10.1111/j.1751-0813.1994.tb03447.x [DOI] [PubMed] [Google Scholar]
- Virbac Animal Health (2012) Leptospirosis Vaccination with Lepto-7 – a commonsense guide. TechTalk, TT026-342. www.virbac.com.au
- Worthington R. W. (1982) Serology as an aid to diagnosis: uses and abuses. New Zealand Veterinary Journal 30, 93–97 doi:10.1080/00480169.1982.34894 [DOI] [PubMed] [Google Scholar]
- Wynwood S. J., Burns M. A., Graham G. C., Weier S. L., McKay D. B., Craig S. B (2015). Validation of a microsphere immunoassay for serological leptospirosis diagnosis in human serum by comparison to the current gold standard. PLOS Tropical Neglected Diseases 9, e0003636 doi:10.1371/journal.pntd.0003636 [DOI] [PMC free article] [PubMed] [Google Scholar]



