Abstract
Football is the most popular sport worldwide and is associated with a high injury rate, most of which are the result of trauma from player contact. Ankle injuries are among the most commonly diagnosed injuries in the game. The result is reduced physical activity and endurance levels, lost game time, and considerable medical cost. Sports medicine professionals must employ the correct diagnostic tools and effective treatments and rehabilitation protocols to minimize the impact of these injuries on the player. This review examines the diagnosis, treatment, and postoperative rehabilitation for common football injuries of the ankle based on the clinical evidence provided in the current literature.
Keywords: Management, Soccer, Football, Ankle, Injury
Core tip: Injury prevention is paramount in optimizing function and decreasing time lost from sport in footballers. Early recognition of foot and ankle injuries allows implementation of conservative measures aimed at improving function and reducing the risk of re-injury or development of concomitant pathologies. Treatment, whether conservative or surgical, requires an understanding of the mechanical component of injury (e.g., ligament tear, osteophytes), while additionally addressing the biological components affecting healing. This includes restoration of normal proprioceptive pathways through physical therapy programs while also treating the catabolic biochemical environment through selected use of biological adjuncts including platelet-rich plasma and bone marrow aspirate concentrate.
INTRODUCTION
Football is the most popular sport in the world, while also being associated with a high injury rate both at professional and amateur levels[1-3]. Elite soccer players experience between 13 and 35 injuries per 1000 competitive player-hours, with up to 74% resulting from direct player contact. When cause is analyzed, approximately 80% are traumatic in origin and 20% are overuse injuries[4,5]. The lower limb is most commonly affected with the ankle accounting for up to a third of all injuries[1-3,6]. At the 2004 Olympics, foot and ankle injuries were encountered in football proportionally more than any other team sport[7]. During the 2010 FIFA world cup, ankle sprains were among the most prevalent diagnoses and of these, approximately 50% prevented participation in training or competition[8]. Additionally a recent study of an English Premier League (EPL) club revealed over a four year period, 20% of injuries were of the foot and ankle with a resultant mean return to sport time of 54 d[9].
The consequences of ankle injuries include reduced physical activity and endurance levels, lost game time, and considerable medical cost[3,10,11]. Due to the frequency and debilitating nature of these injuries it is critical for trainers, therapists, and team physicians to correctly diagnose injuries as early as possible and apply the most effective treatments to return athletes to the field expeditiously.
This article reviews the mechanisms of injury and highlights appropriate examination, diagnostics, treatment, and postoperative rehabilitation for common soccer injuries of the ankle.
ANKLE SPRAINS AND ANKLE INSTABILITY
Ankle sprains are the most common pathology accounting for up to 67% of all soccer related ankle injuries[12,13]. Analyzing ankle sprains in players from the English football league over a 2-year period, Woods et al[13] found the majority were sustained during player contact (59%) except for goalkeepers in whom 79% occurred during non-contact situations. In addition, Jain et al[9] showed a 28.6% recurrence in anterior talofibular ligament (ATFL) injury in their EPL cohort. In a typical sprain, forced ankle inversion-supination precipitates tearing of the ATFL to varying degrees. Video analysis of ankle injuries in professional soccer players has shown that direct contact with a laterally directed force on the medial aspect of the lower leg just before or at foot strike can causes the player to land with the ankle in this vulnerable inverted position[14]. The injury tends to be more severe if the affected foot is planted and weight-bearing at the time of impact[6]. The peroneal tendons are also at risk in a combination of mechanical stretch and overload as they attempt to evert the foot back into neutral alignment.
Mechanical instability occurs when ligaments fail to remodel to normal length, allowing motion beyond normal physiological limits. The ankle joint capsule and soft tissues about the joint are often stretched or torn at the time of injury, disrupting the proprioceptive nerve fibers that run through them. This can produce a functional instability where the player may be mechanically stable but unable to maintain balance when in unilateral foot stance[15]. Both mechanical and functional instability may be present independently or in combination in any player and if untreated can potentiate additional sprains and the development of chronic ankle instability[16].
Clinical examination may be difficult in the immediate period following an acute injury. If there is concern for a ligamentous ankle injury, consideration can be given to delaying a definitive examination for up top 5 d in the off season as this permits the partial resolution of swelling and inflammation. van Dijk et al[17] have reported a diagnostic sensitivity of 96% with a delayed assessment protocol. The anterior drawer and valgus stress test can be useful in the delayed or chronic setting, however, these tests have been shown to have limited sensitivity and significant variability in differing examiners hands[18]. The modified Romberg test can demonstrate proprioceptive deficiencies of the ankle, indicating the presence of functional instability[19].
During the active playing season consideration should be given for early and accurate diagnosis. Clinical diagnosis will direct further diagnostic tests including plain radiographs, magnetic resonance imaging (MRI) and computed tomography (CT). Ankle sprain is not a benign injury and up to 75% of these injuries will have an associated soft tissue pathology[20]. MRI has the sensitivity required to detect these associated injuries including osteochondral lesions (OCL’s), tendinous and syndesmotic tears, and associated fractures. Plain radiographs may miss up to 50% of OCL’s of the talus following ankle sprain and CT scan, while useful in evaluating bony injury, lacks the sensitivity for diagnosis of soft tissue pathology[21].
Specific clinical tests can direct the radiographic diagnostic intervention. The so called “high ankle sprain” or syndesmotic injury is typically identified by pain over the anterior inferior tibiofibular ligament (AITFL) and interosseous membrane. More specific and sensitive evaluations are the squeeze test of the mid-fibula and resisted external rotation test respectively[22,23]. Augmenting clinical assessment and subtle injuries diagnosed by plain radiograph with MRI is recommended to fully evaluate the injury with a reported accuracy of 97% (sensitivity: 100%; specificity: 97%) (Figures 1-3)[22,24].
Figure 1.
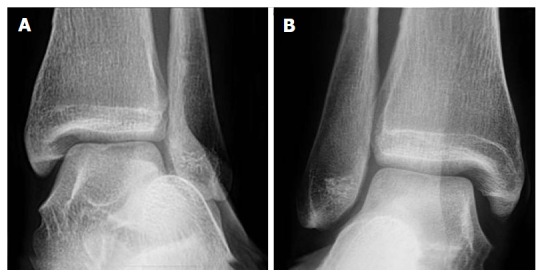
Plain radiographs of the left (A) and right (B) ankles of a single patient in the coronal plane. Both ankles are under eversion stress. The right ankle was symptomatic. Only subtle syndesmotic gapping and widening of the medial clear space can be appreciated on the right compared to the left ankle.
Figure 3.
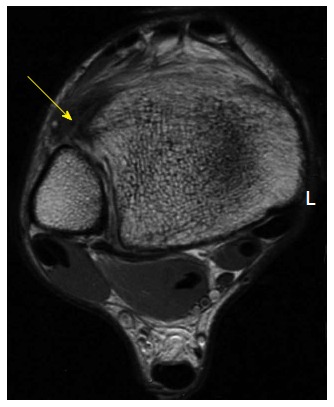
Axial fast-spin echo proton density magnetic resonance images of the right ankle seen in Figures 1 and 2. Disruption and remodeling of the anterior inferior tibiofibular ligament (yellow arrow) can be appreciated.
Figure 2.
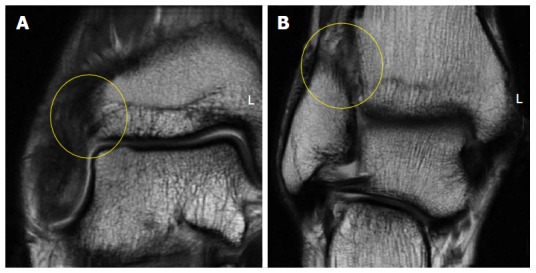
Coronal fast-spin echo proton density magnetic resonance images of the right ankle seen in Figure 1. Disruption and remodeling of the anterior inferior tibiofibular ligament (A; yellow circle) and interosseous ligament (B; yellow circle) can be appreciated.
The peroneal tendons are injured in up to 25% of acute ankle sprains acting as secondary stabilizers[20]. Resisted eversion is useful to assess the integrity of the peroneal tendons[25]. MRI is again useful in detecting any peroneal pathology and dynamic ultrasound can be a useful adjunct in detecting subtle peroneal injury[26,27].
The so called “low ankle sprain” or pain in the sinus tarsi secondary to a torn interosseous ligament in the acute phase and scar formation in the chronic phase can be examined by direct local pressure in the sinus tarsi while inverting the mid-tarsal joint. Radiographic evaluation including MRI and ultrasound are often less sensitive and specific for the low ankle sprain than for other ankle pathology and a small local anesthetic injection to the area can often be helpful in elucidating the source of pain.
The majority of simple ankle sprains heal with non-operative treatment, however, there is no consensus on the ideal rehabilitation protocol[20]. Early mobilization followed by phased rehabilitation is advocated by most authors as beneficial in minimizing time lost[28-30]. A multicentre study of 584 patients suggested there is faster recovery with a short period of immobilization in a below the knee cast or removable boot when compared to treatment in a compression bandage[31]. It must be noted that no information was provided on additional interventions, the study utilized a postal questionnaire and there was a 17% drop-out rate. But overall, the results indicate initial immobilization can be beneficial. Conversely, prolonged immobilization of greater than 2 wk has a detrimental effect on muscles, ligaments, and joint surfaces and may result in longer return to play time[32].
A rehabilitation protocol should be divided into specific stages: Acute and subacute pain and swelling control; range of motion and strengthening exercises; soccer specific functional training; and prophylactic intervention with balance and proprioception stimulating exercises. Upon return to play, continued proprioceptive training is vital to minimize recurrence[33-36]. Semi-rigid orthoses and air-cast braces may help prevent ankle sprains, especially in athletes with a history of recurrent instability[37-39]. Bracing has a mechanical advantage over simple taping, as tape loses its ability to restrict inversion and eversion approximately 20 min after starting activity[39].
Surgery is indicated in patients with chronic mechanical instability. Traditionally, two forms of repair are considered: An anatomic reconstruction such as the Brostrom or Gould modification (Figure 4), or a non-anatomic checkrein tenodesis such as the Chrisman-Snook procedure[20,40]. Anatomical repairs appear to produce better outcomes and there is additional concern that some checkrein procedures can restrict subtalar motion and prevent normal agility on playing surfaces by altering hindfoot biomechanics[38,41-43]. As over 90% of patients with chronic ankle instability have additional intra-articular lesions, arthroscopic ankle evaluation, and treatment where necessary, can be performed at the same time as open lateral ankle ligament repair[44,45].
Figure 4.
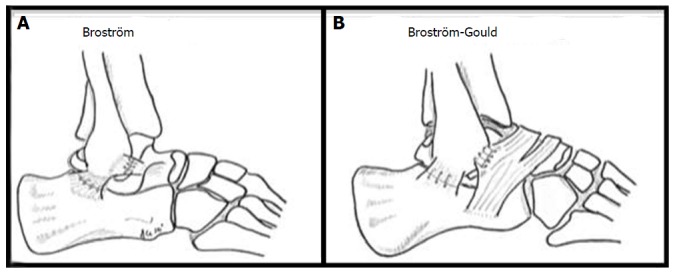
Illustrations of the Brostrom (A) and Modified Brostrom-Gould (B) surgical technique for lateral ligament reconstruction of the ankle. Reproduced, with permission, from Prisk et al[108].
With acute ankle ligament instability the traditional treatment paradigm of triple phase physical therapy and avoiding surgical intervention has been recently questioned. A report from van Dijk demonstrated superior results with acute surgical repair in a cohort of athletes[46]. It consisted of a subgroup analysis in which only a single surgeon series of acute operative repair was conducted. Objective instability, as defined by a positive talar tilt on stress radiographs or positive anterior drawer sign, was significantly less when compared to non-operative treatment. Because increased objective instability is a predictor for future ankle sprains, an acute reconstruction may be preferred in professional athletes[47,48]. The outcome of a recent consensus meeting also suggested a role for selective operative treatment in athletic populations[43].
Surgical outcomes in acute and chronic lateral ankle ligament repairs are generally good and most athletes are able to return to their pre-injury level of function[20]. Kennedy et al[40] reported an anatomic repair augmented with a portion of peroneus longus used as an ATFL checkrein in 57 athletes, including 11 soccer players (Figure 5). Although all patients achieved mechanical stability, five patients did not return to their pre-injury level for reasons unrelated to their ankle. Maffulli et al[49] recently reported outcomes of ankle arthroscopy and Broström repair in 38 athletes at an average 8.7 years follow-up. Laxity grade, American Orthopaedic Foot and Ankle Society Ankle-Hindfoot scores, and Kaikkonen scales all improved significantly at last follow up. Return to high contact sport was allowed six months postoperatively. Fifty-eight percent of patients returned to their pre-injury level of activity, 16% decreased their activity but maintained activity in less demanding sports, and 26% abandoned sport participation but remained physically active. Arthroscopic assisted techniques are emerging and may play a role in some cases, but its exact role has yet to be determined and open techniques are currently considered the gold standard[50].
Figure 5.
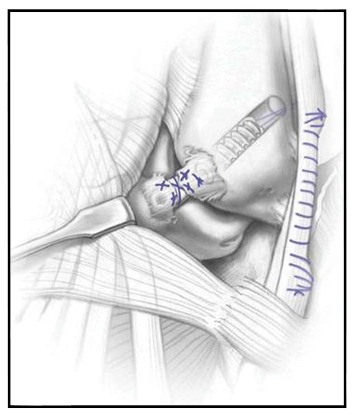
Illustration of the hybrid anatomic lateral ligament reconstruction[27]. A tendon autograft taken from the peroneus longus has been docked in the talus and distal fibula and remaining anterior talofibular ligament fibers have been sutured over the reconstruction, theoretically allowing proprioceptive fibers to aid in regaining functional stability. Illustration copyright of and reproduced with permission from Kennedy JG, MD. Reproduction without express written consent is prohibited. Reproduced, with permission, from Kennedy et al[40].
Acute syndesmotic injuries (high ankle sprains) are less common than lateral ankle sprains and if missed can potentiate persistent morbidity and early degeneration of the ankle joint[51,52]. Mild syndesmotic sprains can be managed conservatively with protected weight-bearing and functional rehabilitation. Compared with lateral ankle sprains, syndesmosis injuries typically require longer rehabilitation programs[52]. A recent study of National Football League players, however, did indicate an earlier return to play (4-6 wk) is possible with milder injuries[53]. Due to the potential for prolonged rehabilitation, some clinicians advocate surgical intervention in professional athletes with mild sprains to expedite a return to play[52].
Surgical intervention is necessary in severe acute injuries where there is tibio-fibular diastasis. Screw fixation has traditionally been utilized with most physicians advocating screw removal between 7 and 12 wk postoperatively[51,52]. Non-absorbable suture-button fixation devices have more recently emerged as a fixation technique[54]. They have the advantage of obviating hardware removal and can allow earlier weightbearing[55]. Chronic syndesmotic injuries can be treated with screw fixation, arthrodesis and arthroscopic debridement. While there are no papers specifically assessing outcomes in an athletic populations, a recent systematic review and meta-analysis reported screw fixation as the most successful treatment option[51].
OCL’s
OCL’s of the ankle have an incidence between 50% and 70% of all acute ankle sprains and fractures[56-59]. Unfortunately, cartilage injuries have a poor spontaneous healing response. Therefore the role of surgical management involves the repair an acute lesion when possible, initiating fibrocartilage formation with marrow stimulation techniques in small lesions, with consideration given to cartilage transplant or other modalities for larger lesions. The ultimate aim is to return the athlete to their pre-injury level of play.
Symptoms of an OCL may be mechanical and include clicking or locking of the ankle. Typically however, talar OCL’s present localized deep joint pain without any mechanical symptoms. Physical examination may reveal swelling, localized tenderness along the joint line, and limited motion. Plain radiographs may miss up to 50% of OCL’s and CT is only useful in obtaining the degree of bone injury as both are unable to assess overlying cartilage[21,60]. Magnetic resonance imaging is recommended for a definitive diagnosis and T2 Mapping sequences can be useful with increased sensitivity to cartilage change (Figure 6)[61].
Figure 6.
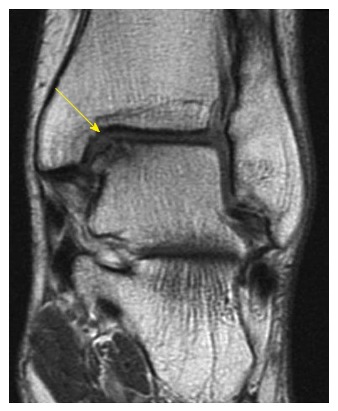
Coronal fast-spin echo proton density magnetic resonance image demonstrating an uncontained osteochondral lesion of the medial talar dome.
Lesion size and location determine the most appropriate treatment strategy. Most OCL’s will not heal with conservative treatment and therefore surgery is typically recommended[62]. Reparative surgical techniques such as bone marrow stimulation for lesions of less than 15 mm in diameter yield good functional outcomes in short term follow-up[56,63]. Larger lesions are best treated with replacement strategies including autologous osteochondral transplantion (AOT) (Figure 7) or other techniques including autologous chondrocyte implantation[64-66].
Figure 7.
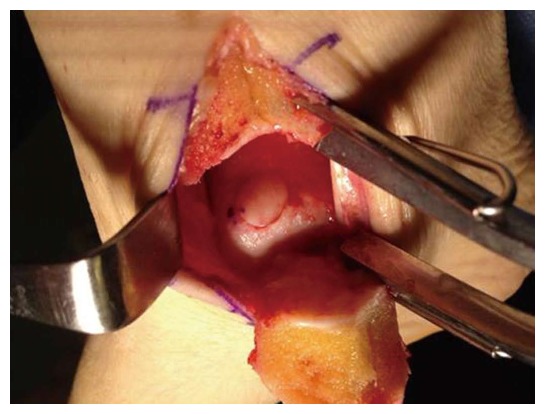
Intraoperative photograph of an autologous osteochondral graft transplanted into the medial talar dome. Access was achieved via a medial malleolar osteotomy. The graft was gently tamped into position until flush with the surrounding surface.
Both reparative and replacement strategies have been evaluated in regards to return to soccer however the evidence is limited in both groups. Saxena and Eakin[57] evaluated functional activity after surgical management of OCL’s in 44 athletic subjects of which 18 were considered high level, including six soccer players. At medium term follow-up (32 mo), 17 of 18 high level athletes had returned to their pre-injury level and the other subject had not returned for personal reasons. The mean time to return to sport was 15 wk after marrow stimulation and 19 wk after bone grafting (not AOT’s). More recently, Paul et al[64] assessed sports activity after talar AOT’s in 131 patients with a mean age of 31 years. Seventy percent were either very satisfied or satisfied by the procedure and 85% required no analgesia when currently involved in sports. While 20% were involved in competitive sports preoperatively, only half of these were still competing postoperatively. Age at time of injury may be a factor in a person’s ability to return to sport. For example, Hangody et al[65] reported a combination of talar and knee OCL’s in 354 competitive athletes including 67 soccer players and four Olympians. They found that 63% were able to return to their same activity/sporting level although most were under the age of 30 years. Overall, 9% had to give up sports completely.
The long-term outcome of cartilage repair surgeries has prompted significant interest in biologic augmentation of cartilage healing in the sports world. Concentrated bone-marrow aspirated (CBMA), typically obtained from the players iliac crest at the time of surgery, is a source of mesenchymal stem cells and growth factors. This can be delivered directly to the OCL during arthroscopy and may potentially enhance the healing environment. When combined with microfracture in a large animal model, the resultant cartilage of the CBMA group demonstrated greater type-II collagen, proteoglycan and glysosaminoglycan content consistent with a more normal cartilaginous architecture[67]. At this time no long term data exists to support the use of CBMA and early return to sport following talar OCL surgery.
Platelet-rich plasma (PRP) is an autologous blood product that contains many growth factors that may promote cartilage repair. A recent review of PRP basic science literature found that it promotes chondroctye and mesenchymal stem cell proliferation, type II collagen deposition, and proteoglycan deposition[68]. Results also indicated that PRP may increase chondrocyte viability, promote migration and chondrogenic differentiation of mesenchymal stem cells, as well as inhibit the effects of inflammatory cytokines. Mei-Dan et al[69] recently compared intra-articular injections of PRP and hyaluronic acid (HA) for non-operative management of talar OCL’s in a randomized trial of 30 patients. Fifteen patients were randomized into each group, treated with three consecutive injections of either PRP of HA, and followed for 28 wk. American Orthopaedic Foot and Ankle Society Ankle-Hindfoot scores, visual analogue scale (VAS) pain, VAS stiffness, VAS function, and subjective global function scores all improved significantly more in the PRP group than the HA group. Current clinical studies are limited for PRP, and formal randomized human trials are required to determine the true in-vivo effect.
Postoperative care is dependent on the chosen treatment. After marrow stimulation, the patient is initially non-weight-bearing in a soft leg cast for two weeks during which ankle pump exercises are performed three times daily. At two weeks, the patient is placed in a CAM boot and encouraged to start range of motion exercises. At six weeks, patients commence weight-bearing starting with 10% of their body weight, increasing by 10% daily until full weight-bearing is achieved. Formal rehabilitation concentrates on balance, joint proprioception, and stabilization. Strengthening and sport specific exercises begin at 10 wk. After an AOT procedure, patients are non-weightbearing for six weeks followed by two weeks of progressive weight-bearing as above. At eight weeks, formal physical therapy commences and sport-specific training introduced at 12 wk. Return to soccer is typically at six months following surgery.
ANTEROLATERAL IMPINGEMENT
Anterolateral impingement syndrome (ALI) typically manifests as chronic anterolateral ankle pain following an ankle sprain. It is thought to result from the entrapment of hypertrophic soft tissues or torn and inflamed ligaments in the lateral gutter and anterolateral ankle joint[70]. Following tears of the ATFL, AITFL, and/or CFL, repetitive motion after incomplete healing can lead to inflammation and subsequent synovitis with scar tissue formation[71]. Mild sprains with minimal capsular tearing may also produce an intraarticular hematoma, the reabsorption of which by synovium in the lateral gutter may induce a reactive synovitis[71,72].
Players typically present with chronic ankle pain, limited dorsiflexion, and swelling after activity[73,74]. Tenderness on palpation of the anterolateral gutter is characteristic. ALI can be distinguished from anteromedial impingement (AMI) if pain is elicited on palpation lateral to peroneus tertius[74,75]. Symptoms must also be differentiated from sinus tarsi syndrome and a diagnostic injection of local anesthetic is useful in deducing this.
While standard radiographs are effective in diagnosing the presence of anterolateral osteophytes, MRI can reveal soft tissue impingement[74-76]. However, as false-negative results have been reported with MRI, arthroscopy is generally advocated as the definitive diagnostic and therapeutic modality[71,77].
Initial treatment involves physical therapy modalities with deep tissue massage and other techniques to reduce inflammation. If after one month of therapy no improvement is noted an ultrasound guided injection to the soft tissue impingement with a combination of low dose steroid and local anesthetic may be useful. Surgical treatment is reserved for recalcitrant cases. During the playing season players can be treated with conservative modalities and surgery may be delayed until the off season. Surgical management includes arthroscopic excision of a pathologic fascicle of the ATFL if present as well as hypertrophic synovium in the lateral gutter (Figure 8). At the end of the procedure range of motion of the ankle is checked under direct arthroscopic visualization to ensure no impingement remains during full dorsiflexion[71].
Figure 8.
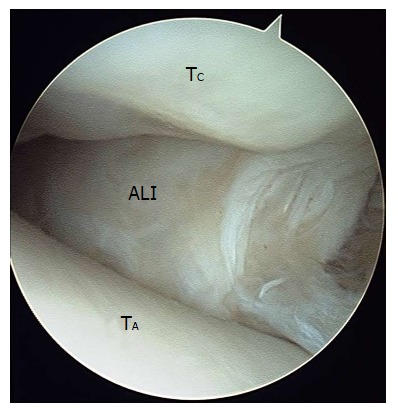
Arthroscopic image of soft tissue impingement the later gutter of the ankle joint. ALI: Anterolateral soft tissue impingement including cicatraized tissue, entrapped lateral ligaments, and hypertrophied synovium; TA: Talar dome; TC: Tibial cartilage.
Following surgery, the player should remain non-weightbearing for two days. Dorsi- and plantarflexion exercises should commence one day postoperatively to minimize arthrofibrosis. Weight bearing is increased as tolerated. Physical therapy begins after one week and soccer-specific training is recommended at 2-3 wk.
There is no published outcome data in a soccer-specific cohort following surgical management for anterolateral ankle impingement; however, reported outcomes in general athletic populations are considered very good[78,79]. In a series of 11 elite dancers, Nihal et al[80] reported postoperative improvement in all performers with 9 returning to full function at a mean of 7 wk. DeBerardino et al[79] reported a series of 60 athletes with chronic anterolateral soft-tissue impingement following ankle inversion trauma treated with arthroscopic debridement. At a mean of 27 mo follow-up, 85% of patients had an excellent outcome on the West Point Ankle Score. Average functional results of the 12 m single-leg hop test was 94% of the time obtained compared to the unaffected side. Eighty-three percent of patients experienced good to excellent subjective pain relief.
ANTEROMEDIAL IMPINGEMENT
First termed footballer’s ankle by McMurray[81] in 1950, AMI is a common cause of ankle pain in soccer players. This is generally a bony rather than soft-tissue impingement and causative factors include direct trauma, recurrent microtrauma, and chronic ankle instability[73,81-83].
Recurrent stressing of the ankle at the extremes of motion was previously thought to induce bone spur formation and soft tissue proliferation due to traction of the capsule[74,75]. Current thoughts on the actual pathogenesis are less clear. Cadaveric analysis has found the anterior joint capsule to be attached more proximally than the site of the tibial spur, and arthroscopic evaluation generally identifies the osteophytes to lie within the joint capsule[84-86]. Microtrauma from recurrent impact of a football on the anterior ankle may also contribute to osteophyte formation[82]. Thickened soft tissue can be compressed between talar and tibial osteophytes (kissing lesions) with ankle dorsiflexion causing focal inflammation and pain[75,85].
AMI commonly presents as anteromedial ankle pain, swelling after activity, and sometimes limited dorsiflexion[73]. Tenderness with palpation medial to the tibialis anterior tendon is considered indicative of AMI[75]. Forced hyperdorsiflexion does not always provoke the players typical pain[75].
The diagnosis of AMI is usually confirmed by plain film radiology[87]. Anterior tibial osteophytes can be seen with standard lateral views. An oblique AMI view (45° craniocaudal, 30° external rotation of the leg with the ankle in full plantarflexion) is recommended when plain radiographs are negative as it can provide specific visualization of anteromedial osteophytes (Figure 9)[75]. CT may aid in confirming the diagnosis and MRI can identify soft-tissue impingement, soft tissue injuries, or OCL’s[76].
Figure 9.
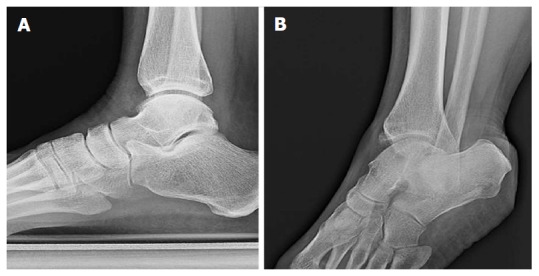
Standard lateral views about anterior tibial osteophytes. Lateral plain radiograph of a right ankle with clinical suspicion of anteromedial impingement (A). An anteromedial radiographic view with 30 degrees of external rotation of the same ankle demonstrated an osteophyte on the anteromedial aspect of the distal tibia (B) that could not be appreciated on the lateral view.
Ultrasound-guided corticosteroid injections can reduce the patients symptoms temporarily, however, definitive treatment is typically achieved surgically[84]. This is in contrast to ALI which will often respond to conservative therapy. Operative treatment involves removal of any cicatrized soft tissue and synovial hyperplasia from the joint capsule and a thorough osseous resection of the anterior distal tibia and talar neck. Careful resection to the anterior border of the medial malleolus is vital. Range of motion assessment under arthroscopic visualization is advised to confirm an adequate debridement. The postoperative treatment course is the same as previously described for ALI.
The largest reported series for surgical management of AMI involved 41 patients of which 16 were active soccer players and one retired professional player[88]. Overall, 93% were satisfied with their outcome. For the athletic population, return to play was 7 wk on average, with longer recovery times in those that required additional procedures, specifically lateral ligament reconstruction (15 wk) and microfracture for OCL’s (14 wk). All but one of the athletic cohort (33 of 34 patients) returned to their same level of play indicating an expectation of return to play following this procedure[40].
POSTERIOR ANKLE IMPINGEMENT
Posterior ankle impingement (PAI) is characterized by posterior ankle pain with plantarflexion. Causative pathologies include a prominent posterior talar process (os trigonum), fracture of the lateral tubercle of the posterior talar process, compression of posterior soft tissues, traction on the posterior talofibular ligament (PTFL) and posterior capsule, and flexor hallucis longus (FHL) tendonitis or tenosynovitis. Repetitive plantarflexion, as seen with ball striking, can precipitate overuse injury[89-91].
Pain from ankle hyperplantarflexion is due to compression of the soft tissue or bony structures between the posterior aspect of the distal tibia and the calcaneus. Zwiers et al[75] described a simple clinical test where rapid passive hyperflexion of the ankle was combined with a rotational grinding motion at maximal plantarflexion. They found a negative test could exclude PAI. Injection of local anesthetic can further aid in confirming the diagnosis.
Maximal ankle dorsiflexion can also reproduce pain due to tension within the posterior joint capsule and PTFL. Depending on the exact underlying pathology, pain can be elicited with focal palpation of the PTFL, transverse tibiofibular ligament, posterior inferior tibiofibular ligament (PITFL), CFL within the posterolateral gutter, or FHL.
Plain film radiology can help confirm PAI. The lateral ankle view is most useful in detecting osteophytes, calcifications, loose bodies, chondromatosis, and retrocalcaneal bursitis (Kager’s triangle) (Figure 10). To differentiate between a hypertrophied posterolateral talar process and an os trigonum, a lateral radiograph with the foot in 25o of external rotation in relation to the standard lateral radiograph is useful[75]. Further assessment with MRI may identify associated flexor tendon injury, most commonly FHL[92,93].
Figure 10.
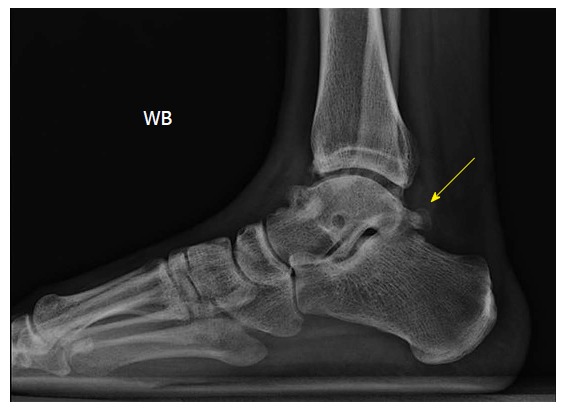
Lateral plain radiograph of a right ankle revealing an os trigonum, which was clinically symptomatic, as well as anterior tibial and talar osteophytes.
Conservative treatment is initially recommended for most patients with additional consideration given to ultrasound-guided corticosteroid injections for more severe cases[94]. Surgery can be offered for recalcitrant conditions. If bone impingement is determined as the underlying cause, we advise a single diagnostic injection of local anesthesia and caution against multiple injections as they are ultimately associated with prolonged postoperative recovery[95]. Elite soccer players require prompt return to play, and surgery should be considered early in the treatment algorithm[96,97].
While arthroscopic procedures can permit earlier rehabilitation than traditional open procedures there is concern regarding the potential risk of neurovascular injury. Several studies have shown that the rate of nerve injury in patients treated with posterior ankle arthroscopy is lower than that found in anterior arthroscopy as long as care is taken to avoid structures medial to the FHL tendon[97-99].
Following posterior ankle arthroscopy players remain non-weightbearing in a compression bandage for 24-28 h. Weight bearing is subsequently increased as tolerated until full-weight bearing by one week[95,100]. Early range-of-motion exercises help prevent cicatrization and stiffness. Physiotherapy is focused on restoring strength and range of motion.
A recent current concepts review illustrates the technique for posterior ankle arthroscopy and management of posterior impingement and co-existing pathologies[98]. As for anterior impingement syndromes, arthroscopic treatment is very effective at restoring an athlete’s ability to return to competitive sport with a very low incidence of postoperative complications[95,96,99,100]. Calder et al[95] reported on hindfoot arthroscopy for 27 professional soccer players experiencing bone and/or soft tissue impingement. Overall, the mean time to return to training was 34 d and return to playing was 41 d. Recovery, measured as return to training, was faster in cases of soft tissue impingement (mean, 28 d) compared with osseus injury (mean, 40 d). The authors reported only one non major complication of portal leakage which resolved with two weeks rest[95].
CONCLUSION
Preventing injuries and minimizing lost playtime is the main goal for physicians and trainers and can be achieved via accurate diagnosis, effective treatment, and proper rehabilitation or postoperative course.
While most injuries in soccer occur as a result of player-to-player contact, a significant number of non-contact injuries also occur. The risk of suffering from non-contact lower extremity injuries may be lowered by implementing in-season neuromuscular training programs aimed at enhancing ankle and knee proprioception. Effective warm-up programs with an emphasis on stretching, regular cool down at the end of training or competition, sufficient recovery and rehabilitation programs, proper equipment, and good field conditions can all contribute to injury prevention[9].
Cleated shoe wear provides traction on the field while running and cutting but high shoe-surface traction has been suggested as a culprit for many ankle injuries. Longer cleats should be avoided due to the increased shoe-surface traction[101,102]. There are inherent advantages and disadvantages to both natural grass and artificial turf. Higher frictional resistance and shoe-surface traction, which is the case in turf fields, correlates with increased performance, but also increases the incidence of injuries[103]. Artificial turf also increases plantar pressures, potentially mediating metatarsalgia and stress fractures[104,105]. Natural grass has a lower friction coefficient than artificial turf but certain species are associated with increased risk of injury[106]. Warm conditions also harden the ground, resulting in increased shoe-surface traction and risk of injury[106]. Preventive measures including selecting appropriate cleats length and softening the field through watering should be considered.
Age and gender represent important risk factors for soccer players[107]. As players age, they should devote more time to appropriate warm-up, stretching and strengthening routines in order to prevent injury. Adhering to a well-designed training program throughout the season and treating injuries promptly and adequately will allow the soccer player to make the most out of the season and minimize lost play time.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest pertaining to this manuscript. Full conflict of interest statement has been provided for all authors.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 21, 2015
First decision: May 13, 2015
Article in press: September 18, 2015
P- Reviewer: Fernandez-Fairen M, Macheras GA
S- Editor: Tian YL L- Editor: A E- Editor: Li D
References
- 1.Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Physical fitness, injuries, and team performance in soccer. Med Sci Sports Exerc. 2004;36:278–285. doi: 10.1249/01.MSS.0000113478.92945.CA. [DOI] [PubMed] [Google Scholar]
- 2.Junge A, Dvorak J, Graf-Baumann T, Peterson L. Football injuries during FIFA tournaments and the Olympic Games, 1998-2001: development and implementation of an injury-reporting system. Am J Sports Med. 2004;32:80S–89S. doi: 10.1177/0363546503261245. [DOI] [PubMed] [Google Scholar]
- 3.Wong P, Hong Y. Soccer injury in the lower extremities. Br J Sports Med. 2005;39:473–482. doi: 10.1136/bjsm.2004.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvorak J, Junge A. Football injuries and physical symptoms. A review of the literature. Am J Sports Med. 2000;28:S3–S9. doi: 10.1177/28.suppl_5.s-3. [DOI] [PubMed] [Google Scholar]
- 5.Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Influencing factors. Am J Sports Med. 2000;28:S58–S68. doi: 10.1177/28.suppl_5.s-58. [DOI] [PubMed] [Google Scholar]
- 6.Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31:550–554. doi: 10.1177/03635465030310041201. [DOI] [PubMed] [Google Scholar]
- 7.Badekas T, Papadakis SA, Vergados N, Galanakos SP, Siapkara A, Forgrave M, Romansky N, Mirones S, Trnka HJ, Delmi M. Foot and ankle injuries during the Athens 2004 Olympic Games. J Foot Ankle Res. 2009;2:9. doi: 10.1186/1757-1146-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorak J, Junge A, Derman W, Schwellnus M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br J Sports Med. 2011;45:626–630. doi: 10.1136/bjsm.2010.079905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain N, Murray D, Kemp S, Calder J. Frequency and trends in foot and ankle injuries within an English Premier League Football Club using a new impact factor of injury to identify a focus for injury prevention. Foot Ankle Surg. 2014;20:237–240. doi: 10.1016/j.fas.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson J. Are sports related injuries expensive. Scand J Med Sci Sports. 2005;15:1–2. doi: 10.1111/j.1600-0838.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 11.Pritchett JW. Cost of high school soccer injuries? Am J Sports Med. 1981;9:64–66. doi: 10.1177/036354658100900116. [DOI] [PubMed] [Google Scholar]
- 12.Kofotolis ND, Kellis E, Vlachopoulos SP. Ankle sprain injuries and risk factors in amateur soccer players during a 2-year period. Am J Sports Med. 2007;35:458–466. doi: 10.1177/0363546506294857. [DOI] [PubMed] [Google Scholar]
- 13.Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37:233–238. doi: 10.1136/bjsm.37.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen TE, Floerenes TW, Arnason A, Bahr R. Video analysis of the mechanisms for ankle injuries in football. Am J Sports Med. 2004;32:69S–79S. doi: 10.1177/0363546503262023. [DOI] [PubMed] [Google Scholar]
- 15.Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg Br. 1965;47:678–685. [PubMed] [Google Scholar]
- 16.Hubbard-Turner T. Relationship between mechanical ankle joint laxity and subjective function. Foot Ankle Int. 2012;33:852–856. doi: 10.3113/FAI.2012.0852. [DOI] [PubMed] [Google Scholar]
- 17.van Dijk CN, Lim LS, Bossuyt PM, Marti RK. Physical examination is sufficient for the diagnosis of sprained ankles. J Bone Joint Surg Br. 1996;78:958–962. doi: 10.1302/0301-620x78b6.1283. [DOI] [PubMed] [Google Scholar]
- 18.Kerkhoffs GM, Blankevoort L, van Poll D, Marti RK, van Dijk CN. Anterior lateral ankle ligament damage and anterior talocrural-joint laxity: an overview of the in vitro reports in literature. Clin Biomech (Bristol, Avon) 2001;16:635–643. doi: 10.1016/s0268-0033(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal Y, Carey JP, Hoffman HJ, Sklare DA, Schubert MC. The modified Romberg Balance Test: normative data in U.S. adults. Otol Neurotol. 2011;32:1309–1311. doi: 10.1097/MAO.0b013e31822e5bee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23:1–19, v. doi: 10.1016/S0278-5919(03)00095-4. [DOI] [PubMed] [Google Scholar]
- 21.Loomer R, Fisher C, Lloyd-Smith R, Sisler J, Cooney T. Osteochondral lesions of the talus. Am J Sports Med. 1993;21:13–19. doi: 10.1177/036354659302100103. [DOI] [PubMed] [Google Scholar]
- 22.Sman AD, Hiller CE, Refshauge KM. Diagnostic accuracy of clinical tests for diagnosis of ankle syndesmosis injury: a systematic review. Br J Sports Med. 2013;47:620–628. doi: 10.1136/bjsports-2012-091702. [DOI] [PubMed] [Google Scholar]
- 23.Nussbaum ED, Hosea TM, Sieler SD, Incremona BR, Kessler DE. Prospective evaluation of syndesmotic ankle sprains without diastasis. Am J Sports Med. 2001;29:31–35. doi: 10.1177/03635465010290011001. [DOI] [PubMed] [Google Scholar]
- 24.Oae K, Takao M, Naito K, Uchio Y, Kono T, Ishida J, Ochi M. Injury of the tibiofibular syndesmosis: value of MR imaging for diagnosis. Radiology. 2003;227:155–161. doi: 10.1148/radiol.2271011865. [DOI] [PubMed] [Google Scholar]
- 25.Sarig-Bahat H, Krasovsky A, Sprecher E. Evaluation of clinical methods for peroneal muscle testing. Physiother Res Int. 2013;18:55–62. doi: 10.1002/pri.1534. [DOI] [PubMed] [Google Scholar]
- 26.Raikin SM. Intrasheath subluxation of the peroneal tendons. Surgical technique. J Bone Joint Surg Am. 2009;91 Suppl 2 Pt 1:146–155. doi: 10.2106/JBJS.H.01356. [DOI] [PubMed] [Google Scholar]
- 27.Staresinic M, Bakota B, Japjec M, Culjak V, Zgaljardic I, Sebecic B. Isolated inferior peroneal retinculum tear in professional soccer players. Injury. 2013;44 Suppl 3:S67–S70. doi: 10.1016/S0020-1383(13)70202-X. [DOI] [PubMed] [Google Scholar]
- 28.Linde F, Hvass I, Jürgensen U, Madsen F. Early mobilizing treatment in lateral ankle sprains. Course and risk factors for chronic painful or function-limiting ankle. Scand J Rehabil Med. 1986;18:17–21. [PubMed] [Google Scholar]
- 29.Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22:83–88. doi: 10.1177/036354659402200115. [DOI] [PubMed] [Google Scholar]
- 30.Bleakley CM, O’Connor SR, Tully MA, Rocke LG, Macauley DC, Bradbury I, Keegan S, McDonough SM. Effect of accelerated rehabilitation on function after ankle sprain: randomised controlled trial. BMJ. 2010;340:c1964. doi: 10.1136/bmj.c1964. [DOI] [PubMed] [Google Scholar]
- 31.Lamb SE, Marsh JL, Hutton JL, Nakash R, Cooke MW. Mechanical supports for acute, severe ankle sprain: a pragmatic, multicentre, randomised controlled trial. Lancet. 2009;373:575–581. doi: 10.1016/S0140-6736(09)60206-3. [DOI] [PubMed] [Google Scholar]
- 32.Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly K, Struijs PA, van Dijk CN. Immobilisation and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002;(3):CD003762. doi: 10.1002/14651858.CD003762. [DOI] [PubMed] [Google Scholar]
- 33.Balduini FC, Vegso JJ, Torg JS, Torg E. Management and rehabilitation of ligamentous injuries to the ankle. Sports Med. 1987;4:364–380. doi: 10.2165/00007256-198704050-00004. [DOI] [PubMed] [Google Scholar]
- 34.Safran MR, Zachazewski JE, Benedetti RS, Bartolozzi AR, Mandelbaum R. Lateral ankle sprains: a comprehensive review part 2: treatment and rehabilitation with an emphasis on the athlete. Med Sci Sports Exerc. 1999;31:S438–S447. doi: 10.1097/00005768-199907001-00005. [DOI] [PubMed] [Google Scholar]
- 35.McGuine TA, Keene JS. The effect of a balance training program on the risk of ankle sprains in high school athletes. Am J Sports Med. 2006;34:1103–1111. doi: 10.1177/0363546505284191. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35:922–926. doi: 10.1177/0363546507299259. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson J. [Ankle braces prevent ligament injuries] Lakartidningen. 2002;99:3486–3489. [PubMed] [Google Scholar]
- 38.Sammarco VJ. Complications of lateral ankle ligament reconstruction. Clin Orthop Relat Res. 2001;(391):123–132. doi: 10.1097/00003086-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Gross MT, Liu HY. The role of ankle bracing for prevention of ankle sprain injuries. J Orthop Sports Phys Ther. 2003;33:572–577. doi: 10.2519/jospt.2003.33.10.572. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy JG, Smyth NA, Fansa AM, Murawski CD. Anatomic lateral ligament reconstruction in the ankle: a hybrid technique in the athletic population. Am J Sports Med. 2012;40:2309–2317. doi: 10.1177/0363546512455397. [DOI] [PubMed] [Google Scholar]
- 41.Liu SH, Baker CL. Comparison of lateral ankle ligamentous reconstruction procedures. Am J Sports Med. 1994;22:313–317. doi: 10.1177/036354659402200303. [DOI] [PubMed] [Google Scholar]
- 42.Maffulli N, Ferran NA. Management of acute and chronic ankle instability. J Am Acad Orthop Surg. 2008;16:608–615. doi: 10.5435/00124635-200810000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Guillo S, Bauer T, Lee JW, Takao M, Kong SW, Stone JW, Mangone PG, Molloy A, Perera A, Pearce CJ, et al. Consensus in chronic ankle instability: aetiology, assessment, surgical indications and place for arthroscopy. Orthop Traumatol Surg Res. 2013;99:S411–S419. doi: 10.1016/j.otsr.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Hua Y, Chen S, Li Y, Chen J, Li H. Combination of modified Broström procedure with ankle arthroscopy for chronic ankle instability accompanied by intra-articular symptoms. Arthroscopy. 2010;26:524–528. doi: 10.1016/j.arthro.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Choi WJ, Lee JW, Han SH, Kim BS, Lee SK. Chronic lateral ankle instability: the effect of intra-articular lesions on clinical outcome. Am J Sports Med. 2008;36:2167–2172. doi: 10.1177/0363546508319050. [DOI] [PubMed] [Google Scholar]
- 46.Kerkhoffs GM, Tol JL. A twist on the athlete’s ankle twist: some ankles are more equal than others. Br J Sports Med. 2012;46:835–836. doi: 10.1136/bjsports-2012-091493. [DOI] [PubMed] [Google Scholar]
- 47.Verhagen EA, Van der Beek AJ, Bouter LM, Bahr RM, Van Mechelen W. A one season prospective cohort study of volleyball injuries. Br J Sports Med. 2004;38:477–481. doi: 10.1136/bjsm.2003.005785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van den Bekerom MP, Kerkhoffs GM, McCollum GA, Calder JD, van Dijk CN. Management of acute lateral ankle ligament injury in the athlete. Knee Surg Sports Traumatol Arthrosc. 2013;21:1390–1395. doi: 10.1007/s00167-012-2252-7. [DOI] [PubMed] [Google Scholar]
- 49.Maffulli N, Del Buono A, Maffulli GD, Oliva F, Testa V, Capasso G, Denaro V. Isolated anterior talofibular ligament Broström repair for chronic lateral ankle instability: 9-year follow-up. Am J Sports Med. 2013;41:858–864. doi: 10.1177/0363546512474967. [DOI] [PubMed] [Google Scholar]
- 50.Nery C, Raduan F, Del Buono A, Asaumi ID, Cohen M, Maffulli N. Arthroscopic-assisted Broström-Gould for chronic ankle instability: a long-term follow-up. Am J Sports Med. 2011;39:2381–2388. doi: 10.1177/0363546511416069. [DOI] [PubMed] [Google Scholar]
- 51.Parlamas G, Hannon CP, Murawski CD, Smyth NA, Ma Y, Kerkhoffs GM, van Dijk CN, Karlsson J, Kennedy JG. Treatment of chronic syndesmotic injury: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21:1931–1939. doi: 10.1007/s00167-013-2515-y. [DOI] [PubMed] [Google Scholar]
- 52.Del Buono A, Florio A, Boccanera MS, Maffulli N. Syndesmosis injuries of the ankle. Curr Rev Musculoskelet Med. 2013;6:313–319. doi: 10.1007/s12178-013-9183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osbahr DC, Drakos MC, O’Loughlin PF, Lyman S, Barnes RP, Kennedy JG, Warren RF. Syndesmosis and lateral ankle sprains in the National Football League. Orthopedics. 2013;36:e1378–e1384. doi: 10.3928/01477447-20131021-18. [DOI] [PubMed] [Google Scholar]
- 54.Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207–212. [PubMed] [Google Scholar]
- 55.Rigby RB, Cottom JM. Does the Arthrex TightRope® provide maintenance of the distal tibiofibular syndesmosis? A 2-year follow-up of 64 TightRopes® in 37 patients. J Foot Ankle Surg. 2013;52:563–567. doi: 10.1053/j.jfas.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 56.Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24:106–112. doi: 10.1016/j.arthro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 57.Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35:1680–1687. doi: 10.1177/0363546507303561. [DOI] [PubMed] [Google Scholar]
- 58.O’Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38:392–404. doi: 10.1177/0363546509336336. [DOI] [PubMed] [Google Scholar]
- 59.Hintermann B, Regazzoni P, Lampert C, Stutz G, Gächter A. Arthroscopic findings in acute fractures of the ankle. J Bone Joint Surg Br. 2000;82:345–351. doi: 10.1302/0301-620x.82b3.10064. [DOI] [PubMed] [Google Scholar]
- 60.Ferkel RD, Flannigan BD, Elkins BS. Magnetic resonance imaging of the foot and ankle: correlation of normal anatomy with pathologic conditions. Foot Ankle. 1991;11:289–305. doi: 10.1177/107110079101100506. [DOI] [PubMed] [Google Scholar]
- 61.Potter HG, Chong le R. Magnetic resonance imaging assessment of chondral lesions and repair. J Bone Joint Surg Am. 2009;91 Suppl 1:126–131. doi: 10.2106/JBJS.H.01386. [DOI] [PubMed] [Google Scholar]
- 62.Hannon CP, Smyth NA, Murawski CD, Savage-Elliott I, Deyer TW, Calder JD, Kennedy JG. Osteochondral lesions of the talus: aspects of current management. Bone Joint J. 2014;96-B:164–171. doi: 10.1302/0301-620X.96B2.31637. [DOI] [PubMed] [Google Scholar]
- 63.Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37:1974–1980. doi: 10.1177/0363546509335765. [DOI] [PubMed] [Google Scholar]
- 64.Paul J, Sagstetter M, Lämmle L, Spang J, El-Azab H, Imhoff AB, Hinterwimmer S. Sports activity after osteochondral transplantation of the talus. Am J Sports Med. 2012;40:870–874. doi: 10.1177/0363546511435084. [DOI] [PubMed] [Google Scholar]
- 65.Hangody L, Dobos J, Baló E, Pánics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38:1125–1133. doi: 10.1177/0363546509360405. [DOI] [PubMed] [Google Scholar]
- 66.Niemeyer P, Salzmann G, Schmal H, Mayr H, Südkamp NP. Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc. 2012;20:1696–1703. doi: 10.1007/s00167-011-1729-0. [DOI] [PubMed] [Google Scholar]
- 67.Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, Stokol T, Cheetham J, Nixon AJ. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927–1937. doi: 10.2106/JBJS.I.01284. [DOI] [PubMed] [Google Scholar]
- 68.Smyth NA, Murawski CD, Fortier LA, Cole BJ, Kennedy JG. Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy. 2013;29:1399–1409. doi: 10.1016/j.arthro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40:534–541. doi: 10.1177/0363546511431238. [DOI] [PubMed] [Google Scholar]
- 70.Moustafa El-Sayed AM. Arthroscopic treatment of anterolateral impingement of the ankle. J Foot Ankle Surg. 2010;49:219–223. doi: 10.1053/j.jfas.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Ferkel RD, Karzel RP, Del Pizzo W, Friedman MJ, Fischer SP. Arthroscopic treatment of anterolateral impingement of the ankle. Am J Sports Med. 1991;19:440–446. doi: 10.1177/036354659101900504. [DOI] [PubMed] [Google Scholar]
- 72.Guhl JF. New concepts (distraction) in ankle arthroscopy. Arthroscopy. 1988;4:160–167. doi: 10.1016/s0749-8063(88)80020-3. [DOI] [PubMed] [Google Scholar]
- 73.Cutsuries AM, Saltrick KR, Wagner J, Catanzariti AR. Arthroscopic arthroplasty of the ankle joint. Clin Podiatr Med Surg. 1994;11:449–467. [PubMed] [Google Scholar]
- 74.Tol JL, van Dijk CN. Anterior ankle impingement. Foot Ankle Clin. 2006;11:297–310, vi. doi: 10.1016/j.fcl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Zwiers R, Wiegerinck JI, Murawski CD, Smyth NA, Kennedy JG, van Dijk CN. Surgical treatment for posterior ankle impingement. Arthroscopy. 2013;29:1263–1270. doi: 10.1016/j.arthro.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 76.Endele D, Jung C, Bauer G, Mauch F. Value of MRI in diagnosing injuries after ankle sprains in children. Foot Ankle Int. 2012;33:1063–1068. doi: 10.3113/FAI.2012.1063. [DOI] [PubMed] [Google Scholar]
- 77.Ferkel RD, Tyorkin M, Applegate GR, Heinen GT. MRI evaluation of anterolateral soft tissue impingement of the ankle. Foot Ankle Int. 2010;31:655–661. doi: 10.3113/FAI.2010.0655. [DOI] [PubMed] [Google Scholar]
- 78.Villarreal JM, Cerecedo RB, Cal y Mayor FF, González IL. [Arthroscopic treatment for anterolateral ankle impingement of athletes] Acta Ortop Mex. 2008;22:103–106. [PubMed] [Google Scholar]
- 79.DeBerardino TM, Arciero RA, Taylor DC. Arthroscopic treatment of soft-tissue impingement of the ankle in athletes. Arthroscopy. 1997;13:492–498. doi: 10.1016/s0749-8063(97)90129-8. [DOI] [PubMed] [Google Scholar]
- 80.Nihal A, Rose DJ, Trepman E. Arthroscopic treatment of anterior ankle impingement syndrome in dancers. Foot Ankle Int. 2005;26:908–912. doi: 10.1177/107110070502601102. [DOI] [PubMed] [Google Scholar]
- 81.McMurray T. Footballer’s ankle. J Bone Joint Surg BR. 1950;32:68–69. [Google Scholar]
- 82.Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med. 2002;30:45–50. doi: 10.1177/03635465020300012101. [DOI] [PubMed] [Google Scholar]
- 83.Biedert R. Anterior ankle pain in sports medicine: aetiology and indications for arthroscopy. Arch Orthop Trauma Surg. 1991;110:293–297. doi: 10.1007/BF00443461. [DOI] [PubMed] [Google Scholar]
- 84.Tol JL, Verheyen CP, van Dijk CN. Arthroscopic treatment of anterior impingement in the ankle. J Bone Joint Surg Br. 2001;83:9–13. doi: 10.1302/0301-620x.83b1.10571. [DOI] [PubMed] [Google Scholar]
- 85.Tol JL, van Dijk CN. Etiology of the anterior ankle impingement syndrome: a descriptive anatomical study. Foot Ankle Int. 2004;25:382–386. doi: 10.1177/107110070402500603. [DOI] [PubMed] [Google Scholar]
- 86.van Dijk CN, Tol JL, Verheyen CC. A prospective study of prognostic factors concerning the outcome of arthroscopic surgery for anterior ankle impingement. Am J Sports Med. 1997;25:737–745. doi: 10.1177/036354659702500603. [DOI] [PubMed] [Google Scholar]
- 87.van Dijk CN, Wessel RN, Tol JL, Maas M. Oblique radiograph for the detection of bone spurs in anterior ankle impingement. Skeletal Radiol. 2002;31:214–221. doi: 10.1007/s00256-002-0477-0. [DOI] [PubMed] [Google Scholar]
- 88.Murawski CD, Kennedy JG. Anteromedial impingement in the ankle joint: outcomes following arthroscopy. Am J Sports Med. 2010;38:2017–2024. doi: 10.1177/0363546510369335. [DOI] [PubMed] [Google Scholar]
- 89.Hamilton WG, Geppert MJ, Thompson FM. Pain in the posterior aspect of the ankle in dancers. Differential diagnosis and operative treatment. J Bone Joint Surg Am. 1996;78:1491–1500. doi: 10.2106/00004623-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Hedrick MR, McBryde AM. Posterior ankle impingement. Foot Ankle Int. 1994;15:2–8. doi: 10.1177/107110079401500102. [DOI] [PubMed] [Google Scholar]
- 91.van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc. 2010;18:570–580. doi: 10.1007/s00167-010-1064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bureau NJ, Cardinal E, Hobden R, Aubin B. Posterior ankle impingement syndrome: MR imaging findings in seven patients. Radiology. 2000;215:497–503. doi: 10.1148/radiology.215.2.r00ma01497. [DOI] [PubMed] [Google Scholar]
- 93.Dipaola JD, Nelson DW, Colville MR. Characterizing osteochondral lesions by magnetic resonance imaging. Arthroscopy. 1991;7:101–104. doi: 10.1016/0749-8063(91)90087-e. [DOI] [PubMed] [Google Scholar]
- 94.Robinson P, Bollen SR. Posterior ankle impingement in professional soccer players: effectiveness of sonographically guided therapy. AJR Am J Roentgenol. 2006;187:W53–W58. doi: 10.2214/AJR.05.0614. [DOI] [PubMed] [Google Scholar]
- 95.Calder JD, Sexton SA, Pearce CJ. Return to training and playing after posterior ankle arthroscopy for posterior impingement in elite professional soccer. Am J Sports Med. 2010;38:120–124. doi: 10.1177/0363546509346390. [DOI] [PubMed] [Google Scholar]
- 96.Smyth NA, Murawski CD, Levine DS, Kennedy JG. Hindfoot arthroscopic surgery for posterior ankle impingement: a systematic surgical approach and case series. Am J Sports Med. 2013;41:1869–1876. doi: 10.1177/0363546513489489. [DOI] [PubMed] [Google Scholar]
- 97.van Dijk CN, de Leeuw PA, Scholten PE. Hindfoot endoscopy for posterior ankle impingement. Surgical technique. J Bone Joint Surg Am. 2009;91 Suppl 2:287–298. doi: 10.2106/JBJS.I.00445. [DOI] [PubMed] [Google Scholar]
- 98.Smyth NA, Zwiers R, Wiegerinck JI, Hannon CP, Murawski CD, van Dijk CN, Kennedy JG. Posterior hindfoot arthroscopy: a review. Am J Sports Med. 2014;42:225–234. doi: 10.1177/0363546513491213. [DOI] [PubMed] [Google Scholar]
- 99.Scholten PE, Sierevelt IN, van Dijk CN. Hindfoot endoscopy for posterior ankle impingement. J Bone Joint Surg Am. 2008;90:2665–2672. doi: 10.2106/JBJS.F.00188. [DOI] [PubMed] [Google Scholar]
- 100.Willits K, Sonneveld H, Amendola A, Giffin JR, Griffin S, Fowler PJ. Outcome of posterior ankle arthroscopy for hindfoot impingement. Arthroscopy. 2008;24:196–202. doi: 10.1016/j.arthro.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 101.Orchard J. Is there a relationship between ground and climatic conditions and injuries in football? Sports Med. 2002;32:419–432. doi: 10.2165/00007256-200232070-00002. [DOI] [PubMed] [Google Scholar]
- 102.Gehring D, Rott F, Stapelfeldt B, Gollhofer A. Effect of soccer shoe cleats on knee joint loads. Int J Sports Med. 2007;28:1030–1034. doi: 10.1055/s-2007-965000. [DOI] [PubMed] [Google Scholar]
- 103.Nigg BM, Segesser B. The influence of playing surfaces on the load on the locomotor system and on football and tennis injuries. Sports Med. 1988;5:375–385. doi: 10.2165/00007256-198805060-00003. [DOI] [PubMed] [Google Scholar]
- 104.Ford KR, Manson NA, Evans BJ, Myer GD, Gwin RC, Heidt RS, Hewett TE. Comparison of in-shoe foot loading patterns on natural grass and synthetic turf. J Sci Med Sport. 2006;9:433–440. doi: 10.1016/j.jsams.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 105.Queen RM, Charnock BL, Garrett WE, Hardaker WM, Sims EL, Moorman CT. A comparison of cleat types during two football-specific tasks on FieldTurf. Br J Sports Med. 2008;42:278–284; discussion 284. doi: 10.1136/bjsm.2007.036517. [DOI] [PubMed] [Google Scholar]
- 106.Orchard JW, Chivers I, Aldous D, Bennell K, Seward H. Rye grass is associated with fewer non-contact anterior cruciate ligament injuries than bermuda grass. Br J Sports Med. 2005;39:704–709. doi: 10.1136/bjsm.2004.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women’s professional soccer. Br J Sports Med. 2005;39:212–216; discussion 212-216. doi: 10.1136/bjsm.2004.011973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prisk VR, Imhauser CW, O’Loughlin PF, Kennedy JG. Lateral ligament repair and reconstruction restore neither contact mechanics of the ankle joint nor motion patterns of the hindfoot. J Bone Joint Surg Am. 2010;92:2375–2386. doi: 10.2106/JBJS.I.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]


