Abstract
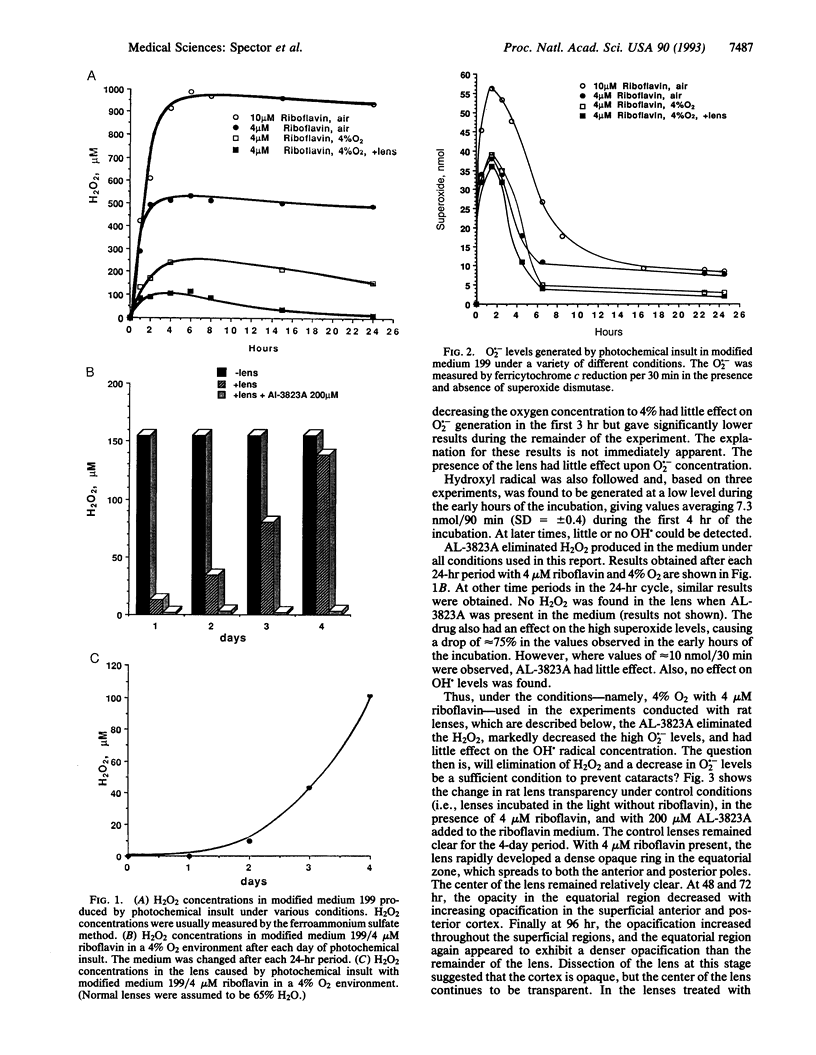
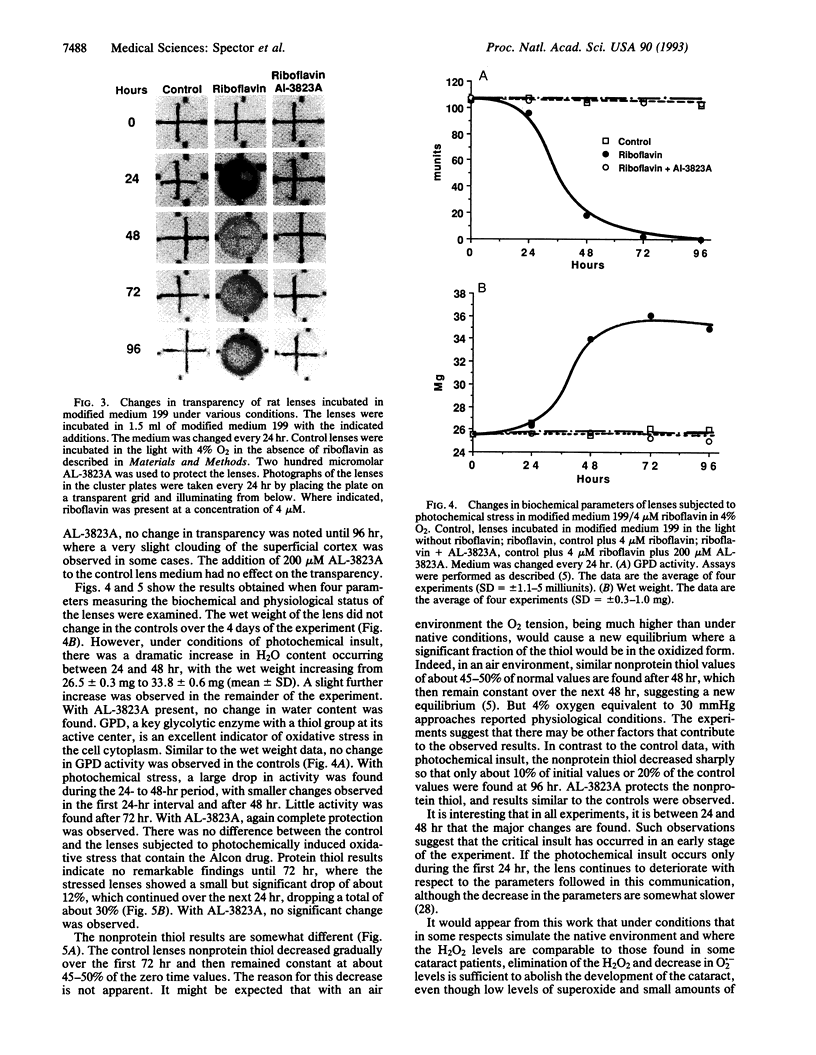
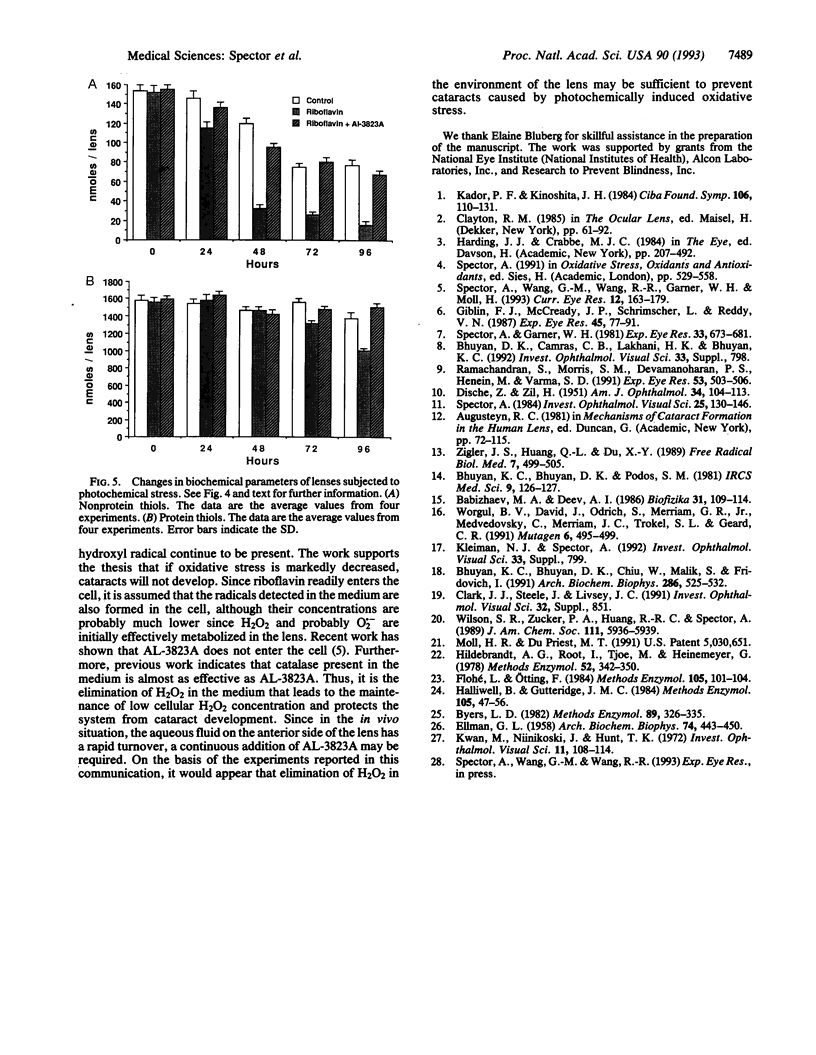
Oxidative stress is known to cause cataracts in lens culture systems and is believed to be an important factor contributing to human cataracts. In this communication, it is demonstrated that cataract development of cultured rat lenses produced as a result of photochemically induced oxidation in a 4% oxygen atmosphere similar to the native environment of the lens can be blocked by the transition metal complex AL-3823A. In this system, riboflavin is added to the medium as a photosensitizer. AL-3823A acts primarily as a glutathione peroxidase mimic, which catalytically metabolizes H2O2 and also has low superoxide dismutase-like activity. Measurements of H2O2, O2.-, and OH. indicate that appreciable levels of the first two of these oxidants and low levels of OH. are produced by this photochemical stressing system. The H2O2 concentrations are similar to those found in some patients with cataracts. The development of cataracts was followed over a 96-hr period. Transparency, hydration, glyceraldehyde-3-phosphate dehydrogenase activity, and protein and nonprotein thiol were monitored. All parameters show marked changes during the 96-hr period. However, in the presence of 200 microM AL-3823A, no difference between control and light-exposed lenses was observed with respect to these parameters. The results suggest that in vivo human cataract development caused by oxidative stress may be prevented by compounds of this type.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babizhaev M. A., Deev A. I. Svobodnoradikal'noe okislenie lipidov i tiolovykh grupp pri kataraktogeneze. Biofizika. 1986 Jan-Feb;31(1):109–114. [PubMed] [Google Scholar]
- Bhuyan K. C., Bhuyan D. K., Chiu W., Malik S., Fridovich I. Desferal-Mn(III) in the therapy of diquat-induced cataract in rabbit. Arch Biochem Biophys. 1991 Aug 1;288(2):525–532. doi: 10.1016/0003-9861(91)90230-g. [DOI] [PubMed] [Google Scholar]
- Byers L. D. Glyceraldehyde-3-phosphate dehydrogenase from yeast. Methods Enzymol. 1982;89(Pt 500):326–335. doi: 10.1016/s0076-6879(82)89059-9. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., ZIL H. Studies on the oxidation of cysteine to cystine in lens proteins during cataract formation. Am J Ophthalmol. 1951 May;34(5 2):104–113. doi: 10.1016/0002-9394(51)90013-x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys. 1958 Apr;74(2):443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- Flohé L., Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- Giblin F. J., McCready J. P., Schrimscher L., Reddy V. N. Peroxide-induced effects on lens cation transport following inhibition of glutathione reductase activity in vitro. Exp Eye Res. 1987 Jul;45(1):77–91. doi: 10.1016/s0014-4835(87)80080-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of iron in oxygen radical reactions. Methods Enzymol. 1984;105:47–56. doi: 10.1016/s0076-6879(84)05007-2. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A. G., Roots I., Tjoe M., Heinemeyer G. Hydrogen peroxide in hepatic microsomes. Methods Enzymol. 1978;52:342–350. doi: 10.1016/s0076-6879(78)52037-5. [DOI] [PubMed] [Google Scholar]
- Kador P. F., Kinoshita J. H. Diabetic and galactosaemic cataracts. Ciba Found Symp. 1984;106:110–131. doi: 10.1002/9780470720875.ch7. [DOI] [PubMed] [Google Scholar]
- Kwan M., Niinikoski J., Hunt T. K. In vivo measurements of oxygen tension in the cornea, aqueous humor, and anterior lens of the open eye. Invest Ophthalmol. 1972 Feb;11(2):108–114. [PubMed] [Google Scholar]
- Ramachandran S., Morris S. M., Devamanoharan P., Henein M., Varma S. D. Radio-isotopic determination of hydrogen peroxide in aqueous humor and urine. Exp Eye Res. 1991 Oct;53(4):503–506. doi: 10.1016/0014-4835(91)90167-d. [DOI] [PubMed] [Google Scholar]
- Spector A., Garner W. H. Hydrogen peroxide and human cataract. Exp Eye Res. 1981 Dec;33(6):673–681. doi: 10.1016/s0014-4835(81)80107-8. [DOI] [PubMed] [Google Scholar]
- Spector A. The search for a solution to senile cataracts. Proctor lecture. Invest Ophthalmol Vis Sci. 1984 Feb;25(2):130–146. [PubMed] [Google Scholar]
- Spector A., Wang G. M., Wang R. R., Garner W. H., Moll H. The prevention of cataract caused by oxidative stress in cultured rat lenses. I. H2O2 and photochemically induced cataract. Curr Eye Res. 1993 Feb;12(2):163–179. doi: 10.3109/02713689308999484. [DOI] [PubMed] [Google Scholar]
- Worgul B. V., David J., Odrich S., Merriam G. R., Jr, Medvedovsky C., Merriam J. C., Trokel S. L., Geard C. R. Evidence of genotoxic damage in human cataractous lenses. Mutagenesis. 1991 Nov;6(6):495–499. doi: 10.1093/mutage/6.6.495. [DOI] [PubMed] [Google Scholar]
- Zigler J. S., Jr, Huang Q. L., Du X. Y. Oxidative modification of lens crystallins by H2O2 and chelated iron. Free Radic Biol Med. 1989;7(5):499–505. doi: 10.1016/0891-5849(89)90025-7. [DOI] [PubMed] [Google Scholar]






