Abstract
KU-32 and KU-596 are novobiocin-derived, C-terminal heat shock protein 90 (Hsp90) modulators that induce Hsp70 levels and manifest neuroprotective activity. However, the synthetically complex noviose sugar requires 10 steps to prepare, which makes translational development difficult. In this study, we developed a series of “noviomimetic” analogues of KU-596, which contain noviose surrogates that can be easily prepared, while maintaining the ability to induce Hsp70 levels. Both sugar and sugar analogues were designed, synthesized, and evaluated in a luciferase reporter assay, which identified compound 37, a benzyl containing noviomimetic, as the most potent inducer of Hsp70.
Keywords: Heat Shock Protein 90, Heat Shock Protein 70, C-terminal inhibition, neuroprotection, noviomimetics
Heat shock protein 90 (Hsp90) is a 90 kDa molecular chaperone that represents a promising biological target for the treatment of cancer and/or neurodegenerative diseases. It exhibits a wide range of functions stemming from its ability to assist in the folding, stability, and rematuration of proteins. Hsp90 interacts with more than 200 client proteins, many of which are oncoproteins that contribute to cancer growth and/or resistance.1−6
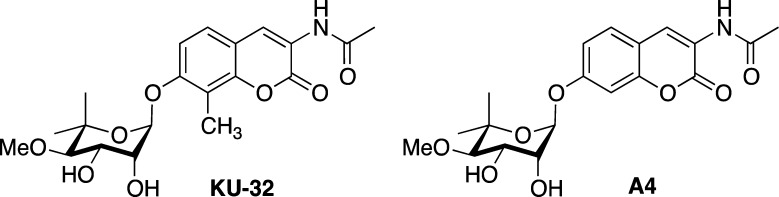
There has been a tremendous focus to develop Hsp90 inhibitors for the treatment of cancer. In fact, 17 small molecules that bind the N-terminus of Hsp90 have entered clinical trials.7,8 These drugs preferentially inhibit Hsp90 and induce client protein degradation in malignant versus normal cells.9−11 Although this selectivity aids the clinical efficacy of N-terminal Hsp90 inhibitors, enthusiasm for their use has been dampened because they also induce the pro-survival heat shock response at the same concentration needed to inhibit client protein folding, which may limit their clinical potential.2 In contrast to N-terminal inhibitors, we have developed novobiocin-based C-terminal inhibitors such as KU-32 and A4 (Figure 1) that can segregate induction of the heat shock response (and subsequent cytoprotection) from client protein degradation (and cytotoxicity).12−19 In fact, KU-32 was found to protect against neuronal glucotoxicity and to reverse clinical end points of diabetic peripheral neuropathy in mice.20,21
Figure 1.
Chemical structures of KU-32 and A4.
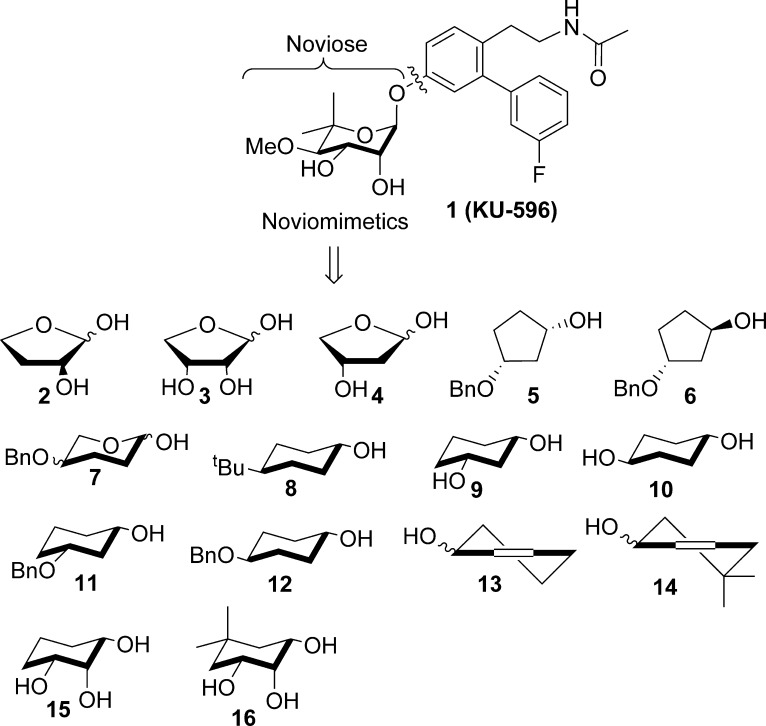
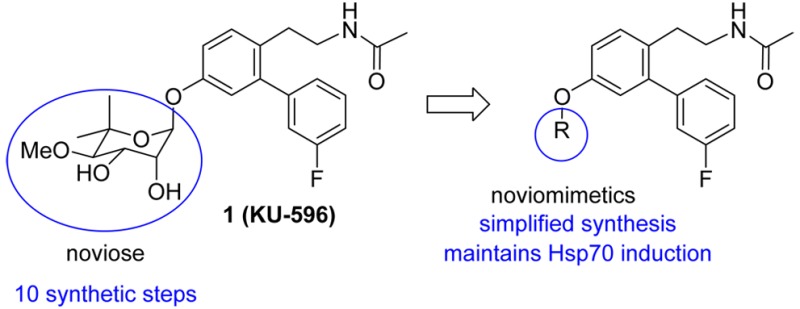
In an ongoing effort to develop C-terminal Hsp90 inhibitors that manifest neuroprotective activity, KU-32 has undergone modifications to produce a second generation of noviosylated analogues, termed novologues. Novologues are small molecules that replaced the coumarin core of KU-32 with a biphenyl ring system and flexible side chain such as KU-596 (1), which manifests an enhancement in neuroprotective activity upon biological evaluation against primary sensory neurons.22 Since novologues contain noviose, a synthetically complex sugar that requires ten steps to prepare, this sugar represents a potential impediment for the translational advancement of such compounds.23−25 To circumvent this concern, a library of succinctly prepared KU-596 analogues containing both sugar and nonsugar surrogates was generated. Previous molecular modeling studies suggested that 1 and KU-32 bind the C-terminal binding site in a manner that projects the noviose sugar into a pocket that could accommodate additional substitutions.22 Therefore, the design of noviose replacements that extend into this pocket and introduce additional interactions within the binding site were pursued. The ability of the non-noviosylated compounds to function as “noviomimetics” by promoting the induction of Hsp70 was assessed.
The noviose surrogates chosen for this study are shown in Figure 2 and include a series of simplified pyranoses that closely mimic the noviose chair conformation. Ring contracted furanose analogues in the envelope conformation that can project substituents into unexplored regions of the Hsp90 C-terminal binding pocket were also investigated.
Figure 2.
Structure of KU-596 (1) and sugar analogues selected for noviose replacement.
As the syntheses of sugars often require many steps, simplified analogues containing a cyclohexyl or cyclopentyl ring were also investigated to determine whether a carbocyclic analogue could exhibit beneficial activity. Analogues containing an alkyl or aryl substituent were also probed to determine constraints within the binding pocket. A racemic mixture of the compounds were used in these studies.
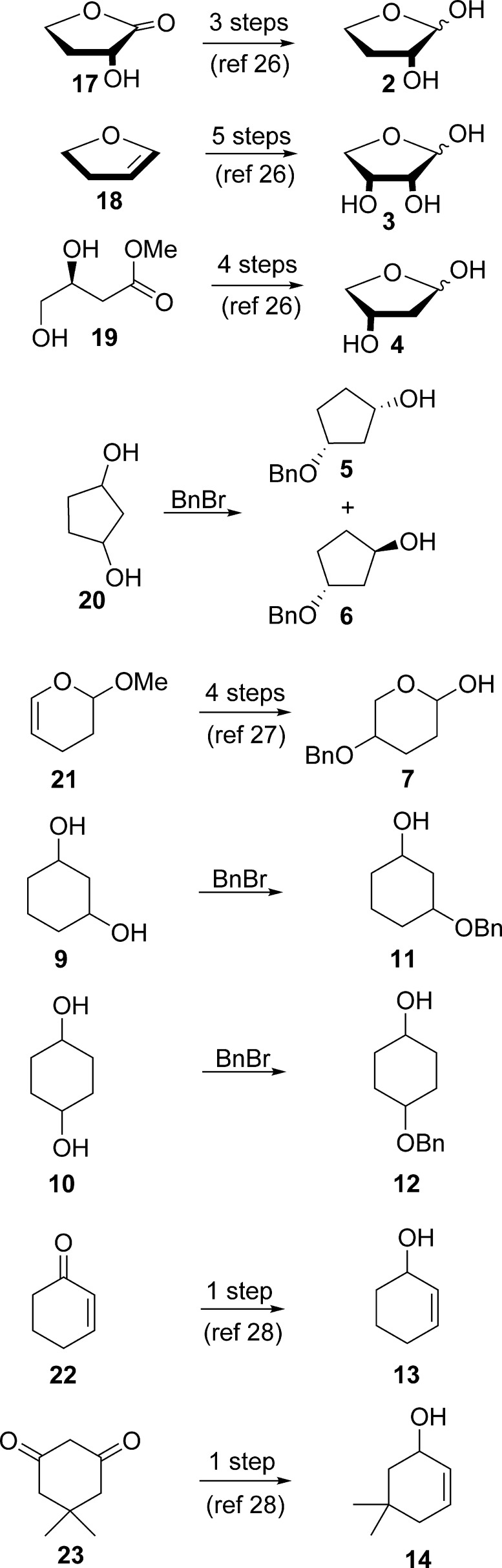
As shown in Scheme 1, furanose derivatives 2, 3, and 4, were synthesized from commercially available (S)-(−)-α-hydroxy-γ-butyrolactone, 17, 2,3-dihydrofuran, 18, and (S)-methyl 3,4-dihydroxybutanoate, 19, respectively, via reported procedures,26 whereas cyclopentanes 5 and 6 were obtained from monobenzylation of commercially available 1,3-cyclopentadiol, 20. The resulting syn- and anti-isomers were easily separated by column chromatography. Sugar 7 was obtained from commercially available 3,4-dihydro-2-methoxypyran, 21, via a four-step procedure.27 Cyclohexane derivatives 8, 9, and 10 are commercially available, whereas 11 and 12 were obtained from monobenzylation of 9 and 10, respectively. Cyclohexene derivatives 13 and 14 were synthesized via published procedures from commercially available 2-cyclohexen-1-one, 22, and 5,5-dimethyl-1,3-cyclohexanedione, 23, respectively (Scheme 1).28 Aglycone 24 was synthesized via our previously reported procedure.22
Scheme 1. Synthesis of 5- and 6-Membered Ring Analogues.
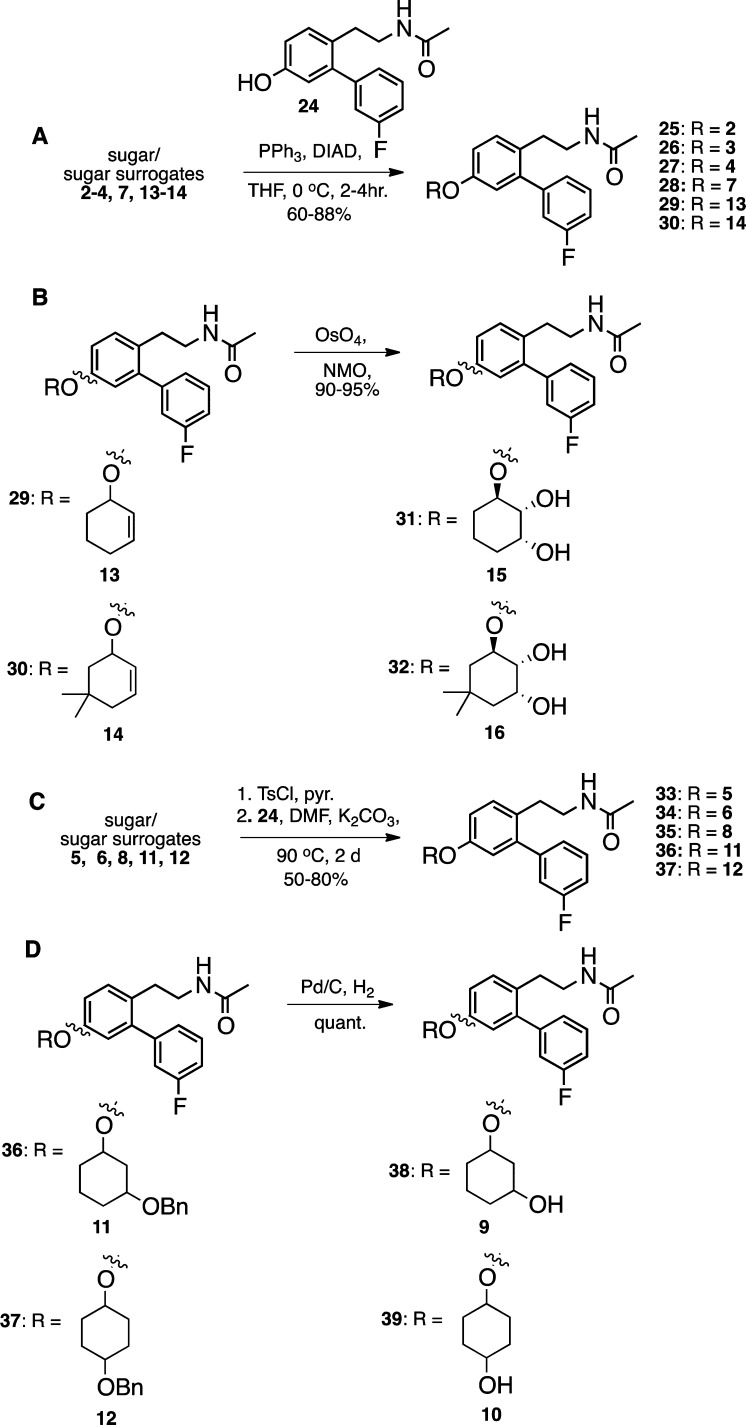
Sugars and sugar surrogates 2, 3, 4, 7, 13, and 14 were linked to aglycone 24 via a Mitsunobu reaction, employing triphenyl phosphine and DIAD, to obtain compounds 25–30 (Scheme 2A). Noviomimetics 29 and 30 were subsequently subjected to an osmium tetroxide-catalyzed dihydroxylation reaction in order to obtain the corresponding diols, 31 and 32, which contain the sugar surrogates, 15 and 16, respectively (Scheme 2B). To obtain compounds 33–37, sugar surrogates 5, 6, 8, 11, and 12 were first converted to the corresponding toluenesulfonates using 4-toluenesulfonyl chloride, after which they were subjected to an SN2 substitution reaction with aglycone 24 to obtain compounds 33–37 (Scheme 2C). The benzyl ether-containing noviomimetics 36 and 37, were cleaved via hydrogenolysis to afford compounds 38 and 39, which contain the sugar surrogates, 9 and 10, respectively (Scheme 2D).
Scheme 2. Synthesis of Noviomimetics 25–39.
We have previously shown that the cytoprotective activity manifested by KU-32 is dependent upon expression of Hsp70, which occurs upon Hsp90 inhibition.21 Therefore, upon construction of the library of noviomimetics, we determined their ability to induce Hsp70 via a luciferase reporter assay. In this assay, an Hsp70 promoter, which contains a heat shock binding element, is subcloned in front of a luciferase reporter gene and the resulting vector transfected into an immortalized sensory neuronal cell line (50B11 cells). The transformed cells are subsequently treated with Hsp90 inhibitors, which displace the transcription factor, heat shock factor 1 (HSF1), from the Hsp90 complex. Upon activation, HSF1 translocates to the nucleus, wherein it binds the Hsp70 promoter and leads to increased luciferase activity that is easily quantified. An increase in luciferase activity represents activation of the Hsp70 promoter.
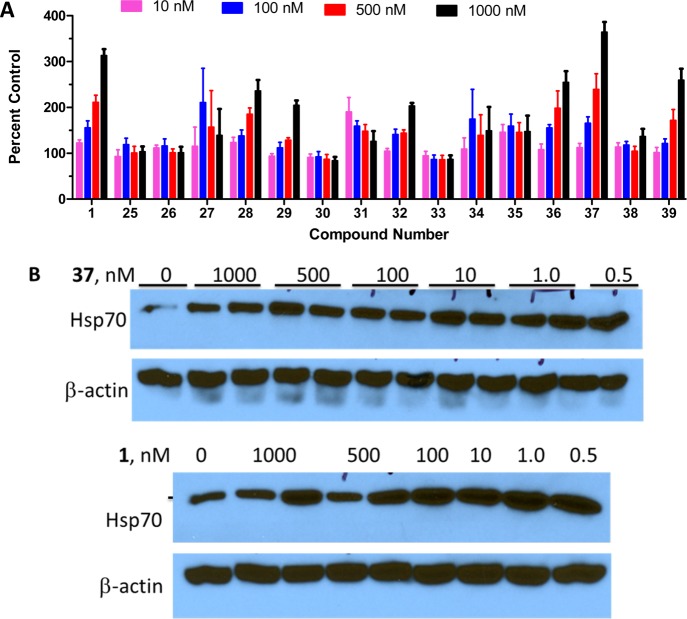
Based on the results shown in Figure 3A, the derivatives that closely mimicked the chair conformation of the noviose sugar, resulted in greater Hsp70 induction. The furanose novologues 25, 26, and 27 were relatively inactive in the luciferase reporter assay, which suggests that the conformation manifested by the furanose derivatives does not project substituents into a favorable region of the binding pocket and, consequently, minimizes Hsp70 induction. Similarly cyclopentanes 33 and 34 did not result in significant induction. The novologues with constrained rings, 29 and 30, were also relatively inactive in the luciferase reporter assay, and do not mimic the chair conformation exhibited by the noviose sugar. These results collectively suggest that the novologues that mimic the chair conformation of the noviose sugar is required for Hsp70 induction.
Figure 3.
(A) Luciferase assay assessing Hsp70 induction by analogues 25–39. Results are mean ± SEM (n = 5–13). (B) Western Blot analysis of Hsp70 induction for 37 and 1 in nontransfected 50B11 cells.
Novologues that contain simplified pyranose derivatives produced varying degrees of luciferase induction. Generally, the more simplified the pyranose, the greater the Hsp70 induction. Compounds 36 and 37 were most active in the luciferase assay and represent simplified pyranose derivatives. Their nonbenzylated derivatives 38 and 39 were less active but exhibit the same trend wherein para substituents result in greater luciferase induction when compared to the meta substituent. These results suggest that not only is the chair conformation of the pyranose important for activity but larger substituents improve activity. As shown in Figure 3A, compound 37, which contains the 4-benzyl ether on the carbocyclic ring, induced the highest level of luciferase in the 50B11 transformed cellular assay and, consequently, was further investigated.
Using nontransfected 50B11 cells, compound 37 was shown to increase Hsp70 levels at concentrations similar to KU-596 (Figure 3B). In fact, 37 continued to induce a robust heat shock response even at subnanomolar concentrations.
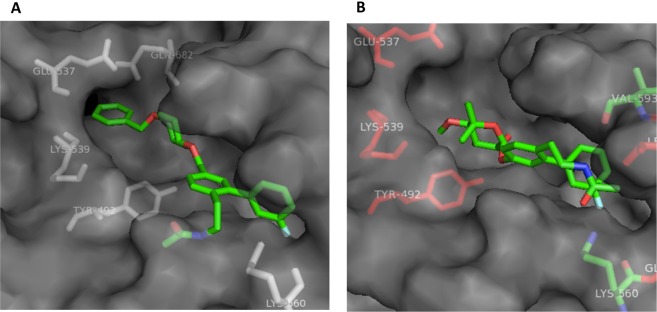
As depicted in Figure 4A, compound 37 is suspected to bind the Hsp90 C-terminal binding site and project the aryl ring further into the pocket, which is left unoccupied in the case of KU-596, Figure 4B. Studies are currently underway to probe for additional interactions between substitutions on the aryl ring of 37 and the binding site.
Figure 4.
(A) Compound 37 docked to Hsp90 C-terminal binding site. (B) KU-596, 1, docked to the Hsp90 C-terminal binding site.
In conclusion, a library of noviomimetics was designed to replace the synthetically complex noviose sugar of 1 with various sugar surrogates. It was determined that a cyclohexyl derivative containing a 4-benzyl ether (37) manifested equipotent activity as KU-596, which significantly simplifies the preparation of such compounds. Furthermore, these studies suggest that noviomimetics can successfully retain the ability to induce Hsp70, a key mechanistic feature that is associated with the neuroprotective activity manifested by the novologue class of compounds, such as 1, as well as the novobiocin-based compounds (e.g., KU-32). Thus, noviomimetics may represent a new series of synthetically simple neuroprotective compounds for the treatment of neurodegenerative diseases.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00331.
Preparation and evaluation of reported compounds (PDF)
This work was supported by grants [CA109265] to B.S.J.B.; [DK095911] to R.T.D. and [NS075311] to B.S.J.B. and R.T.D. from The National Institutes of Health.
The authors declare no competing financial interest.
Supplementary Material
References
- Blagg B. S. J.; Kerr T. A. Hsp90 inhibitors: Small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med. Res. Rev. 2006, 26, 310–338. 10.1002/med.20052. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Michaelis M. L.; Blagg B. S. J. Hsp90 modulation for the treatment of Alzheimer’s disease. Adv. Pharmacol. 2012, 64, 1–25. 10.1016/B978-0-12-394816-8.00001-5. [DOI] [PubMed] [Google Scholar]
- Soti C.; Nagy E.; Giricz Z.; Vigh L.; Csermely P.; Ferdinandy P. Heat shock proteins as emerging therapeutic targets. Br. J. Pharmacol. 2005, 146, 769–780. 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury S.; Welch T. R.; Blagg B. S. J. Hsp90 as a target for drug development. ChemMedChem 2006, 1, 1331–1340. 10.1002/cmdc.200600112. [DOI] [PubMed] [Google Scholar]
- Chiosis G.; Rodina A.; Moulick K. Emerging Hsp90 inhibitors: From discovery to clinic. Anti-Cancer Agents Med. Chem. 2006, 6, 1–8. 10.2174/187152006774755483. [DOI] [PubMed] [Google Scholar]
- Peterson L. B.; Blagg B. S. J. To fold or not to fold: Modulation and consequences of Hsp90 inhibition. Future Med. Chem. 2009, 1, 267–283. 10.4155/fmc.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L.; Workman P. Hsp90 molecular chaperone Inhibitors: Are we there yet?. Clin. Cancer Res. 2012, 18, 64–76. 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biamonte M. A.; Van de Water R.; Arndt J. W.; Scannevin R. H.; Perret D.; Lee W. Heat Shock Protein 90: Inhibitors in clinical trials. J. Med. Chem. 2010, 53, 3–17. 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- Luo W.; Rodina A.; Chiosis G. Heat shock protein 90: translation from cancer to Alzheimer’s disease treatment?. BMC Neurosci. 2008, 9 (Suppl 2), S7. 10.1186/1471-2202-9-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A.; Thao L.; Sensintaffar J.; Zhang L.; Boehm M. F.; Fritz L. C.; Burrows F. J. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003, 425, 407–410. 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- Chiosis G.; Huezo H.; Rosen N.; Mimnaugh E.; Whitesell L.; Neckers L. 17AAG: Low target binding affinity and potent cell activity - finding an explanation. Mol. Cancer Ther. 2003, 2, 123–129. [PubMed] [Google Scholar]
- Zhao H. and Blagg B. S. J.. Inhibitors of the Hsp90 C-terminus. In Inhibitors of Molecular Chaperones As Therapeutic Agents; Machajewski T. D., Gao Z., Eds.; RSC Publishing: London, 2014; pp 259–301. [Google Scholar]
- Shelton S. N.; Shawgo M. E.; Matthews S. B.; Lu Y.; Donnelly A. C.; Szabla K.; Tanol M.; Vielhauer G. A.; Rajewski R. A.; Matts R. L.; Blagg B. S. J.; Robertson J. D. KU135, a novel novobiocin-derived C-Terminal inhibitor of the 90-kDa heat shock protein, exerts potent antiproliferative effects in human leukemic cells. Mol. Pharmacol. 2009, 76, 1314–1322. 10.1124/mol.109.058545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M.; Mukerji R.; Samadi A. K.; Zhang X.; Zhao H.; Blagg B. S. J.; Cohen M. Novel C-Terminal Hsp90 inhibitor for head and neck squamous cell cancer (HNSCC) with in vivo efficacy and improved toxicity profiles compared with standard agents. Ann. Surg. Oncol. 2012, 19 (Suppl 3), S483–S490. 10.1245/s10434-011-1971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlison J. A.; Avila C.; Vielhauer G.; Lubbers D. J.; Holzbeierlein J.; Blagg B. S. J. Development of novobiocin analogues that manifest anti-proliferative activity against several cancer cell lines. J. Org. Chem. 2008, 73, 2130–2137. 10.1021/jo702191a. [DOI] [PubMed] [Google Scholar]
- Burlison J. A.; Blagg B. S. J. Synthesis and evaluation of coumermycin A1 analogues that inhibit the Hsp90 protein folding machinery. Org. Lett. 2006, 8, 4855–4858. 10.1021/ol061918j. [DOI] [PubMed] [Google Scholar]
- Burlison J. A.; Neckers L.; Smith A. B.; Maxwell A.; Blagg B. S. J. Novobiocin: Redesigning a DNA gyrase inhibitor for selective inhibition of Hsp90. J. Am. Chem. Soc. 2006, 128, 15529–15536. 10.1021/ja065793p. [DOI] [PubMed] [Google Scholar]
- Donnelly A.; Mays J. R.; Burlison J. A.; Nelson J. T.; Vielhauer G.; Holzbeierlein J.; Blagg B. S. J. The design, synthesis, and evaluation of coumarin ring derivatives of the novobiocin scaffold that exhibit antiproliferative activity. J. Org. Chem. 2008, 73, 8901–8920. 10.1021/jo801312r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. M.; Shen G.; Neckers L.; Blake H.; Holzbeierlein J.; Cronk B.; Blagg B. S. J. Hsp90 inhibitors identified from a library of novobiocin analogues. J. Am. Chem. Soc. 2005, 127, 12778–12779. 10.1021/ja0535864. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Ansar S.; Michaelis M. L.; Blagg B. S. J. Neuroprotective activity and evaluation of Hsp90 inhibitors in an immortalized neuronal cell line. Bioorg. Med. Chem. 2009, 17, 1709–1715. 10.1016/j.bmc.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban M. J.; Li C.; Yu C.; Lu Y.; Krise J. M.; McIntosh M. P.; Rajewski R. A.; Blagg B. S. J.; Dobrowsky R. T. Inhibiting heat-shock protein 90 reverses sensory hypoalgesia in diabetic mice. ASN Neuro 2010, 2, 189–199. 10.1042/AN20100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma B. R.; Zhang L.; Sundstrom T.; Peterson L. B.; Dobrowsky R. T.; Blagg B. S. J. Synthesis and evaluation of novologues as C-terminal Hsp90 inhibitors with cytoprotective activity against sensory neuron glucotoxicity. J. Med. Chem. 2012, 55, 5797–5812. 10.1021/jm300544c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh B. M.; Shinde M. V.; Kannan M.; Srinivas G.; Iqbal J.; Reddy D. S. Enantiodivergent routes to (+) and (−)-novioses from (−)-pantolactone. RSC Adv. 2013, 3, 20291–20297. 10.1039/c3ra42891e. [DOI] [Google Scholar]
- Matsushima K.; Kino J. Novel concise synthesis of (±)-Noviose and L-(+)-Noviose by palladium-catalyzed epoxide opening. Synthesis 2011, 2011, 1290–1294. 10.1055/s-0030-1258475. [DOI] [Google Scholar]
- Schmidt B.; Hauke S. Metathesis-based de novo synthesis of Noviose. Eur. J. Org. Chem. 2014, 2014, 1951–1960. 10.1002/ejoc.201301615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. M.; Han H.; Blagg B. S. J. Synthesis of mono- and dihydroxylated furanoses, pyranoses, and an oxepanose for the preparation of natural product analogue libraries. J. Org. Chem. 2005, 70, 5599–5605. 10.1021/jo050558v. [DOI] [PubMed] [Google Scholar]
- Beaver M. G.; Billings S. B.; Woerpel K. A. C-Glycosylation reactions of sulfur-substituted glycosyl donors: evidence against the role of neighboring-group participation. J. Am. Chem. Soc. 2008, 130, 2082–2086. 10.1021/ja0767783. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Donnelly A. C.; Kusuma B. R.; Brandt G. E. L.; Brown D.; Rajewski R. A.; Vielhauer G.; Holzbeierlein J.; Cohen M. S.; Blagg B. S. J. Engineering an antibiotic to fight cancer: optimization of the novobiocin scaffold to produce anti-proliferative agents. J. Med. Chem. 2011, 54, 3839–3853. 10.1021/jm200148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Mi R.; Haughey N.; Oz M.; Höke A. Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J. Peripher. Nerv. Syst. 2007, 12, 121–130. 10.1111/j.1529-8027.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen L. N.; Robin P. A. Alicyclic glycols. Part II. Derivatives of cyclohexane-1:4-diol. J. Chem. Soc. 1949, 320–326. 10.1039/jr9490000320. [DOI] [Google Scholar]
- Blagg B. S. J.; Kusuma B. R. Sundstrom T. C-terminal Hsp90 inhibitors. PCT Int. Appl. 2013119985, 2013.
- Kim J. D.; Han G.; Zee O. P.; Jung Y. H. Deprotection of benzyl and p-methoxybenzyl ethers by chlorosulfonyl isocyanate-sodium hydroxide. Tetrahedron Lett. 2003, 44, 733–735. 10.1016/S0040-4039(02)02648-5. [DOI] [Google Scholar]
- Eckhardt M.; Himmelsbach F.; Eickelmann P. Thomas L.. Glucopyranosyl-substituted benzyl-benzene derivatives, medicaments containing such compounds, their use and process for their manufacture. U.S. Pat. Appl. Publ. 20070049537, 2007.
- Matts R. L.; Dixit A.; Peterson L. B.; Sun L.; Voruganti S.; Kalyanaraman P.; Hartson S. D.; Verkhivker G. M.; Blagg B. S. J. Elucidation of the Hsp90 C-Terminal Inhibitor Binding Site. ACS Chem. Biol. 2011, 6, 800–807. 10.1021/cb200052x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.